Confirmed COVID-19 cases are in the millions, and with hundreds of thousands of deaths [1] from the disease the social, healthcare and economic consequences are likely to persist for years. While drug treatments may emerge, which reduce the impact of the disease, vaccination represents the most appropriate means to bring the virus under control in developed and developing countries equally. Multiple technologies are being deployed to identify a safe and effective vaccine.
In the case of COVID-19 most vaccines in early development are targeting the so-called spike protein. This protein is expressed on the surface of the virus and allows the virus to bind to the ACE2 receptor on the cell surface, initiating fusion with and uptake by the cell, followed by the chain of events leading to virus replication.
A vaccine typically contains an antigen made from weakened or killed forms of the microbe/virus, its toxins, or one of its surface proteins. The antigen stimulates the body’s immune system to recognise the agent as a threat, and destroy the associated virus. The antigen protein(s) can be administered directly, usually together with an adjuvant to enhance the immune response. In the case of COVID-19, the spike protein or epitopes, including the receptor binding domain, are used as the antigen.
However, rather than administer the protein antigen, alternative techniques have been developed to administer the DNA or RNA which encodes the antigenic protein. A DNA/RNA vaccine introduces the DNA/RNA into the cell to produce the spike protein, which will then be internally processed by the cell and presented on the cell surface to immune cells. This way, the virus or its antigen is presented in a similar manner to during infection with the wildtype virus and thus will trigger a more natural immune response. By doing so, the immune system should activate both killer T cells but also neutralise antibodies to combat the virus.
The biggest challenge facing DNA/RNA vaccines is ensuring the host cell accepts the introduced genetic material. There are two common methods of introducing the DNA/RNA into the host immune cells, namely (1) using viral vectors or (2) by using a delivery system to carry the DNA/RNA across the cell membrane and promote synthesis of the spike protein. A novel silica nanopoarticle, Nuvec®, has the potential to be important in both approaches. A third method, electroporation, is also considered in this article.
Oligonucleotide (DNA/RNA) delivery systems
Many industry leaders believe an efficient nanoparticle delivery system is the key to mount an effective DNA/RNA vaccine response. Any ideal delivery system needs to demonstrate a combination of high loading capacity, controlled release with extended half-life, no leakage, no interference with the stability of the therapeutic molecule, and a straightforward and consistent manufacturing process. Good biocompatibility, low toxicity, and biodegradability, as well as a clear understanding of the mode of action of the delivery system, are also desirable factors.
Adeno-associated virus (AAV) and lentivirus-based vaccines
The pressure to find and administer a COVID-19 vaccine has sparked interest in finding a safe and effective viral delivery system, or most likely, more than one delivery system. As of April 2020, the World Health Organisation (WHO) has identified 89 vaccine candidates in various stages of (pre) clinical development. Of those 89, the seven front runners (in clinical stage) are predominantly nucleic acid vaccines using non-replicating viral vectors (such as adeno-associated virus or lentivirus) for the vaccine delivery.
Many companies developing COVID-19 vaccines are using viral vectors to ensure effective delivery of the vaccine. However, production of the viral vectors can be a cumbersome and challenging process and any improvement to that process could result in more vaccines being available faster. The viral vector-producing cells need to be transfected with multiple plasmids carrying various viral genes and the ‘payload’, the vaccine.
Electroporation
Among the clinical candidates for COVID-19 vaccine delivery systems is an experimental DNA vaccine that uses electroporation, which is the process of applying a high-voltage electrical pulse to a living cell, causing temporary permeability of the cell membrane, through which a foreign material such as DNA may pass.
However, researchers noted that “a primary drawback of electroporation is pain and discomfort at the application site compared with conventional injections” [2]. There were also reaction reports of involuntary muscle contraction and mild to severe asymptomatic increases in CPK (creatine phosphokinase) levels in the blood of six participants. The researchers acknowledged that “The electroporation procedure has been shown to carry some potential of transient muscle damage in animal models, evident as increased numbers of fibres with central nucleoli and damaged myofibrillar bundles” [3]. In addition, the equipment required together with the training of medical staff in its use will preclude widespread adoption of electroporation as a mass vaccination methodology.
Lipid nanoparticle (LNP) systems
Liposomal encapsulation of drugs with bioavailability issues was a proven approach in small molecule pharma, and it was apparent to biopharma R&D scientists that LNPs could meet the basic requirements of an RNA/DNA delivery system too, namely to protect nucleic acid from in vivo digestion as it travels to the target. Additionally, LNPs can be produced with catatonic outer membranes to allow cell entry.
However, solid LNPs [4] have some significant disadvantages to be overcome, including cell toxicity; stimulating the release of systemic inflammatory cytokines; possible accumulation in the liver and spleen, with the resulting possibility of hepato-toxicity; low drug payload for hydrophilic molecules; and the potential of the reticuloendothelial system (RES) as a major route of clearance if liposomes are administered systemically. The challenge is delivering enough nucleic acid into the cells without unwanted side effects. This level of variation in performance has led scientists to consider alternatives to LNPs.
Nanosilica for DNA and RNA delivery
Widely used in many different pharmaceutical and food situations, silica and has been proven to be safe in these various uses. As scientists looked to alternative carriers that could be adapted to encapsulate and protect nucleic acids, mesoporous silica nanoparticles (MSNs) – silica-based nanostructured materials with excellent biocompatibility and chemical stability – emerged as a suitable candidate 5, 6. In particular, nanoparticulate silica can be re-engineered to bind oligonucleotides of a range of sizes including DNA, RNA and SiRNA.
N4 Pharma is developing a novel silica nanoparticle technology for the delivery of vaccines and drugs, with a particular focus on supporting the development of cancer treatments and viral vaccines based on mRNA and pDNA. The original technology, licensed from researchers at the University of Queensland (UQ) in Australia, was developed as a nanosilica system for the delivery of a hepatitis B vaccine that would reduce the number of doses per day from three to one.
Building on this experience, the founders of N4 Pharma licensed the UQ technology and jointly developed a novel silica nanoparticle specifically designed for nucleic acid delivery has an irregular (spiky) surface structure – functionalised by coupling with polyethyleneimine (PEI). This surface simply and effectively traps and protects nucleic acids (such as mRNA/pDNA) from nuclease enzymes as it travels to the cells. Once inside the cell, the DNA/RNA is released and will result in synthesis of the foreign/target protein which will activate the immune system, leading to both a cellular and humoral immune response. The cellular response (T cells) can sense and kill infected cells and the humoral response (antibodies) will bind to the virus and (1) neutralise its capability to bind to the target cell and (2) be increasingly cleared by phagocytic mechanisms.
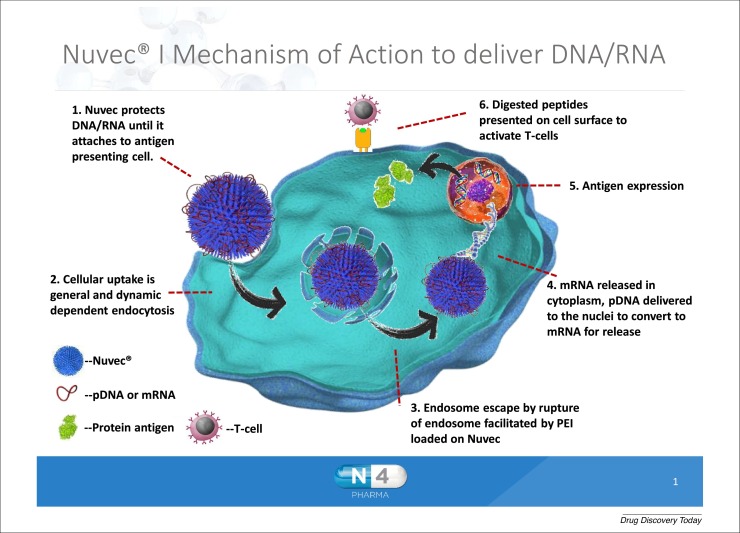
The new delivery system is called Nuvec®. Figure 1 shows the end-to-end mode of action for Nuvec® as it delivers DNA/RNA to antigen presenting cells: the Nuvec® particle carrying the DNA/RNA attaches to the cell membrane, by charge attraction, and is taken up into the cell via general and dynamin endocytosis. Once inside the endosome, the association of DNA with Nuvec changes as a consequence of the acidic environment of the endosome, releasing some DNA/RNA.
Figure 1.
Nuvec® mode of action as it delivers DNA/RNA to antigen presenting cells.
A crucial feature of Nuvec® is that, compared to lipid-based delivery systems, it does not disrupt the cell membrane as it enters the cell and does not exhibit inflammatory response at the site of injection or any unwanted systemic side-effects. A series of studies have supported the safety, efficacy and mode of action of Nuvec® and the particle is being used in late stage pre-clinical studies.
In addition, Nuvec®’s capability to bind more than one plasmid has the potential to assist viral vector delivery. This ensures that in a multi-plasmid transfection approach, each cell will be exposed to all plasmids at the same time and can lead to less plasmid necessary to achieve the same transfection efficacy. Also during in vivo delivery of the vaccine, Nuvec® and the viral vector can work synergistically to deliver the payload.
Nuvec®’s particle will be sensed and picked up by macrophages and antigen-presenting cells. The presence of PEI will facilitate the endosomal disruption and release of nucleic acid but also presents a danger signal that will up regulate surface markers and hence attract immune cells and boost the immune response.
Delivering the vaccine
The global urgency of the COVID-19 pandemic has brought more than 25 years of research into nucleic acid-based vaccines and therapeutics into the limelight. The pharmaceutical industry, government agencies and WHO are working hard to find a COVID-19 vaccine. As researchers search for their ideal delivery platforms, safe and effective alternatives to established technologies – including emerging technologies like such as Nuvec® – can be considered.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- 2.Tolerability of intramuscular and intradermal delivery by CELLECTRA® adaptive constant current electroporation device in healthy volunteers. Hum. Vaccin. Immunother. 2013;9(October (10)):2246–2252. doi: 10.4161/hv.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzuto G. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6417–6422. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharm. Sci. 2018;13(4):288–303. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarn D. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc. Chem. Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.E. Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc. Chem. Res. 2011;44:893–902. doi: 10.1021/ar2000259. [DOI] [PubMed] [Google Scholar]