Abstract
It has been demonstrated from previous studies about the killing effect of dihydroartemisinin (DHA) on glioblastoma, which involves multiple aspects: cytotoxicity, cell cycle arrest and invasion inhibition. DHA has the advantages of low cytotoxicity to normal cells, selective killing effect and low drug resistance, making it one of the popular anti-tumor research directions. Ferroptosis is a newly discovered form of cell death characterized by iron dependence and lipid reactive oxygen species (ROS) accumulation. In the present study, we found differences in the expression of transferrin receptors in normal human astrocytes (NHA) and glioblastoma cells (U87 and A172), which may be one of the mechanisms of DHA selective killing effect. Through the determination of ferroptosis-related protein expression, we found that the significant decrease of GPX4, accompanied by the constant expression of xCT and ACSL4, suggesting GPX4 was a pivotal target for DHA-activated ferroptosis in glioblastoma. Total and lipid ROS levels were increased and all these results could be reversed by the ferroptosis inhibitor, ferrostatin-1. These findings demonstrated ferroptosis would be a critical component of cell death caused by DHA and GPX4 was the main target. All these results provide a novel treatment direction to glioblastoma. The association between ferroptosis and polyamines is also discussed, which will provide new research directions for ferroptosis caused by DHA in glioblastoma.
Keywords: dihydroartemisinin, ferroptosis, glioma, selective, transferrin receptors
Introduction
Glioblastoma is the most malignant glioma with high mortality and recurrence rates, with the average survival time of less than 18 months [1]. At present, surgical treatment combined with temozolomide chemotherapy and radiotherapy are the main methods [2]. However, temozolomide-based chemotherapy has developed drug resistance and serious side effects [3], radiotherapy has the disadvantage that large dose of radiotherapy would cause damage to normal brain tissue [4], which suggests other adjuvant or alternative chemotherapy methods are urgently needed.
In recent years, researches on molecular therapies and natural plant extracts have been widely carried out. In terms of molecular therapies, the role of micro-RNA has been extensively studied. For example, it has been shown that miR-5096 could initiate invasion inhibition in glioblastoma through decline of channel Kir4.1 [5]. To plant extracts, various effective anti-cancer ingredients have been found, including taxol, cryptotanshinone, baicalin and artemisinin [6–9]. Artemisinin is an active ingredient extracted from the natural plant Artemisia annua and currently widely used in the treatment of malaria [10]. In recent years, artemisinin has been found to be other than anti-malarial effects, including anti-tumor, anti-neurodegeneration [11] and anti-systemic lupus erythematosus effects [12]. Based on the high safety of artemisinin, there are many anti-tumor studies for artemisinin, including lung cancer [13], hepatocellular carcinoma [14], chronic leukemia [15] and glioblastoma [16–19]. According to previous studies, the killing effect of artemisinin on tumors was selective, which may be related to the increased expression of transferrin receptor on the cell membrane [21]. Dihydroartemisinin (DHA) is the metabolic form of artemisinin in vivo, which is several times more potent than artemisinin.
The Cytotoxicity mechanism of artemisinin on glioblastoma has been studied in some studies. Artemisinin and its derivatives play an anti-glioblastoma role through multiple mechanisms such as apoptosis [19], autophagy [18] and invasion inhibition [16]. The artemisinin contains an endoperoxide bridge that reacts with a ferrous iron atom to form free radicals, which then cause damage to cells [10]. It seems that artemisinin may be related to the ferroptosis. Ferroptosis is a newly discovered mode of programmed cell death [20], of which the death process is different from that of apoptosis, autophagy and necrosis [22]. Previous studies have shown that high-grade tumors express higher ferroptosis-resistance proteins and enhanced ferroptosis can significantly increase tumor inhibition [23]. Studies have found that temozolomide and pseudolaric acid B have an anti-tumor effect in glioblastoma through promoting ferroptosis [24,25]. In addition, it has been shown that artemisinin and its derivatives activate ferroptosis and then inhibit head and neck carcinoma and fibrosarcoma [26,27]. To date, there is no literature to verify whether ferroptosis exists in the cell death of glioblastoma caused by artemisinin and its status and importance in it.
Materials and methods
Reagents
DHA and ferrostatin-1 were purchased from Sigma-Aldrich Co. (St Louis, MO, U.S.A.) and was dissolved in DMSO. In all experiments, the final DMSO concentration was 0.1%(v/v) and DMSO alone had no demonstrable effect on cultured cells.
Cell culture
Glioblastoma U87, A172 cell lines were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Normal human astrocyte (NHA) was obtained from the Institute of Basic Medical Sciences (Beijing, China). U87 and A172 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Waltham, MA, U.S.A.) and NHA were grown in the Astrocyte Medium (AM; Sciencell, San Diego, CA, U.S.A.) both containing 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, U.S.A.) and penicillin (100 U/ml)/streptomycin (100 μg/ml) (HyClone, GE Healthcare Life Sciences, Logan, UT, U.S.A.) in an incubator with humidified atmosphere of 5% CO2/ 95% air at 37°C.
Cell proliferation assay
Cells were plated in 96-well plates at a density of 5 × 104 and treated with different concentrations of DHA (0, 25, 50, 66, 100, 200 and 300 μM) for 24 h. The effects of DHA on U87 and A172 cell proliferation were evaluated by the Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) viability assay according to the manufacturer’s instruction.
Western blot analysis
Cells with the treatment of DHA were harvested and rinsed with PBS, then extracted in 300 μl radio immunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China). Proteins were determined by the bicinchoninic acid assay (BCA) kits (Beyotime, Shanghai, China). The proteins were loaded and size-fractionated by SDS-PAGE gels, then transferred onto PVDF membranes (EMD Millipore, Billerica, MA, U.S.A.). After blocking, the membranes were incubated with primary antibodies at 4°C overnight, including anti-GPX4 (1:1000), ACSL4 (1:1000), xCT (1:1000), HO-1 (1:1000), TfR (1:500) and β-actin (1:5000) or GAPDH (1:5000). All these antibodies were purchased from Abcam (Cambridge, MA, U.S.A.). After TBST washes, the membranes were incubated with the appropriate peroxidase conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, U.S.A.). At last, protein bands were visualized using the enhanced chemiluminescence detection reagents (EMD Millipore) by a chemiluminescence imaging system (Tanon, Shanghai, China).
Measurement of total and lipid ROS levels
Total ROS levels were measured by DCFH-DA (Beyotime, Shanghai, China) and lipid ROS levels were tested using BODIPY 581/591 C11 Lipid Peroxidation Sensor (Thermo Fisher Scientific, Waltham, MA, U.S.A.). After indicated treatments for 8 h, cells were washed with serum-free culture medium and incubated with 5 μM DCFH-DA or BODIPY for 30 min, followed by determination of the fluorescence intensity by a FACS-Calibur flow cytometer (BD Biosciences, San Jose, CA, U.S.A.). Results were analyzed using FlowJo software.
Immunofluorescence staining
Cells were seeded on coverslips. After treatment of DHA, the cells were fixed with 4% formaldehyde for 15 min, then washed with PBS, permeabilized in 0.3% Triton-X100 for 20 min, blocked with 10% normal goat serum for 1 h. Coverslips were incubated with the GPX4 antibody overnight, washed with PBST and incubated with Alexa Fluor 488 anti-rabbit secondary antibody for 1 h. Then, they were incubated with DAPI for 5 min and fixed on glass slides. The coverslips were scanned with a ZEISS immunofluorescence microscope.
Transmission electron microscopy (TEM)
The u87 and A172 cell lines treated with DHA were harvested, washed and fixed with 2.5% glutaraldehyde for overnight, then fixed in 1% osmium tetroxide for 1 h at 4°C. After washing, the cell pellets were embedded in epon araldite. The ultrathin sections were observed with a Hitachi-h7650 electron microscope and representative images were analyzed.
Statistical analysis
Data are expressed as mean ± SD. All statistical analyses were performed using Statistical Package for Social Sciences software (SPSS 22.0). Statistical significance was calculated using the Student’s t-test or one-way analysis of variance (ANOVA). Differences were considered significant if P<0.05 (*P<0.05; **P<0.01).
Results
DHA produced different cytotoxic effects on astrocytes and glioblastoma
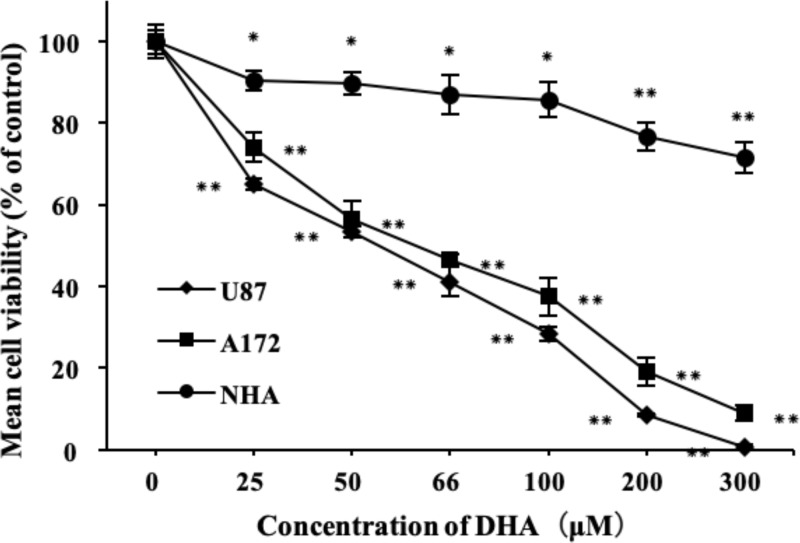
Glioblastoma cell lines U87, A172 and normal human astrocyte (NHA) were treated with different concentrations of DHA (0–300 μM) for 24 h. The effect of DHA on viability was detected by CCK-8 test kits. As shown in Figure 1, DHA reduced the viability of U87 and A172 in a dose-dependent manner in vitro, but had almost no effect on NHA. At concentration of about 300 μM, both U87 and A172 cells were almost completely killed, whereas NHA cells still survived (almost 70%). These results suggested that DHA had a selective cytotoxic effect on glioblastoma compared with normal human astrocytes. The IC50 of DHA for U87 and A172 was 50 and 66 μM, respectively. Based on these data, we carried out the subsequent experiments to detail the mechanisms underlying the cytotoxic effect.
Figure 1. Effects of DHA on the viability of glioblastoma cell lines.
U87, A172 and NHA cells were treated with different concertrations of DHA (0–300 μM) for 24 h. Cell viability was measured by CCK-8 assay. Data are expressed as mean ± S.E.M. (n=3 for each group). *P<0.05; **P<0.01 versus control group.
The expression of TfR in glioblastoma was higher than that in astrocytes
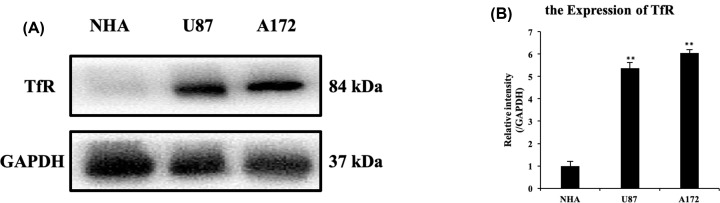
According to the previous studies, iron plays an important role in the process of cell metabolism [28]. The concentration of iron atom in tumor cells is much higher than that of normal cells, which may be related to the high expression of transferrin receptor (TfR) on the surface of tumor cells [29]. Targeted drug delivery through transferrin receptors is also a hot spot in recent years. To confirm the selective cytotoxic effect of DHA between two glioblastoma cell lines and NHA, we performed a Western blot analysis of TfR. It could be seen from the Figure 2 that TfR expression of U87 and A172 was significantly higher than that of NHA (with more than five times), suggesting that transferrin receptor in glioblastoma was much higher than that in normal astrocytes, and it may be one of the mechanisms for selective killing effect of DHA.
Figure 2. The expression difference of transferrin receptor between glioblastoma and astrocyte cells.
(A and B) The proteins of U87, A172 and NHA cells were extracted without any treatment. The expression of proteins was analyzed by Western blot analysis with indicated antibodies. The bands were then quantified by ImageJ software. Results are the mean ± S.E.M. (n=3 for each group). *P<0.05; **P<0.01 versus control group.
DHA promoted ferroptosis-related ROS formation
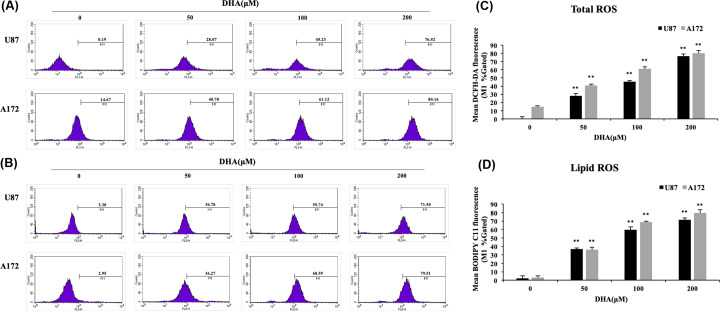
As we have described above, lipid reactive oxygen species (ROS) accumulation is one of the characteristics of ferroptosis, so we tested total ROS levels by DCFH-DA and lipid ROS levels by BODIPY C11 in the groups with different concentrations of DHA. Flow cytometry analysis revealed that DHA treatment resulted in both total and lipid increased ROS levels compared with the control group and the difference was dose dependent (Figure 3). These results indicated that the cytotoxicity of DHA was dependent on total and lipid ROS generation.
Figure 3. The reactive oxygen species levels in U87 and A172 cells with the treatment of DHA.
(A and C) The total ROS levels in U87 and A172 cells with DHA (0, 50, 100, 200 μM) were measured by DCFH-DA kits. (B and D) The lipid ROS levels by BODIPY C11 kits. Results are the mean ± S.E.M. (n=3 for each group). *P<0.05; **P<0.01 versus control group.
DHA modulated the expression of ferroptosis-related proteins in glioblastoma
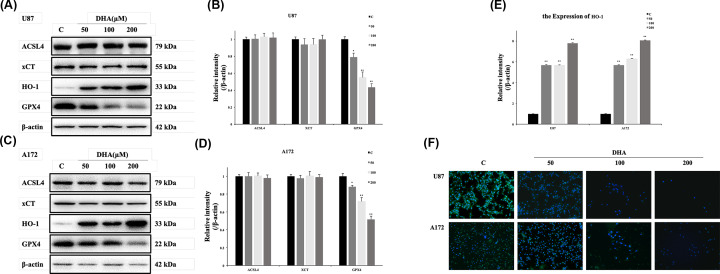
It has been described that GPX4, xCT and ACSL-4 are the main targets in the regulation of ferroptosis [30–32] and GPX4 is the key in the ferroptosis of head and neck carcinoma cells [26]. To clarify the specific target of DHA-initiated ferroptosis in glioblastoma, ferroptosis-related protein expression was determined. Compared with control groups, the expression of GPX4 in both U87 and A172 cells were down-regulated in the DHA-treated groups, accompanied by a constant expression of ACSL-4 and xCT (Figure 4A–D). These suggested that DHA activated ferroptosis by the inhibition of GPX4 in glioblastoma. For further verification, immunofluorescence of GPX4 was also performed (Figure 4F). Images suggested GPX4 decreased with DHA-concentration increasing, which is the same as above. In addition, up-regulation of HO-1 expression was also observed in the treatment group (Figure 4E), which was thought to have both a dual role in anti-oxidation and ferroptosis promoting [33]. Further research is needed to clarify the specific role of HO-1 in ferroptosis.
Figure 4. DHA initiated ferroptosis in glioblastoma by down-regulation of GPX4.
(A–E) U87 and A172 cells were treated with the indicated concentration of DHA for 24 h. The expression of proteins was analyzed by Western blot analysis with indicated antibodies. The bands were then quantified by ImageJ software. Results are the mean ± S.E.M. (n=3 for each group). *P<0.05; **P<0.01 versus control group. (F) U87 and A172 cells were treated with the indicated concentration of DHA for 24 h. The expression and position of GPX4 in two glioblastoma cells were detected by Immunofluorescence staining. The green fluorescence represented GPX4 and blue fluorescence represented nucleus.
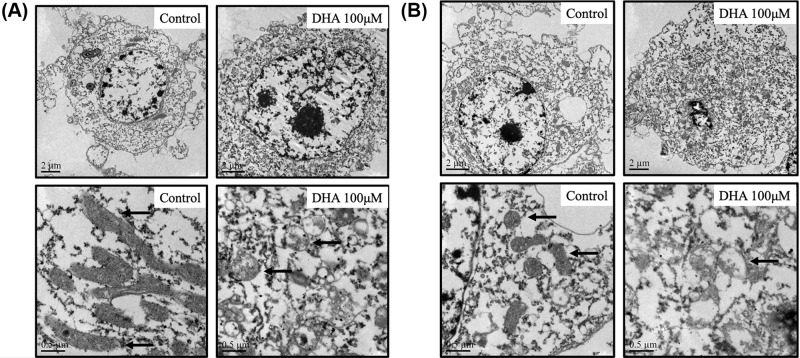
DHA caused the ferroptosis-related structural changes in TEM
The ultramorphological features of ferroptosis are considered as cell membrane rupture and blistering, mitochondria becoming smaller, membrane density increasing, mitochondrial ridges decreasing or disappearing, mitochondrial outer membrane rupture, normal nucleus size, but lack of chromatin condensation [30–32]. To confirm the initiation of ferroptosis, we observed the cell morphology in the control groups and DHA treatment groups (100 μM) under transmission electron microscope (TEM). It was found in both U87 and A172 cells that mitochondria became smaller with the mitochondrial ridges decreasing. The bilayer membrane density was also increased, which was considered as the symbol of ferroptosis (Figure 5).
Figure 5. The ultramorphological structures of two glioblastoma cells in TEM with DHA treatment.
(A and B) The ultramorphological structure difference between control groups and DHA groups was presented. Diversification in mitochondrial structures was primarily observed.
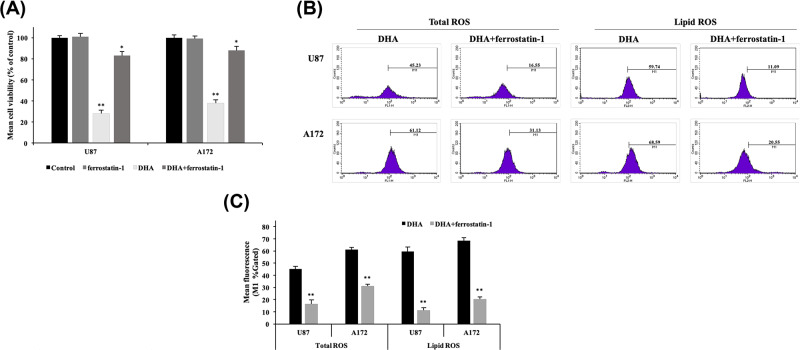
Effect of DHA could be reversed by the ferroptosis inhibitor (ferrostatin-1)
It has been shown that ferrostatin-1 is the specific inhibitor of ferroptosis by inhibiting lipid ROS accumulation, which is an alternative to GPX4 and has no influence on other kinds of cell death. In addition, it has no effect when applied alone to cells [34,35]. To confirm that ferroptosis was responsible for the above results, we compared the results of treatment with 100 μM DHA and 100 μM DHA + 20 μM ferrostatin-1 [36] in the cell survival experiment and two kinds of ROS levels assays. In the cell survival experiment by CCK-8 assay, it was found that ferrostatin-1 treament alone had no influence in both two glioblastoma cell lines, whereas DHA+ferrostatin-1 treatment showed a significant reversal effect compared with the DHA treatment (viability increasing over 40%) (Figure 6A). From the Figure 6B,C, it was obvious that ferrostatin-1 significantly reversed DHA-induced climb of total and lipid ROS levels, which verified ferroptosis initiated the above results.
Figure 6. Effect of ferrostatin-1 on DHA-treated cells.
(A) U87 and A172 cells were treated with ferrostatin-1 (20 μM) alone, DHA (100 μM) alone and DHA+ferrostatin-1 for 24 h. Cell viability was measured by CCK-8 assay. Data are expressed as mean ± S.E.M. (n=3 for each group). *P<0.05; **P<0.01 versus control group. (B and C) The comparison of total and lipid ROS levels between DHA groups and DHA+ferrostatin-1 groups was measured by DCFH-DA and BODIPY C11 kits, respectively. Results are the mean ± S.E.M. (n=3 for each group). *P<0.05; **P<0.01 versus control group.
Discussion
Previous studies have demonstrated the possibility of DHA as an anticancer drug, including glioblastoma [16–18]. DHA has a selective cytotoxic effect, which is thought to be related to the transferrin receptor on the cell membrane surface [28]. Artemisinin plays its role by oxidizing free radicals by Fenton reaction with iron ions. In addition, transportation of iron ions depends on the involvement of transferrin receptor [37]. Therefore, the increased expression of transferrin receptor causes an increase in iron ion uptake, then enhancing the effect of artemisinin on tumor cells. In the present study, we demonstrated the cytotoxic effect of DHA on glioblastoma, accompanied with weak effect on normal astrocytes by CCK8 assay. We first compared the difference of transferrin receptors expression in normal astrocytes and glioblastomas by Western blot, validating the origin of DHA selectivity, and the results revealed, as we thought, TfR expression in two glioblastoma cell lines was much higher than that in the normal astrocytes.
There are many mechanisms underlying cell death. Ferroptosis is a unique form of death, which is distinguished from other kinds of cell death such as apoptosis and necrosis by its characteristics of iron dependence and lipid ROS accumulation [22]. There have been some studies that confirmed the activation of ferroptosis in glioblastoma [38,39]. In the present study, we first discovered the effect of DHA in the glioblastoma lines U87, A172 by ferroptosis through Western blot, we found that DHA induced ferroptosis by down-regulating glutathione peroxidase 4 (GPX4), and this result was verified in immunofluorescence staining. One of the functions of GPX4 is to eliminate lipid ROS formation and its decrease would cause accumulation of lipid ROS, then causing ferroptosis. Other proteins associated with ferroptosis did not show significant differences with treatments of DHA. xCT is a constituent protein of cystine/glutamate transporter, and its function is mainly to provide a substrate for the synthesis of glutathione. The down-regulation of xCT would lead to a decrease in ROS clearance of GPX4 through insufficient synthesis of glutathione, and finally cause cell death [40,41]. ACSL4 is one of the essential components for triggering ferroptosis by participating in the synthesis of the easily oxidized membrane phospholipids, making cells sensitive to RSL3 and other inducing factors [42,43]. Interestingly, an up-regulation of HO-1 expression was also observed from the result of Western blot. As we mentioned above, HO-1 is not only an antioxidant enzyme but also a necessary factor in ferroptosis [33]. The raise of HO-1 expression is closely related to the up-regulation of iron ions dose, which is an important foundation of ferroptosis. When HO-1 is at a normal level, it mainly exerts an antioxidant effect, and when it is excessively elevated, it causes an accumulation of iron ions. The role of elevated HO-1 expression in DHA requires further investigation to validate. By flow cytometry, we demonstrated the up-regulation of total ROS levels and lipid ROS levels in U87 and A172 after DHA treatment. The accumulation of lipid ROS is the ultimate cause of ferroptosis, so this result fully confirms the value of ferroptosis as a necessary role in the cell death by DHA treatment. Through transmission electron microscope (TEM), we observed that the mitochondria in U87 and A172 cells became smaller and mitochondrial ridges were decreasd, as well as the density of the bilayer membrane was increased, confirming the existence of ferroptosis. To further validate the initiation of ferroptosis in DHA-induced glioblastoma death, we used the ferroptosis inhibitor ferrostatin-1. This inhibitor specifically protects cells from ferroptosis without affecting apoptosis, necrosis, etc. Ferrostatin-1 inhibits the synthesis of lipid peroxides in ferroptosis, playing a role like GPX4. Comparison of DHA groups and DHA+ferrostatin-1 groups further confirmed that ferroptosis was a pivotal part of the DHA-produced cytotoxicity.
It is needed to note that association between iron and polyamines has been studied in recent years. Polyamines are a kind of molecules with biological activity which exist widely in organisms. It has been found that polyamine content in cancer is up-regulated, and polyamine inhibition could inhibit tumor growth [44]. In the nervous system, polyamines are found mainly in astrocytes, and that in other glial cells is relatively low, such as Bergmann and Müller glial cells. However, it is found to be rare in neurons [45,46]. 5′-Methylthioribose (MTA), by-product in the polyamine pathway, which can inhibit polyamine accumulation, is the substrate of methionine salvage pathway, and the process requires the participation of ferrous ions [44]. Other studies have found that, after using of O-trensox and Deferasirox, in addition to the down-regulation of iron concentration, the polyamine content showed a downward trend [47]. This demonstrates a preliminary association between iron and polyamines, so it makes sense for further experiments to investigate the effects of DHA on polyamines. Based on the synchrony, we hypothesize that differences in polyamine degradation between astrocyte and glioblastoma might be another explanation for selective killing effect. Further research is needed to confirm this conclusion. Then, the association between ferroptosis and polyamines is considered. Previous studies have shown that key enzymes in polyamine metabolism are closely related to ferroptosis. The Activation of SAT1, the speed limit of polyamine metabolism enzyme, caused the decrease of polyamine content and the increase of lipid peroxides(ferroptosis) [48]. The other two enzymes in the metabolic process, polyamine oxidase (PAOX) and spermine oxidase (SMOX), could produce reactive oxygen species while degrading polyamines [44]. These studies demonstrate the interaction between polyamine and ferroptosis. It is important to notice that whether PAOX and SMOX affect lipid peroxide levels and there are other interactions in this process. In addition, when intracellular polyamine synthesis is blocked or degraded, SLC22A transporters would transport polyamines into the cell [44], so whether DHA has an impact on SLC22A needs be further studied. To verify the independence of ferroptosis, inhibition of polyamines needs be included in subsequent experiments, such as polyamine oxidase inactivator MDL72527 [49].
In conclusion, our study demonstrated that DHA had a selective killing effect on glioblastoma, which was associated with over-expression of transferrin receptors. The primary mechanism by which DHA caused ferroptosis was down-regulation of GPX4 and the following lipid ROS accumulation. All these changes would be reversed by ferrostatin-1, a specific ferroptosis inhibitor. It was suggested that ferroptosis was a pivotal part and GPX4 was the critical target in the cell death on glioblastoma by DHA treatment, which provided the possibility and basis for the subsequent use of DHA for alternative or adjuvant therapy for glioblastoma. We also discuss the association between ferroptosis and polyamines, which will provide directions for further research on ferroptosis caused by DHA in glioblastoma.
Abbreviations
- ACSL4
long-chain acyl-CoA synthetases 4
- DHA
dihydroartemisinin
- GPX4
glutathione peroxidase 4
- NHA
normal human astrocytes
- ROS
reactive oxygen species
- SMOX
spermine oxidase
- TEM
transmission electron microscope
- TfR
transferrin receptor
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This study was grant supported by the National Natural Science Foundation of China [grant numbers 81672503, 81571162 and 81401026].
Author Contribution
Conceived and designed the experiments: Renxin Yi. Performed the experiments: Renxin Yi, Chulei Deng and Xinyue Wang. Analyzed the data: Renxin Yi, Lei Yao, Wenhao Niu, Maoxing Fei and Wangdui Zhaba. Wrote original draft: Renxin Yi and Xinyue Wang, review and edited: Handong Wang, Contributed reagents/materials/analysis tools: Handong Wang
References
- 1.Prelaj A., Rebuzzi S.E., Grassi M. et al. (2019) Non-conventional fotemustine schedule as second-line treatment in recurrent malignant gliomas: Survival across disease and treatment subgroup analysis and review of the literature. Mol. Clin. Oncol. 10, 58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittal S., Pradhan S. and Srivastava T. (2015) Recent advances in targeted therapy for glioblastoma. Exp. Rev. Neurother. 15, 1–12 10.1586/14737175.2015.1061934 [DOI] [PubMed] [Google Scholar]
- 3.Jiapaer S., Furuta T., Tanaka S. et al. (2018) Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma. Neurol. Med. Chir. (Tokyo) 58, 405–421 10.2176/nmc.ra.2018-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Chen D., Zhu H. et al. (2019) LITAF Enhances Radiosensitivity of Human Glioma Cells via the FoxO1 Pathway. Cell. Mol. Neurobiol. 39, 871–882 10.1007/s10571-019-00686-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thuringer D., Chanteloup G., Boucher J. et al. (2017) Modulation of the inwardly rectifying potassium channel Kir4.1 by the pro-invasive miR-5096 in glioblastoma cells. Oncotarget 8, 37681 10.18632/oncotarget.16949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M.C., Chen C.A., Hsieh C.Y. et al. (2009) Mesothelin inhibits paclitaxel-induced apoptosis through the PI3K pathway. Biochem. J. 424, 449–458 10.1042/BJ20082196 [DOI] [PubMed] [Google Scholar]
- 7.Lu L., Zhang S., Li C. et al. (2017) Cryptotanshinone inhibits human glioma cell proliferation in vitro and in vivo through SHP-2-dependent inhibition of STAT3 activation. Cell Death Dis. 8, e2767 10.1038/cddis.2017.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y., Fang J., Wang H. et al. (2018) Baicalin suppresses proliferation, migration, and invasion in human glioblastoma cells via Ca2+-dependent pathway. Drug Des. Dev. Ther. 12, 3247 10.2147/DDDT.S176403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Que Z., Wang P., Hu Y. et al. (2017) Dihydroartemisin inhibits glioma invasiveness via a ROS to P53 to β-catenin signaling. Pharmacol. Res. 119, 72–88 10.1016/j.phrs.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 10.Kannan R., Kumar K., Sahal D. et al. (2005) Reaction of artemisinin with haemoglobin: implications for antimalarial activity. Biochem. J. 385, 409–418 10.1042/BJ20041170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu B.W., Baum L., So K.F. et al. (2019) More than anti-malarial agents: therapeutic potential of artemisinins in neurodegeneration. Neural Regen. Res. 14, 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Dong Y., Tu Y. et al. (2006) Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int. Immunopharmacol. 6, 1243–1250 10.1016/j.intimp.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Zhang B., Zhang Z., Wang J. et al. (2018) Dihydroartemisinin sensitizes Lewis lung carcinoma cells to carboplatin therapy via p38 mitogen-activated protein kinase activation. Oncol. Lett. 15, 7531–7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Im E., Yeo C., Lee H.J. et al. (2018) Dihydroartemisinin induced caspase-dependent apoptosis through inhibiting the specificity protein 1 pathway in hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 192, 286–292 10.1016/j.lfs.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 15.Li X., Gao Y., Zhang Q. et al. (2018) Dihydroartemisinin-regulated mRNAs and lncRNAs in chronic myeloid leukemia. Oncotarget 9, 2543 10.18632/oncotarget.23274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Chen X., Wang F. et al. (2015) Dihydroartemisinin suppresses glioma proliferation and invasion via inhibition of the ADAM17 pathway. Neurol. Sci. 36, 435–440 10.1007/s10072-014-1963-6 [DOI] [PubMed] [Google Scholar]
- 17.Shao Y.Y., Zhang T.L., Wu L.X. et al. (2017) AKT axis, miR-21, and RECK play pivotal roles in dihydroartemisinin killing malignant glioma cells. Int. J. Mol. Sci. 18, 350 10.3390/ijms18020350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z.S., Wang J., Shen Y.B. et al. (2015) Dihydroartemisinin increases temozolomide efficacy in glioma cells by inducing autophagy. Oncol. Lett. 10, 379–383 10.3892/ol.2015.3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu C.H., Liu Y., Xiao L.M. et al. (2017) Dihydroartemisinin treatment exhibits antitumor effects in glioma cells through induction of apoptosis. Mol. Med. Rep. 16, 9528–9532 10.3892/mmr.2017.7832 [DOI] [PubMed] [Google Scholar]
- 20.Lőrincz T., Jemnitz K., Kardon T. et al. (2015) Ferroptosis is involved in acetaminophen induced cell death. Pathol. Oncol. Res. 21, 1115–1121 [DOI] [PubMed] [Google Scholar]
- 21.Ba Q., Zhou N., Duan J. et al. (2012) Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PLoS ONE 7, e42703 10.1371/journal.pone.0042703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y., Hou W., Song X. et al. (2016) Ferroptosis: process and function. Cell Death Differ. 23, 369 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H., Guo P., Xie X. et al. (2017) Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 21, 648–657 10.1111/jcmm.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehm T., Rauh M., Wiendieck K. et al. (2016) Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget 7, 74630 10.18632/oncotarget.11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Ding Y., Wang X. et al. (2018) Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 428, 21–33 10.1016/j.canlet.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 26.Lin R., Zhang Z., Chen L. et al. (2016) Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 381, 165–175 10.1016/j.canlet.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 27.Chen G.Q., Benthani F.A., Wu J. et al. (2019) Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 27, 242–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauckman K., Haller E., Taran N. et al. (2015) Iron alters cell survival in a mitochondria-dependent pathway in ovarian cancer cells. Biochem. J. 466, 401–413 10.1042/BJ20140878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prior R., Reifenberger G. and Wechsler W. (1990) Transferrin receptor expression in tumours of the human nervous system: relation to tumour type, grading and tumour growth fraction. Virchows Archiv A 416, 491–496 10.1007/BF01600299 [DOI] [PubMed] [Google Scholar]
- 30.Hirschhorn T. and Stockwell B.R. (2019) The development of the concept of ferroptosis. Free Radic. Biol. Med. 133, 130–143 10.1016/j.freeradbiomed.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H., Guo P., Xie X. et al. (2017) Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 21, 648–657 10.1111/jcmm.13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seibt T.M., Proneth B. and Conrad M. (2019) Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 133, 144–152 10.1016/j.freeradbiomed.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 33.Chiang S.K., Chen S.E. and Chang L.C. (2019) A dual role of Heme Oxygenase-1 in cancer cells. Int. J. Mol. Sci. 20, 39 10.3390/ijms20010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miotto G., Rossetto M., Roveri A. et al. (2018) Insight the mechanism of ferroptosis inhibition by ferrostatin-1. Free Radic. Biol. Med. 120, S120–S121 10.1016/j.freeradbiomed.2018.04.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang H.M. and Tang H.L. (2019) Cell recovery by reversal of ferroptosis. Biology Open 8, bio043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin D., Kim E.H., Lee J. et al. (2018) Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 129, 454–462 10.1016/j.freeradbiomed.2018.10.426 [DOI] [PubMed] [Google Scholar]
- 37.Liu K., Dai L., Li C. et al. (2016) Self-assembled targeted nanoparticles based on transferrin-modified eight-arm-polyethylene glycol–dihydroartemisinin conjugate. Sci. Rep. 6, 29461 10.1038/srep29461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y., Song X., Sun X. et al. (2016) Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 473, 775–780 10.1016/j.bbrc.2016.03.052 [DOI] [PubMed] [Google Scholar]
- 39.Fan Z., Wirth A.K., Chen D. et al. (2017) Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6, e371 10.1038/oncsis.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer E., Judkins J., Salomonis N. et al. (2015) Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 6, e1601 10.1038/cddis.2014.566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khamari R., Trinh A., Gabert P.E. et al. (2018) Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis. 9, 325 10.1038/s41419-018-0340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan H., Li X., Zhang X. et al. (2016) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478, 1338–1343 10.1016/j.bbrc.2016.08.124 [DOI] [PubMed] [Google Scholar]
- 43.Doll S., Proneth B., Tyurina Y.Y. et al. (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91 10.1038/nchembio.2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bae D.H., Lane D.J., Jansson P.J. et al. (2018) The old and new biochemistry of polyamines. Biochim. Biophys. Acta (BBA) 1862, 2053–2068 10.1016/j.bbagen.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 45.Biedermann B., Skatchkov S.N., Brunk I. et al. (1998) Spermine/spermidine is expressed by retinal glial (müller) cells and controls distinct k+ channels of their membrane. Glia 23, 209–220 [DOI] [PubMed] [Google Scholar]
- 46.Laube G. and Veh R.W. (1997) Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 19, 171–179 [DOI] [PubMed] [Google Scholar]
- 47.Gaboriau F., Laupen-Chassay C., Pasdeloup N. et al. (2010) Modulation of cell proliferation and polyamine metabolism in rat liver cell cultures by the iron chelator o-trensox. Biometals 19, 623–632 10.1007/s10534-006-6888-y [DOI] [PubMed] [Google Scholar]
- 48.Ou Y., Wang S.J., Li D. et al. (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. 113, E6806–E6812 10.1073/pnas.1607152113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francine G., Roussi S., Guyot S. et al. (2006) Potentiation of apple procyanidin-triggered apoptosis by polyamine oxidase inactivator MDL72527 in human colon cancer-derived metastatic cells. Int. J. Oncol. 29, 423–428 [PubMed] [Google Scholar]