Abstract
Background: At present, the infection and prevalence rates of tuberculosis (TB) are still high in worldwide. The Xpert MTB/RIF technology has improved the diagnosis speed of Mycobacterium tuberculosis (MTB) and facilitated the rapid treatment of TB patients.
Methods: We searched experimental data derived from Xpert MTB/RIF for detecting MTB in gastric aspirates in PubMed, Embase, Web Of Science, and the Cochrane Library databases between January 2012 to April 2019. A summary receiver operating characteristic curve (SROC curve) was used to analyze the pooled sensitivity, pooled specificity, PLR, NLR, and DOR for determining the accuracy of the test.
Results: Our database search resulted in 10 relevant articles. The pooled sensitivity of Xpert MTB/RIF for detecting TB in GA was 86% (95% CI, 83–89%), and I2 = 93.4%. The pooled specificity was 92% (95% CI, 90–93%) and I2 = 97.8%. In addition, the positive LR was 12.12 (95% CI, 5.60–26.21), negative LR was 0.20 (95% CI, 0.11–0.36), and the diagnostic odds ratio (DOR) was 147.04 (95% CI, 37.20–581.19). Using the SROC curve, the AUC was 0.9730 and Q* was 0.9248 (SE = 0.0261). The publication bias was P=0.517 (P>0.05).
Conclusions: The Xpert MTB/RIF for detecting MTB in gastric aspirates was highly accurate. In addition, we observed that the publication bias in the present study was low. Hence, the Xpert MTB/RIF technology is highly accurate and has the advantage of rapid testing for MTB in clinical samples.
Keywords: Gastric-Aspirate, Mycobacterium-tuberculosis, Sensitivity, Specificity, Xpert MTB/RIF
Introduction
Tuberculosis (TB) remains a globally essential and leading cause of infectious disease. It was estimated that around 3 million TB patients were undiagnosed in 2013, with 10 million people diagnosed with TB in 2017 [1]. Approximately, 95% of TB infections and 99% of deaths due to TB infections occur in developing countries, with South Africa being the most affected country [2]. Hence, the early MTB diagnosis is very important. Sputum samples are the most commonly used material for diagnosis. Culturing MTB is the gold standard for TB diagnosis. However, it usually takes several weeks to produce the results needed for a proper diagnosis. Because the results cannot be quickly generated, timely diagnosis and patient treatment are delayed [3–5]. The WHO recommended method for the initial diagnosis of MTB is smear microscopy. However, due to the low sensitivity of microscopic examinations, the majority of patients will be misdiagnosed [6]. Hence, new methods are urgently needed to develop early diagnosis methods, shorten treatment times, and improve treatment efficacy and prevention.
In 2011, the MTB rapid molecular diagnostic Xpert MTB/RIF test was introduced. It has improved diagnostic sensitivity for detecting MTB [7]. At present, the Xpert MTB/RIF is used to diagnose bacterial infections in patients more accurately. Globally, the Xpert MTB/RIF test has reduced clinical misdiagnosis and has diminished the empirical treatment of patients with negative results. As previously demonstrated by Penz et al. [8], the Xpert MTB/RIF test has different sensitivities and specificities for detecting MTB obtained from lymphatic, pleural effusion, gastrointestinal tract, genitourinary system, cerebrospinal fluid, and other samples. Hence, the purpose of the present study was to perform a research from recently published studies that used the Xpert MTB/RIF test. The overall diagnostic accuracy for detecting MTB in gastric aspirates was then determined for its suitability for clinical diagnosis.
Method
Data sources and search strategy
The following search terms were used: Gastro Enteric, Gastrointestinal, Gastro-Intestinal, GI, Gastric Aspirate, GA, Xpert, Xpert MTB/RIF, Xpert RIF/MTB. The search strategy was performed independently by the authors using Pubmed, Embase, Web Of Science, and the Cochrane Library. Afterwards, the authors presented and compared their individual results. If they were inconsistencies, the search strategy was re-evaluated to resolve any differences. Through discussions, relevant studies were selected and used for the analysis.
Study selection and data extraction
The inclusion and exclusion criteria are formulated first before reading the retrieved studies. The inclusion criteria as follow: (1) Each included study used Xpert MTB/RIF for detection of MTB and a standard test (culture). (2) Study assessing the accuracy of Xpert in gastric TB detection with reliable data to calculate true positive (TP), false positive (FP), false negative (FN), true negative (TN). (3) Humans samples are detected and analyzed. The exclusion criteria as follow: (1) duplicate studies, (2) animal experiments, (3) experiments of two groups of patients without contrasting and unrelated studies, (4) reviews, conference abstracts, case reports, and studies that cannot extract data.
Based on pre-established inclusion and exclusion criteria, the authors independently read the article title, abstract, keywords, etc. to determine whether the article was relevant. Afterwards, the authors compared their results. Any inconsistencies in article selection required all authors to read the full manuscript. The article in question was included or excluded after a consensus was reached through discussions.
The authors independently read the full article after the initial screening to determine whether the data were relevant and extractable. The extractable data included the name of the first author, publication year, country, sample size, gold standard, and true positive (TP), false positive (FP), false negative (FN), and true negative (TN) compared with Xpert and gold standard data. After data extraction, the authors collectively discussed the results and re-read articles with conflicting results. The authors sought additional advice from experts to resolve any differences. Afterwards, the extracted data were used to formulate the data feature table.
Statistical analysis
Meta-disc (version 1.4) was used to analyze the data and determine the sensitivity, specificity, positive LR, negative LR, diagnostic OR, and SROC curve tables.
Each included study was quality assessed using Review Manager (RevMan V5.3, Cochrane Collaboration, Oxford, U.K.) software.
Publication bias was determined using STATA (version 12.0) at a test level of α = 0.05.
Results
Study identification and characteristics
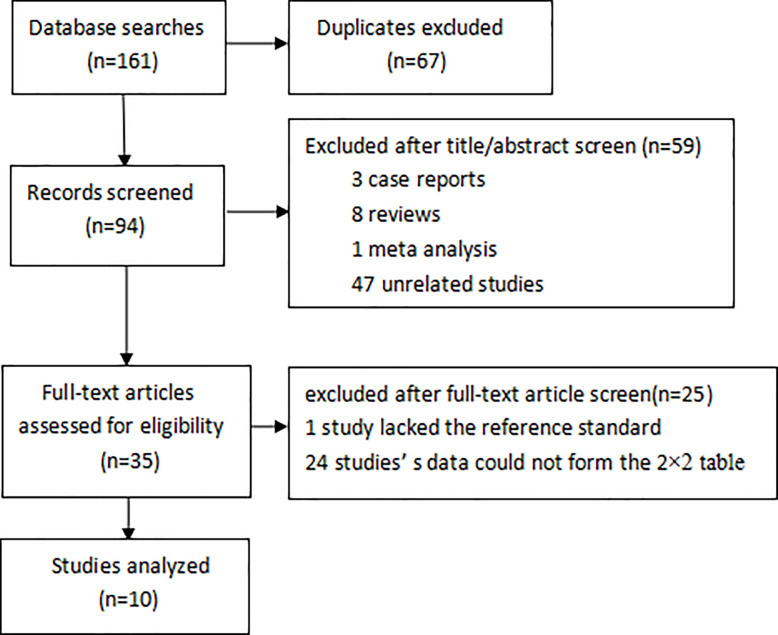
A total of 161 studies are retrieved: 48 studies from PubMed, 54 studies from WOS, 50 studies from Embase, 9 studies from the Cochrance Library, and 67 of the 161 studies are duplicates. The remaining 94 articles are excluded after reading the title and abstract of the article based on the inclusion and exclusion criteria, including 3 case reports, 8 reviews, 1 meta-analysis, and 47 unrelated studies. The remaining 35 articles exclude 25 articles after reading the full text, including 1 article lacking a gold standard comparison, and 24 articles cannot be used to form a 2 × 2 table for analysis based on their data. In the end, we got 10 articles for analysis (Figure 1).
Figure 1. Flowchart of article retrieval.
The 10 selected articles [9–18] spanned from 2012 to 2018, of which 6 were published in Asia [9–14], 2 were published in Europe [15,16], and 2 were published in South Africa (Table 1) [17,18]. The number of study samples in each article ranged from 48 to 788, with a median of 245.5.
Table 1. Study characteristics of the selected publications.
| First Author | Year | Country | Specimen | Patients enrolled | Reference standard | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|
| Myo | 2018 | Myanmar | GA | 231 | culture | 16 | 20 | 0 | 195 |
| Hasan | 2017 | Pakistan | GA | 48 | culture | 9 | 2 | 0 | 37 |
| Aslam | 2017 | Pakistan | GA | 267 | culture | 181 | 40 | 0 | 46 |
| Lu | 2017 | China | GA | 127 | culture | 25 | 40 | 0 | 62 |
| Walters | 2017 | South Africa | GA | 262 | culture | 18 | 3 | 15 | 226 |
| Mazzola | 2016 | Italy | GA | 356 | culture | 51 | 4 | 0 | 301 |
| Singh | 2015 | India | GA | 260 | culture | 52 | 19 | 25 | 164 |
| Pang | 2014 | China | GA | 211 | culture | 11 | 58 | 6 | 136 |
| Bates | 2013 | Zambia | GA | 788 | culture | 33 | 5 | 15 | 735 |
| Tortoli | 2012 | Europe | GA | 224 | culture | 48 | 0 | 10 | 166 |
Study quality
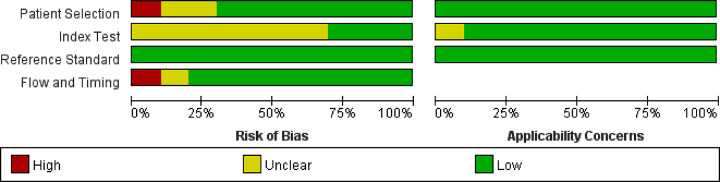
The overall methodological quality evaluation of the Xpert MTB/RIF and bacterial cultures used in the study are summarized in Figure 2. The risk of bias due to patient selection, index testing, reference standards, procedures, and time were considered low.
Figure 2. Risk of bias and applicability concerns graph for tuberculosis detection.
Sensitivity and specificity of Xpert MTB/RIF for diagnosis of MTB in GA
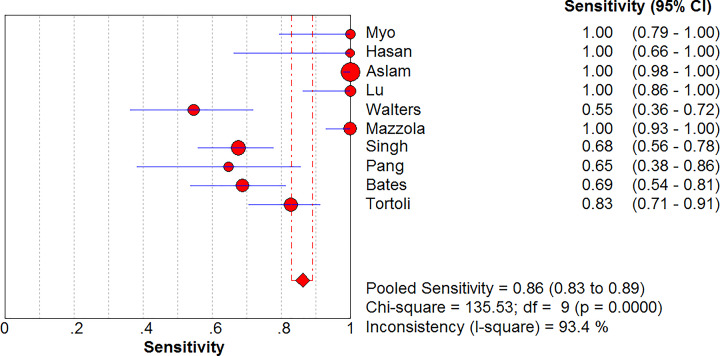
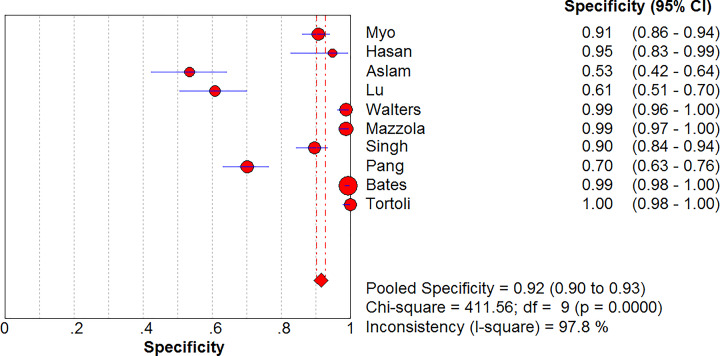
Ten studies included a comparison of the Xpert MTB/RIF versus cultures in 2774 GA samples for detecting tuberculosis (Figures 3 and 4). The sensitivity of Xpert MTB/RIF ranged from 55% (95% CI, 36–72%) to 100% (95% CI, 98–100%). The pooled sensitivity of Xpert MTB/RIF for MTB was 86% (95% CI, 83–89%), and the statistical value of I2 was 93.4%. The specificity of Xpert MTB/RIF ranged from 53% (95% CI, 42–64%) to 100% (95% CI, 98–100%). The pooled specificity of the Xpert MTB/RIF test for MTB was 92% (95% CI, 90–93%), and the statistical value of I2 was 97.8%.
Figure 3. Forest plot of Xpert MTB/RIF sensitivity for tuberculosis detection in gastric aspirates.
Figure 4. Forest plot of Xpert MTB/RIF specificity for tuberculosis detection in gastric aspirates.
Combined positive LR and combined negative LR
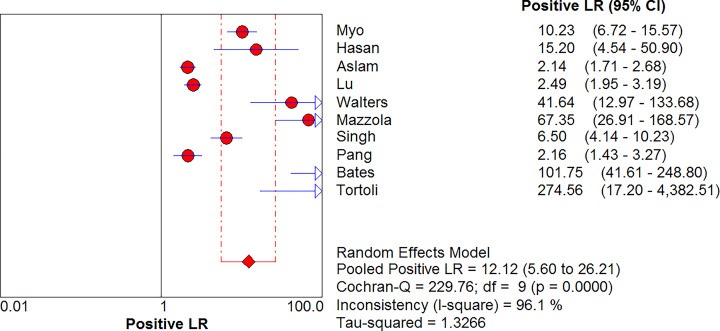
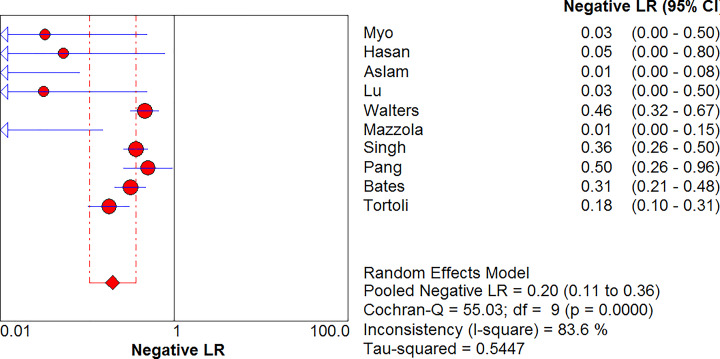
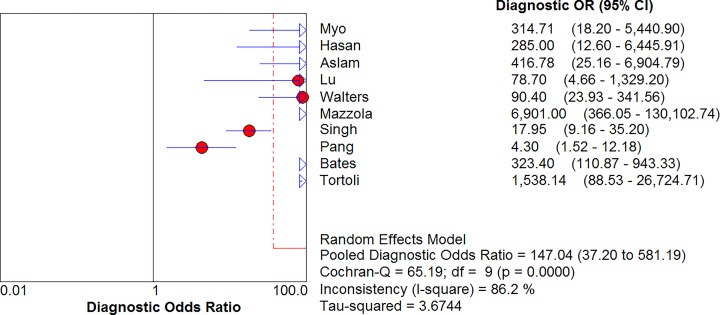
MTB in GA was detected using Xpert MTB/RIF, with a positive LR of 12.12 (95% CI, 5.60–26.21) and a negative LR of 0.20 (95% CI, 0.11–0.36) (Figures 5 and 6). The diagnostic odds ratio (DOR) was 147.04 (95% CI, 37.20–581.19) (Figure 7).
Figure 5. Forest plot of Xpert MTB/RIF positive LR for tuberculosis detection in gastric aspirates.
Figure 6. Forest plot of Xpert MTB/RIF negative LR for tuberculosis detection in gastric aspirates.
Figure 7. Forest plot of Xpert MTB/RIF diagnostic OR for tuberculosis detection in gastric aspirates.
SROC curve
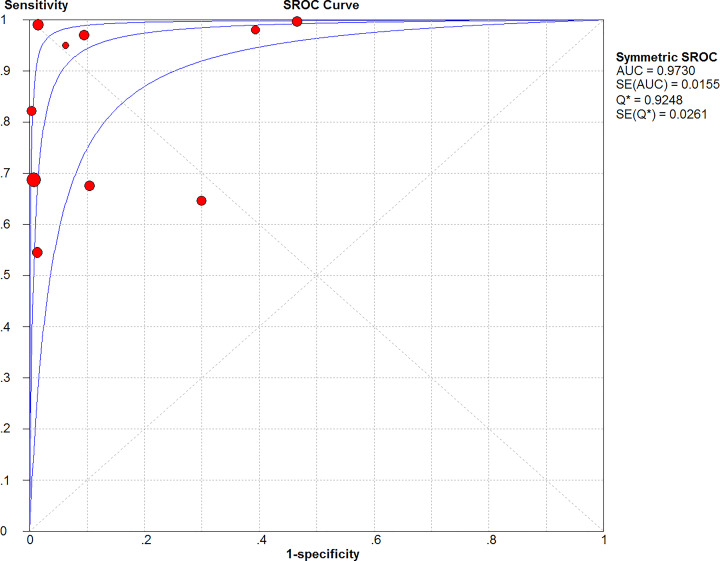
The AUC was 0.9730 determined from the SROC curve, indicating a good diagnostic value (Figure 8). In addition, Q* was 0.9248 (SE = 0.0261). The Q * value was close to 1, which indicated the high accuracy of Xpert MTB/RIF for MTB detection.
Figure 8. Summary receiver operating characteristic curves of Xpert MTB/RIF for tuberculosis detection in gastric aspirates.
Publication bias
The Deeks funnel plot was generated using the STATAS software. The P-value was 0.517 (P>0.05), indicating low publication bias (Figure 9).
Figure 9. Deeks’ funnel plot indicating a low risk of publication bias (P=0.517).
Discussion
In the present study, we extracted Xpert MTB/RIF data from 2774 GA samples obtained from 10 studies. The TB test was performed using GA samples, with bacterial cultures used as the standard. We found that the pooled sensitivity of the Xpert test was 86% (95% CI, 83–89%), and the pooled specificity was 92% (95% CI, 90–93%). In general, the Xpert MTB/RIF TB test performed using GA samples generated results that were relatively accurate compared with the culture method. The research performed by Penz et al. [8] in 2015, demonstrated that the Xpert MTB/RIF test had a sensitivity of 86% (95%CI, 67–98%), and specificity of 98% (95% CI, 98–100%) for analyzing GA samples. This was comparable to our results. However, their positive LR was 65 and negative LR = 0.18, which was different from our results (positive LR = 12.12). The reason may be that we included 10 studies for systematic analysis, while Penz el al. had only 5 studies in their research. In addition, the total sample size was much higher in our study. Hence, the use of the Xpert MTB/RIF test for detecting MTB in GA samples was highly accurate and could be used in clinical settings or public health systems to detect MTB infections in patients.
The standard diagnostic method for detecting MTB is through bacterial cultures [3–5]. We compared the Xpert MTB/RIF technology with the culture method. The Xpert MTB/RIF detects GA differently from the culture method. It is not affected by the acidity of the GA fluid, treatment delay of the bacteria, and excessive bacterial growth [9]. The diagnostic yield of Xpert MTB/RIF (31.4%) was significantly higher compared with the culture method (24.0%) [9]. Singh et al. [14] demonstrated that Xpert MTB / RIF could be used for diagnosing childhood TB even when the GA samples were stored for 4 years. Singh et al. also said in smear and culture-positive GA samples, the sensitivity of the Xpert MTB/RIF assay was 95.6% and was similar to the sensitivity reported for fresh clinical samples. Previous studies have demonstrated that Xpert MTB/RIF had a high detection rate and accuracy. We performed a rigorous search, using stringent screening criteria, and analyzed the data using strict statistical methods to improve the accuracy of our results.
Based on previous studies, the detection rate of MTB by microscopy was 48.94% and was prone to be affected by several factors [19,20]. The detection rate of Xpert MTB/RIF greatly exceeded the detection rate of microscopic examinations. Besides, the Xpert MTB/RIF test is convenient and rapid to perform.
Our work had a few limitations. The selected publications generated inconsistent data due to different instruments and technical protocols used. The low sensitivity and specificity caused from some of the studies had a significant influence on our research. Walters et al. [18] did not describe the technical details of processing their gastrointestinal fluid samples. Hence, we could not make a more objective evaluation of the low sensitivities they observed in their study. Aslams et al. [9] found that the specificity was only 53%. This could probably have been due to patients receiving anti-tuberculosis drugs. After treatment, some patients may have been cured. Hence there is a need to carefully evaluate the positive results from the Xpert MTB/RIF test [21]. Pang et al. [10] demonstrated relatively low levels of sensitivity and specificity. This may have been due to the long storage time of the samples (>3 days), and the MTB in the GA fluids being partially inactivated by the high acidic environment of the samples. It has been proven that MTB may be inactivated in an acid environment [22,23]. Their positive results from bacterial cultures were relatively low.
Additional studies using larger patient cohort samples should be performed to determine whether the Xpert MTB/RIF test could accurately detect MTB in GA samples.
The advantages of the Xpert MTB/RIF test are the reduced cost and faster turn-around times to generate results. This will prevent patients from being misdiagnosed and receive the appropriate treatment in a shorter period. Quicker and a more accurate diagnosis will lead to better patient prognosis, a better quality of life and reduced mortality.
Acknowledgements
We show our great gratitude to all members of our work term for their cooperation.
Abbreviations
- DOR
diagnostic odds ratio
- MTB
Mycobacterium tuberculosis
- SROC
summary receiver operating characteristic
- TB
tuberculosis
- WHO
World Health Organization
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported from the Third affiliated hospital of Guangzhou Medical University [grant number 2019A026].
Author Contribution
Xu-Guang Guo conceived the study. Hong-Kun Tan, Shu-Jin Fan, Yu-Cheng Xu, and Jiong-Jiong Zhou collected the data, analyzed the data, and wrote the manuscript. Yuan-Zhi Chen, Tian-Ao Xie, Zhi-Yong Pan, and Yong Xia participated the data collection and amended the manuscript. The final manuscript was read and approved by all the authors.
References
- 1.Floyd K. et al. (2018) The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir. Med. 6, 299–314 10.1016/S2213-2600(18)30057-2 [DOI] [PubMed] [Google Scholar]
- 2.Dye C. (2006) Global epidemiology of tuberculosis. Lancet 367, 938–940 10.1016/S0140-6736(06)68384-0 [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S. and Rekha B. (2010) Pediatric tuberculosis: global overview and challenges. Clin. Infect. Dis. 50, S184–S194 10.1086/651490 [DOI] [PubMed] [Google Scholar]
- 4.Cruz A.T. and Starke J.R. (2007) Clinical manifestations of tuberculosis in children. Paediatr. Respir. Rev. 8, 107–117 10.1016/j.prrv.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 5.Eamranond P. and Jaramillo E. (2001) Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int. J. Tuberc. Lung Dis. 5, 594–603 [PubMed] [Google Scholar]
- 6.Korenromp E.L. et al. (2012) Implementing the global plan to stop TB, 2011-2015–optimizing allocations and the Global Fund's contribution: a scenario projections study. PLoS ONE 7, e38816 10.1371/journal.pone.0038816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(2011) Policy Statement: Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. WHO Guidelines Approved by the Guidelines Review Committee, World Health Organization, Geneva: [PubMed] [Google Scholar]
- 8.Penz E. et al. (2015) Diagnostic accuracy of the Xpert® MTB/RIF assay for extra-pulmonary tuberculosis: a meta-analysis. Int. J. Tuberc. Lung Dis. 19, 278–284 10.5588/ijtld.14.0262 [DOI] [PubMed] [Google Scholar]
- 9.Aslam W. et al. (2017) Gastric specimens for diagnosing tuberculosis in adults unable to expectorate in Rawalpindi, Pakistan. Public Health Action 7, 141–146 10.5588/pha.16.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang Y. et al. (2014) Evaluation of the Xpert MTB/RIF Assay in Gastric Lavage Aspirates for Diagnosis of Smear-negative Childhood Pulmonary Tuberculosis. Pediatr. Infect. Dis. J. 33, 1047–1051 10.1097/INF.0000000000000403 [DOI] [PubMed] [Google Scholar]
- 11.Myo K. et al. (2018) Evaluation of Xpert((R)) MTB/RIF assay as a diagnostic test for pulmonary tuberculosis in children in Myanmar. Int. J. Tuberc. Lung Dis. 22, 1051–1055 10.5588/ijtld.18.0024 [DOI] [PubMed] [Google Scholar]
- 12.Lu J. et al. (2017) The Feasibility of Xpert MTB/RIF Testing to Detect Rifampicin Resistance among Childhood Tuberculosis for Prevalence Surveys in Northern China. Biomed. Res. Int. 2017, 5857369 10.1155/2017/5857369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasan Z. et al. (2017) Evaluation of Xpert MTB/RIF testing for rapid diagnosis of childhood pulmonary tuberculosis in children by Xpert MTB/RIF testing of stool samples in a low resource setting. BMC Res. Notes 10, 473 10.1186/s13104-017-2806-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S. et al. (2015) Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiol. 15, 191 10.1186/s12866-015-0528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzola E. et al. (2016) Performance of real-time PCR Xpert MTB/RIF in diagnosing extrapulmonary tuberculosis. Le Infezioni in Medicina: Rivista Periodica di Eziologia, Epidemiologia, Diagnostica, Clinica e Terapia Delle Patologie Infettive 24, 304–309 [PubMed] [Google Scholar]
- 16.Tortoli E. et al. (2012) Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur. Respir. J. 40, 442–447 10.1183/09031936.00176311 [DOI] [PubMed] [Google Scholar]
- 17.Bates M. et al. (2013) Assessment of the Xpert MTB/RIF assay for diagnosis of tuberculosis with gastric lavage aspirates in children in sub-Saharan Africa: a prospective descriptive study. Lancet Infect. Dis. 13, 36–42 10.1016/S1473-3099(12)70245-1 [DOI] [PubMed] [Google Scholar]
- 18.Walters E. et al. (2017) Xpert MTB/RIF on Stool Is Useful for the Rapid Diagnosis of Tuberculosis in Young Children With Severe Pulmonary Disease. Pediatr. Infect. Dis. J. 36, 837–843 10.1097/INF.0000000000001563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steingart K.R. et al. (2006) Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect. Dis. 6, 570–581 10.1016/S1473-3099(06)70578-3 [DOI] [PubMed] [Google Scholar]
- 20.Keflie T.S. and Ameni G. (2014) Microscopic examination and smear negative pulmonary tuberculosis in Ethiopia. Pan. Afr. Med. J. 19, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich S.O. et al. (2013) Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med. 1, 462–470 10.1016/S2213-2600(13)70119-X [DOI] [PubMed] [Google Scholar]
- 22.Nhu N.T. et al. (2013) Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC Infect. Dis. 13, 31 10.1186/1471-2334-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parashar D. et al. (2013) Does neutralization of gastric aspirates from children with suspected intrathoracic tuberculosis affect mycobacterial yields on MGIT culture? J. Clin. Microbiol. 51, 1753–1756 10.1128/JCM.00202-13 [DOI] [PMC free article] [PubMed] [Google Scholar]