Graphical abstract
Physiology and pathophysiology of pulmonary alveoli during SARS-CoV-2 infection.
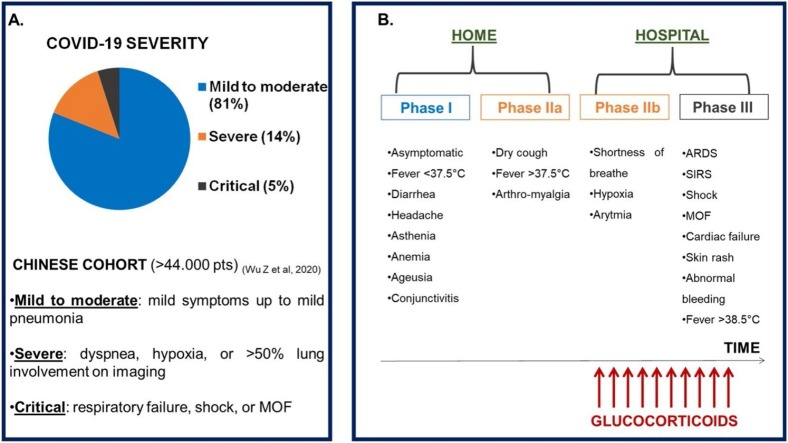
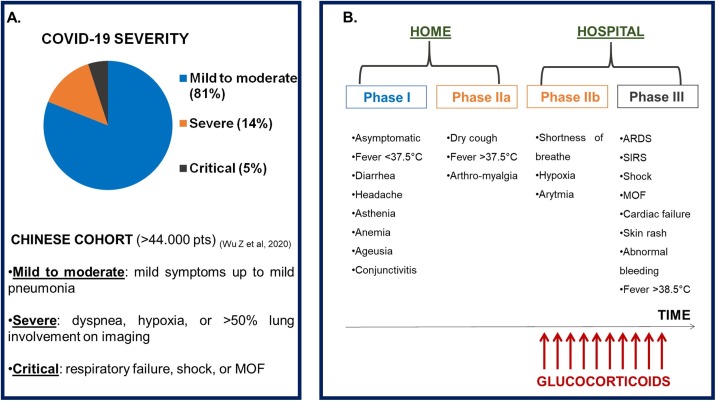
(A) COVID-19 severity: frequency of degrees of clinical manifestations in a Chinese cohort (>44.000 patients) (ref: Wu Z. et al, 2020). (B) Phases of COVID-19 disease and timing for the use of glucocorticoids.
Legend: ARDS: Adult Respiratory Distress Syndrome; COVID-19: CoronaVirus Disease-19; MOF: multi-organ failure; pts: patients; SIRS: systemic inflammatory response syndrome.
Abbreviations: ARDS, Adult Respiratory Distress Syndrome; CAP, Community Acquired Pneumonia; CCL2, CC motif ligand 2; COVID-19, Coronavirus Disease 2019; CRS, Cytokine Release Syndrome; CXCL10, C-X-C motif ligand 10; DX, dexamethasone; GC, glucocorticoid; GCR, glucocorticoid receptor; GM-CSF, Granulocyte Monocyte-Colony Stimulating Factor; ICU, Intensive Care Unit; IL, interleukin; IV, intravenous; MERS-CoV, Middle East Respiratory Syndrome Coronavirus; MP, methylprednisolone; MOF, multi-organ failure; O2, oxygen; RCT, Randomized Controlled Trial; SARS-CoV-2, Severe Acute Respiratory Syndrome-Coronavirus-2; SHLH, Secondary haemophagocytic lymphohistiocytosis; SIRS, Systemic Inflammatory Response Syndrome; TNF-α, tumor necrosis factor-α; ULN, upper limit of normal; WHO, World Health Organization
Keywords: Glucocorticoids, COVID-19, SARS-CoV-2, ARDS, Immune response
Highlights
-
•
Dexamethasone reduces the risk of death in patients critically ill with COVID-19.
-
•
No evidence of benefit from other complementary and/or supportive treatments was seen in critically ill COVID-19 hospitalized patients.
-
•
Synthetic glucocorticoids switch off cytokine storm, and consequent severe respiratory and multi-organ failures in patients with COVID-19.
-
•
Effects of synthetic glucocorticoids might be confounded by the contemporary use of other complementary drugs.
Abstract
The viral infection by SARS-CoV-2 has irrevocably altered the life of the majority of human beings, challenging national health systems worldwide, and pushing researchers to rapidly find adequate preventive and treatment strategies. No therapies have been shown effective with the exception of dexamethasone, a glucocorticoid that was recently proved to be the first life-saving drug in this disease. Remarkably, around 20 % of infected people develop a severe form of COVID-19, giving rise to respiratory and multi-organ failures requiring subintensive and intensive care interventions. This phenomenon is due to an excessive immune response that damages pulmonary alveoli, leading to a cytokine and chemokine storm with systemic effects. Indeed glucocorticoids’ role in regulating this immune response is controversial, and they have been used in clinical practice in a variety of countries, even without a previous clear consensus on their evidence-based benefit.
1. Introduction
The ongoing pandemic of Coronavirus Disease 2019 (COVID-19), caused by the novel Severe Acute Respiratory Syndrome-Coronavirus (CoV)-2 (SARS-CoV-2), was declared a Public Health Emergency of International concern on January 30, 2020 by the World Health Organization (WHO[a]). One month before, pneumonia of unknown origin was detected in Wuhan, China, and was first reported to the WHO Country Office in China. This viral infectious disease is characterized by a high mortality, principally caused by a respiratory failure due to a severe cytokine and chemokine storm, an exaggerated immune response of the host aimed at preventing the invasion of the pathogen. Such a strong reaction is considered responsible for the pathogenesis of the host tissue damage (i.e., of pulmonary alveoli and of pro-inflammatory systemic effects), leading to the principal severe clinical manifestations of COVID-19 (i.e., respiratory and multi-organ failure (MOF)).
Indeed, around 20 % of infected patients develop an Acute Respiratory Distress Syndrome (ARDS) [1,2] (Fig. 1 ), which is thought to be linked to the massive release of pro-inflammatory cytokines (i.e., interleukin (IL)-1β [IL-1β], IL-2, IL-6, IL-7, IL-8, tumor necrosis factor-α [TNF-α]) and chemokines (C-X-C motif ligand 10 [CXCL10] and CC motif ligand 2 [CCL2]), further giving rise to MOF [3,4]. Risk factors for the development of ARDS in COVID-19 were: older age, neutrophilia, organ dysfunction and coagulation dysfunction [5]. The entire phenomenon is named Cytokine Release Syndrome (CRS), or Systemic Inflammatory Response Syndrome (SIRS), or Secondary haemophagocytic lymphohistiocytosis (SHLH). The management of this cytokine and chemokine storm during COVID-19 represents a crucial and controversial point [6], considering that the use of immune suppressive drugs such as glucocorticoids (GCs) can either inhibit the tissue damage and at the same time curb the cell-mediated immunity (i.e., reducing antigen presentation, lymphocyte proliferation, etc.) (Fig. 1) [7].
Fig. 1.
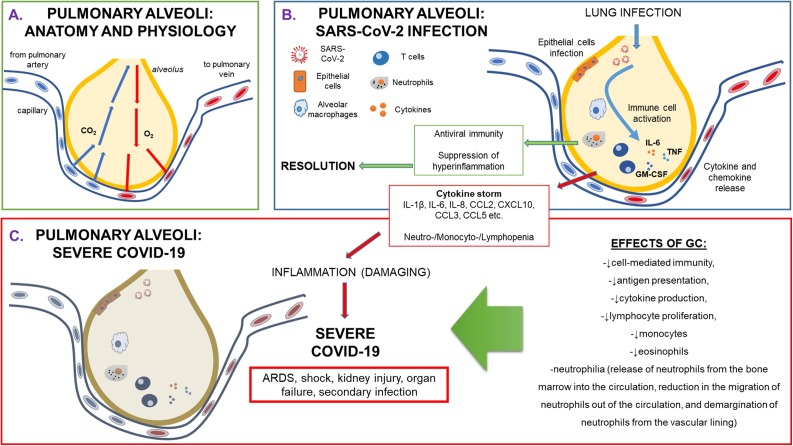
Physiology and pathophysiology of pulmonary alveoli during SARS-CoV-2 infection.
(A) Anatomy and physiology of healthy pulmonary alveoli, representing small air spaces in the lungs where carbon dioxide leaves and oxygen enters the blood. During inhalation air enters the lungs, travels through bronchi, bronchioles and then flows into approximately 300,000,000 alveoli. During exhalation, the carbon-dioxide-laden air is forced out of the alveoli through the same passageways. (B) Pathophysiology of pulmonary alveoli during SARS-CoV-2 infection. Infection of the novel Coronavirus, named SARS-CoV-2 creates an epithelial damage, followed by an immune response that is generated by a cytokine and chemokine release. This could lead to immune cell activation that might give rise to either resolution and severe COVID-19 with the consequent development of ARDS and of multi-organ failure. (C) Severe COVID-19: immune biological aspects. A sustained cytokine storm generates an inflammation that causes damage to the alveolar epithelium, giving rise to ARDS, shock, kidney injury, other organ failure and secondary infection.
Legend: ARDS: Adult Respiratory Distress Syndrome; CCL: C-C motif chemokine ligand; CXCL: C-X-C motif chemokine ligand; CO2:carbon dioxide; COVID-19: CoronaVirus Disease-19; GC: glucocorticoid; GM-CSF: granulocyte-monocyte colony stimulating factor; IL: interleukin; TNF: tumor necrosis factor.
Aims of this narrative review are: to provide a brief synthesis of the rationale for GC use in the management of patients with COVID-19, their principal pharmacological characteristics, their role and the ongoing clinical trials in this setting.
2. Glucocorticoids and COVID-19
Faced with the complex scenario of severely ill patients with COVID-19, a variety of protocols employing complementary treatments (i.e., anti-viral, anti-malarial and anti-rheumatic agents, serine protease inhibitors, IL blockers and low-molecular-weight heparin (LMWH) [8]) have been developed in various countries, some of them using GCs for the treatment of hospitalized patients with phase IIb-III COVID-19 [9,10] (Fig. 2 ). The crucial point here is that GCs might be helpful in preventing the alveolar/pulmonary damage induced by the cytokine and chemokine storm. Although GCs are used in this situation as immune suppressants (i.e., leading to an inhibition of cytokine production), they potentially cause delay in the clearance of the virus and impair lymphocyte proliferation, etc. [10,11] (Fig. 1). Nevertheless, in the past, GCs have been used in the management of ARDS caused by Middle East Respiratory Syndrome (MERS)-CoV[12] and SARS-CoV [13], both characterized by a histological pulmonary inflammation and by a diffuse alveolar damage [14] (Fig. 1). However, there was no consensus in the literature that the treatment with GCs could be beneficial in the management of COVID-19, considering the potential delay in the clearance of the virus, but also the increase of the risk of secondary infections and adverse effects (i.e., hyperglycemia, psychosis, and avascular necrosis), and considering that the effects on patients’ outcome were not that clear [8,12,13,15]. Recently, results from a randomized trial were publicly announced, revealing that low dose dexamethasone (DX) helped save the lives of seriously ill patients with COVID-19[b], cutting the risk of death by a third for patients on ventilators and by a fifth for those on oxygen (O2) therapy.
Fig. 2.
Severity and phases of COVID-19.
(A) COVID-19 severity: frequency of degrees of clinical manifestations in a Chinese cohort (>44.000 patients) (ref: Wu Z. et al., 2020). (B) Phases of COVID-19 disease and timing for the use of glucocorticoids.
Legend: ARDS: Adult Respiratory Distress Syndrome; COVID-19: CoronaVirus Disease-19; MOF: multi-organ failure; pts: patients; SIRS: systemic inflammatory response syndrome.
3. Glucocorticoids: principal mechanisms of action
The mechanisms of action of GCs are characterized as: 1) genomic and 2) non-genomic, as summarized in Fig. 3 . The genomic mechanism of action is mediated by the GC receptor (GCR) that generates the majority of anti-inflammatory and immunosuppressive effects. GCR is localized within the cytoplasm, and the complex generated after its binding with the GC translocates into the nucleus, where it inhibits the transcription of the genes involved in the activation of leukocytes and in the regulation of the function of epithelial, stromal and endothelial cells [16,17]. This generates a reduction of pro-inflammatory cytokines, chemokines, and molecules of cellular adhesion and of other enzymes that are involved in the inflammatory response. Specifically, the effects are: a reduction of the recruitment of white blood cells (including monocytes-macrophages but excluding neutrophils) into affected areas, further inhibiting the release of chemotactic signals and the expression of cytokines regulating the function of macrophages, endothelial cells, lymphocyte activities (i.e.: IL-1, IL-2, IL-3, IL-6, TNF-α and granulocyte monocyte-colony stimulating factor (GM-CSF)) and fibroblast proliferation. Further, release of histamine from basophils is impaired.
Fig. 3.
Mechanisms of action of synthetic glucocorticoids (GC).
(A) Genomic mechanism. (B) Non-genomic mechanism.
Legend: GC: glucocorticoid; GCR: glucocorticoid receptor; mRNA: messanger RNA; TF: transcription factor.
The non-genomic mechanism is more rapid and is mediated by the interactions with the cytoplasmic GCR or with the membrane GCR [18]. Within a few seconds or minutes after GC binding to GCR, a cascade of effects is activated, including the inhibition of phospholipase A2. This phenomenon is mediated by an increased synthesis of lipocortin-1, generating an impaired release of arachidonic acid followed by a decreased production of prostaglandins, leukotrienes and platelet activating factor [16,18].
In addition to the immunosuppressive effects on the proliferation of B and T lymphocytes, as well as the inhibition of monocyte function, high concentrations of GCs reduce complement levels [18,19]. Overall, GC anti-inflammatory and immunosuppressive activity influence the concentration, distribution and function of peripheral leukocytes, with an impairment of the activity of B and T lymphocytes and an increase in the number and activity of neutrophils, whereas in macrophages a reduction of the arachidonic acid metabolites like prostaglandins, leukotrienes and platelet activating factor is observed.
4. Glucocorticoids: treatment of COVID-19
Table 1 summarizes the protocols of GC employed in the treatment of COVID-19 patients.
Table 1.
Synthetic glucocorticoids (GC) employed in the treatment of patients with COVID-19 in China, Italy, United States (US) and United Kingdom (UK).
| Guidelines and therapeutic protocols |
GC | Route of administration | Indications for GC use | Dosing | Type of the study |
|---|---|---|---|---|---|
| CHINA [n] |
MP | IV, OS | *For pts: -in severe and critically ill stage; -with persistent high fever (temperature >39 °C); -whose computerized tomography (CT) demonstrated patchy ground-glass attenuation or having >30 % area of the lungs involved; -whose CT demonstrated rapid progression (>50 % area involved in pulmonary CT images within 48 h); -whose IL-6 is above ≥ 5 ULN. *Appropriate and short-term use of GCs should be considered for pts with severe COVID-19 pneumonia as early as possible. *A high dose of GC should be avoided due to the possible development of adverse events and complications |
*Initial MP at a dose of 0.75∼1.5 mg/kg IV once a day (nearly 40 mg once or twice a day) is recommended. *MP at a dose of 40 mg q12 h can be considered for pts with falling body temperature or for pts with significantly increased cytokines under routine doses of GCs. *MP at a dose of 40 mg–80 mg q12 h can be used for critical cases. *The dosage of MP should be halved every 3–5 days if: -medical conditions of patients are improved; -the body temperature normalizes; -involved lesions on CT are significantly absorbed. *Oral MP once a day is recommended when the IV dose is reduced to 20 mg per day. *The length of GC treatment in not defined; some experts have suggested ceasing GC treatment when patients are nearly recovered. |
Observational/Retrospective |
| ITALY | |||||
| Protocols employed by single institutions | MP PD |
IV OS |
Pts in sub-intensive care units till the development of ARDS | *MP: 1 mg/kg/die for 5 days; *Afterwards MP reduction to 0.5 mg/kg/die for further 5 days. *In some protocols PD is used as a latest step (for dose reduction) and is administered per OS. |
|
| Study promoted by ASUGI (Azienda Sanitaria Universitaria Giuliano Isontina) [o] |
MP | IV, OS | Pts hospitalized in Pneumology/UTIR (Respiratory Intensive Care Unit) matching the criteria established by clinical practice | *Day 1: MP: 80 mg IV as bolus, followed by MP: 80 mg in IV continuous infusion in 240 mL of physiological saline solution (0.9%) in 24 h. *The following 8 days: MP: 80 mg in IV continuous infusion in 240 mL of physiological saline solution (0.9%) in 24 h till reachment of P/F > 350 mmHg and/or CRP 2 ≤ mg/dL. *When P/F > 350 mmHg and/or CRP ≤2 mg/dl (or ≤20 mg/L): oral MP: 16 mg twice a day (or IV MP: 20 mg twice a day) to reduce till withdrawal when CRP is normal (±20 %) and/or P/F > 400 or SatHbO2 ≥ 95 % in air. |
Multicentric non randomized controlled cohort trial, in pts with COVID-19 linked ARDS |
| SIMIT (Società Italiana di Malattie Infettive e Tropicali) [e] [20] |
DX | OS | *Pts with slight respiratory symptoms but aged >70 years and/or with risk factors (COPD, diabetes and cardiopathy); *Symptomatic pts with slight symptoms: fever (temperature >37.5 °C), cough, slight to moderate dyspnea, and standard thorax X-ray with pneumonitis having BCRSS score (Brescia Covid Respiratory Severity Scale) > 2. *Pts with severe pneumonia, ARDS or global respiratory failure, hemodynamic decompensation: it is necessary to start DX24 h after ARDS diagnosis |
*DX: 20 mg/die for 5 days *Afterwards: 10 mg/die for 5 days |
|
| US (Yale University) [i] |
MP | IV | *May be considered for use by critical care team for salvage therapy. *GC should be used if clinically indicated as part of standard of care such as for an asthma or COPD exacerbation, or shock with history of chronic steroid use. *Lack of effectiveness and potential harm shown in literature, specifically inhibition of viral clearance in severe influenza and SARS, though possible benefit with critically ill COVID19 patients. |
*MP: 40 mg q8hr IV for three days, then re-assess | |
| UK [p, q] |
Low-dose DX [other drugs will be tested: Lopinavir-Ritonavir, Hydroxychloroquine, Azithromycin, Tocilizumab] |
IV, OS | *Hospitalized pts diagnosed with SARS-CoV-2 infection | *N.A. | Randomized controlled trial (RECOVERY) EudraCT number: 2020−001113-21 |
| WHO [b] [12,13,25,26] |
Do not routinely give systemic GC for treatment of viral pneumonia outside of clinical trials. A systematic review of observational studies of GC administered to patients with SARS reported no survival benefit and possible harms (avascular necrosis, psychosis, diabetes, and delayed viral clearance). Clinicians considering GC use for a patient with COVID-19 and with sepsis must balance the potential small reduction in mortality with the potential downside of prolonged shedding of CoV in the respiratory tract, as has been observed in patients with MERS-CoV. If GCs are prescribed, monitor and treat hyperglycaemia, hypernatraemia and hypokalaemia. WHO has prioritized the evaluation of GCs in clinical trials to assess safety and efficacy. |
||||
| [US IDSA (Infectious Diseases Society of America) [27] |
Among pts admitted to the hospital with COVID-19 pneumonia, the panel suggests against the use of GC. (Conditional recommendation, very low certainty of evidence). Among pts who have been admitted to the hospital with ARDS due to COVID-19, the panel recommends the use of GC in the context of a clinical trial. (Knowledge gap). |
||||
Legend: ARDS: adult respiratory distress syndrome; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; DX: dexamethasone; GCs: glucocorticoids; h: hours; IV: intravenous; MERS CoV: Middle East Respiratory Syndrome Coronavirus; MP: methilprednisolone; NA: not available; PD: prednisone; P/F: PaO2/FIO2; PO: per os; pts: patients; SARS-CoV-2: Severe Acute Respiratory Syndrome-Coronavirus-2; UK: United Kingdom; US: United States.
Overall, it was not clear whether patients diagnosed with COVID-19 could take advantage or might be harmed by the use of GCs [15]. At the beginnning, WHO did not recommend GCs in this setting[c], considering the available evidence in the literature, arising from non-randomized studies conducted on patients with MERS that revealed a prolonged viral clearance, without influencing or positively impacting the clinical outcome [12]. Nevertheless, WHO considered it a priority to wait for results from randomized controlled trials (RCT) on the use of GCs, since these drugs might represent complementary treatments potentially useful to save human lives during the COVID-19 pandemic in the future[d]. The recently announced results from the RCT on DX will probably change the future scenarios[b].
In some patients affected by SARS-CoV-2-linked pneumonia, the development of an ARDS, potentially responsive to GCs, has been observed. Indeed, a study published in the Journal of American Medical Association has shown favorable data on the use of methylprednisolone (MP), revealing that it might be beneficial in terms of mortality (=mortality reduction: 62 %) [5].
In China MP was employed in hospitalized patients with severe and critically ill stage III, with persistent fever (temperature >39 °C), with peculiar radiological manifestations in Computerized Tomography scans and with IL-6 levels >5 ULN (Table 1).
In Italy, the use of GCs was not clearly recommended by the official guidelines of the principal scientific societies that are involved in the management of COVID-19 patients (i.e., infectious disease[e], anesthesiology[f], pneumology[g], and internal medicine[h]). Nevertheless, the use of DX is suggested for the supportive treatment of patients with confirmed ARDS by an online vademecum published by the SIMIT (Italian Society of Tropical and Infectious Diseases[e]) in the region of Lombardy (the most affected region by COVID-19 in Italy) [20], and also by the recommendations of the Italian National Institute for the Infectious Diseases “L. Spallanzani”, that, as alternative treatment, also suggests the use of MP [21]. Further GCs are used in some Intensive Care Units (ICU), on the basis of the positive results from RCT on patients with severe Community-Acquired Pneumonia (CAP) [22]. Indeed, a number of RCT demonstrated that a prolonged treatment with a moderate dose of GCs, by downregulating the systemic and lung inflammation, is essential for increasing the speed of the resolution of the disease and for restoring tissue homeostasis [23,24].
In United States (US), the use of MP is considered for salvage therapy for the critical care teams, as a part of the standard of care (i.e., for an asthma or chronic obstructive pulmonary disease exacerbation, or shock with history of chronic steroid use[i]).
In United Kingdom, GCs were not recommended and a RCT named RECOVERY (EudraCT number: 2020−001113-21[j]), is testing a variety of drugs, including single agent low dose DX, whose positive results were recently announced[b] (Table 1).
5. Ongoing clinical trials
To date >1300 trials for COVID-19 have been recorded on ClinicalTrials.gov and currently 35 studies explore the use of GCs alone or in association with other drugs for the treatment of patients with SARS-CoV-2 infection (cut-off date: May 10, 2020).
All these studies involving GCs are currently ongoing, with the exception of: 1) a Chinese trial that was permanently suspended because the epidemic of COVID-19 in China (NCT04273321); 2) the NCT04244591 study from Beijing, which started on January 2020 and concluded the final data collection on April 13, 2020) and a 3) recently completed US observational retrospective trial which intends to study the role of early use of MP in the hospitalized patients with a diagnosis of COVID-19 pneumonia (NCT04374071). However, due to the extreme pace and complexity of this disease, no results are yet available from these studies.
Table 2 summarizes the main clinical trials using GCs as single agent therapy for the treatment of COVID-19 in at least one of their treatment arms.
Table 2.
Ongoing clinical trials testing GCs in COVID-19.
| Trial ID/status | Trial phase | Trial design | Drugs | Dose of GC | Phase of disease | Primary endpoint | Statistical method | Patient population | Ref |
|---|---|---|---|---|---|---|---|---|---|
|
NCT04366115 Not yet recruiting |
Phase 1 Phase 2 |
Phase 1: Single-ascending dose Open label Phase 2: Randomized Double-blind Placebo-controlled |
Experimental Arm: AVM0703 plus HCT Standard Arm: Placebo plus HCT |
Not Provided | Severe cases of COVID-19 |
Phase 1:
Phase 2:
|
Phase 1: Dose-Limiting Toxicity [Time Frame: 0-12 months] Phase 1-2: 28-day all-cause mortality [Time Frame: 0-12 months] |
COVID-19 positive adult pts with immediately life-threatening COVID-19, according to COVID-19 Critical Illness Salvage Criteria | NA |
|
NCT04361474 Not yet recruiting |
Phase 3 | Randomized Single-blind Placebo-controlled Multicenter |
Experimental Arm: Nasal irrigation with BUD and physiological saline Standard Arm: Placebo |
BUD 1mg/2mL diluted in 250mL of physiological saline 9°/00 | Mild/Moderate cases of COVID-19 | Efficacy of BUD vs Placebo according to the pre-specified outcome measures | Percentage of pts with an improvement of more than 2 points on the ODORATEST score (5) after 30 days of treatment [Time Frame: 30 Days] |
Adult pts with a suspected SARS-CoV-2 infection, whether or not confirmed by PCR, or close contact with a PCR-confirmed case, typical chest CT scan or positive serology; with isolated sudden onset hyposmia persisting 30 days after the onset of symptoms of SARS-CoV-2 infection | NA |
|
NCT04360876 Not yet recruiting |
Phase 2 | Randomized Double-blind Placebo-controlled Single center |
Experimental Arm: DX Standard Arm: Placebo |
DX 20 mg IV daily for 5 days followed by 10 mg daily for 5 days | Moderate or Severe cases of COVID-19 | Efficacy and Safety of DX vs placebo in mechanically ventilated pts with the hyper-inflammatory sub-phenotype of ARDS due to COVID-19 according to the pre-specified outcome measures | Ventilator Free Days at Day 28 [Time Frame: 28 Days] |
COVID-19 positive adult pts with moderate or severe ARDS (P/F ≤ 200mmHg) requiring mechanical ventilation within 7 days prior to randomization Hyper-inflammatory ARDS Sub-Phenotype |
NA |
|
NCT04359511 Not yet recruiting |
Phase 3 | Randomized Single-blind |
Experimental Arm: PD or HCT Standard Arm: Optimized SoC |
PD 0.7 mg/kg/day PO for 10 days, once a day, or HCT 3.5 mg/kg/day IV by continuous infusion for 10 days, if the patient cannot take drugs by oral route |
Moderate or Severe cases of COVID-19 | Efficacy and Safety of PD or HCT vs SoC according to the WHO Ordinal Scale | Clinical improvement defined by the improvement of 2 points on the WHO 7-category ordinal scale, at 14 days [Time Frame: 14 days] |
COVID-19 positive adult pts with moderate or severe oxygen-dependent disease or with an ARDS with CT scans thoracic images suggestive of diffuse alveolar damage either at the exudative phase or at the pulmonary organization phase | NA |
|
NCT04355637 Recruiting |
Phase 4 | Randomized Open label |
Experimental Arm: BUD Standard Arm: Optimized SoC |
Inhaled BUD | Mild/Moderate cases of COVID-19 | Efficacy of Inhaled BUD vs SoC according to the pre-specified outcome measures | Proportion of pts in both arms fulfilling the criteria for treatment failure:
[Time Frame: 15 days after treatment] |
COVID-19 positive adult pts hospitalized because of pneumonia status #3 - #4 WHO scale |
NA |
|
NCT04355247 Recruiting |
Phase 2 | Non randomized Open label Pilot study Exploratory trial |
Experimental Arm: MP |
MP 80 mg IV bolus injection daily x 5 days starting upon day 1 of admission to hospital. | Moderate or Severe cases of COVID-19 | Efficacy of MP according to the pre-specified clinical complete or partial response criteria | Clinical complete response criteria [Time Frame: 14 days] Clinical Partial Response criteria [Time Frame: 14 days] If < 50% of pts with high risk develop respiratory failure the investigator will consider the treatment as successful |
COVID-19 positive adult pts who meet criteria of high risk for severity | NA |
|
NCT04349410 Enrolling by invitation |
Phase 2 Phase 3 |
Randomized Open label Multicenter |
Following FMTVDM measurements, pts will be randomized into one of the 11 experimental treatment arms including MP 40 to 72 hours later (providing adequate time for treatment effect) FMTVDM will be repeated |
MP 80 mg IV over 30-minutes, BID x 7-days. Then taper off | Mild/Moderate and Severe cases of COVID-19 | Efficacy of FMTVDM to investigate the prevalence and severity of CoVid-19 pneumonia and to provide rapid determination of treatment response in each pts to direct treatment decisions | Improvement in FMTVDM Measurement with nuclear imaging [Time Frame: 72 hours] Measured improvement in tissue as measured using FMTVDM |
COVID-19 positive child and adult pts | NA |
|
NCT04348305 Recruiting |
Phase 3 | Randomized Quadruple-blinded Placebo-controlled Multicenter |
Experimental Arm: HCT Standard Arm: Placebo |
Continuous IV infusion of HCT 200 mg over 24 h (total 104 ml). or bolus injection of HCT 50 mg (10 ml) every 6 h if continuous IV infusion is not possible |
Severe cases of COVID-19 | Efficacy of HCT vs Placebo according to the pre-specified outcome measures | Days alive without life support (i.e. invasive mechanical ventilation, circulatory support or renal replacement therapy) at day 28 [Time Frame: Day 28 after randomization] A total of 1000 pts will be randomised in order to detect a 15% relative reduction in 28-day mortality combined with a 10% reduction in time on life support among the survivors with a power of 85% |
COVID-19 positive adult pts requiring hospitalization and use of one of the following:
|
NA |
|
NCT04345445 Not yet recruiting |
Phase 3 | Randomized Open label Single center |
Experimental Arm: Tocilizumab Standard Arm: MP |
MP 120 mg/day IV over 30 minutes for 3 days | Moderate and Severe cases of COVID-19 | Efficacy and Safety of Tocilizumab vs MPaccording to the pre-specified outcome measures (reducing the need for ventilator support in moderate/severe COVID-19 pts at risk for complications of cytokine storm) | The proportion of pts requiring mechanical ventilation [Time Frame: Through study completion, and average of 6 months] Mean days of ventilation [Time Frame: Through study completion, and average of 6 months] |
Hospitalized symptomatic COVID-19 positive adult pts with the presence of clinical and radiological signs of progressive disease, and laboratory evidence indicative of risk for cytokine storm complications | NA |
|
NCT04344730 Recruiting |
NA | Randomized Quadruple-blinded Placebo-controlled Single center |
Experimental Arm:
Standard O2 and placebo |
DX 20 mg/ 5 ml solution for injection in ampoule of 5mL from day 1 to day 10. | Severe cases of COVID-19 | Efficacy of DX and O2 support strategies vs placebo according to the pre-specified outcome measures | The time-to-death from all causes [Time Frame: day-60] The time to need for mechanical ventilation [Time Frame: day-28.] In non-mechanically ventilated pts, a 2x2 factorial design will be used to assess the two interventions, separately |
ICU COVID-19 positive adult pts with acute hypoxemic respiratory failure | NA |
|
NCT04344288 Recruiting |
Phase 2 | Randomized Open label Multicenter |
Experimental Arm: PD Standard Arm: Optimized SoC |
PD 0.75 mg/kg/day PO for 5 days, then 20 mg/day for 5 more days | Severe cases of COVID-19 | Efficacy of PD vs SoC according to the pre-specified outcome measures | Number of pts with a SpO2 <90% stabilized at rest and under not more than 5 L / min of supplemental O2 using medium concentration mask [Time Frame: 7 days] |
Hospitalized symptomatic COVID-19 positive adult pts with a theoretical respiratory indication for transfer to ICU | NA |
|
NCT04343729 Recruiting |
Phase 2 | Randomized Quadruple-blinded Placebo-controlled Single center |
Experimental Arm: MP Standard Arm: Placebo |
MP 0.5 mg/kg IV BID for 5 days. | Severe cases of COVID-19 | Efficacy and Safety of MP vs Placebo according to the Mortality Rate | Mortality rate at day 28 [Time Frame: on day 28, after randomization] |
COVID-19 positive adult pts with respiratory symptoms suggestive of SARS (cough OR dyspnea) at the time of screening, or diagnosis of SARS, taking into account the following:
|
NA |
|
NCT04330586 Not yet recruiting |
Phase 2 | Randomized Open label Multicenter |
Experimental Arm: CIC Standard Arm: CIC plus hydroxychloroquine |
CIC 320 ug oral inhalation every 12 h for 14 days | Mild/Moderate cases of COVID-19 | Efficacy of CIC alone or in combination with hydroxychloroquine in eradication of SARS-CoV-2 from respiratory tract earlier in pts with mild COVID-19 | Rate of SARS-CoV-2 eradication at day 14 from study enrollment [Time Frame: Hospital day 14] |
COVID-19 positive adult pts with mild COVID-19 according to the NEWS scoring system 0-4 | NA |
|
NCT04329650 Recruiting |
Phase 2 | Randomized Open label Multicenter |
Experimental Arm: Siltuximab Standard Arm: MP |
MP 250 mg/24 h IV for 3 days followed by 30 mg/24 h for 3 days | Mild/Moderate and Severe cases of COVID-19 | Efficacy and Safety of Siltuximab vs MP according to the pre-specified outcome measures | Proportion of pts requiring ICU admission at any time within the study period. [Time Frame: 29 days] |
Hospitalized COVID-19 positive adult pts with at least one of the following requirements:
|
NA |
|
NCT04327401 Recruiting |
Phase 3 | Randomized Open label Multicenter |
Experimental Arm: DX Standard Arm: Optimized SoC |
DX 20 mg IV daily for 5 days, followed by 10 mg IV daily for 5 days | Moderate and Severe cases of COVID-19 | Efficacy and Safety of DX vs SoC according to the pre-specified outcome measures (Ventilator-free days) | Ventilator-free days [Time Frame: 28 days after randomization] |
Probable or confirmed COVID-19 adult pts with
|
NA |
|
NCT04325061 Recruiting |
Phase 4 | Randomized Open label Multicenter |
Experimental Arm: DX Standard Arm: Optimized SoC |
DX 20 mg IV daily for 5 days, followed by 10 mg IV daily for 5 days | Severe cases of COVID-19 | Efficacy of DX vs SoC according to the pre-specified outcome measures (60-day mortality rate) | 60-day mortality [Time Frame: 60 days] |
Intubated and mechanically ventilated COVID-19 adult pts
|
NA |
|
NCT04323592 Recruiting |
Phase 2 | Non Randomized Open label Single center |
Experimental Arm: MP Standard Arm: "historical" but concurrent pts never treated with GC |
MP 80 mg IV bolus at admission followed by 80 mg in 240 ml 0.9% saline administered IV at 10 mL/h speed for at least 7 day or more | Severe cases of COVID-19 | Efficacy of MP vs SoC according to the pre-specified outcome measures | Composite primary endpoint
[Time Frame: 28 days] Comparison of two groups of SARS-CoV-2 positive pts: (i) Consecutively treated with low prolonged doses of MP (ii)Historical pts never treated with GC The two group will be matched (1:1) according to the following criteria:
|
COVID-19 positive young and adult pts with the following requirements:
|
NA |
|
NCT04278404 Recruiting |
Observational | Prospective Multicenter |
The POP02 study is collecting bodily fluid samples of children prescribed some drugs of interest per SoC, including the following GC: BUD DX HCT |
Not Provided | Mild/Moderate and Severe cases of COVID-19 | PK/PD and Safety Profile of under-studied drugs administered to children per SoC | CL or CL/F as measured by PK sampling V or V/F as measured by PK sampling Elimination rate constant as measured by PK sampling Half-life as measured by PK sampling Absorption rate constant as measured by PK sampling AUC as measured by PK sampling Cmax as measured by PK sampling Tmax as measured by PK sampling [Time Frame: Data will be collected up to 90 days from the time of consent] |
Participant with < 21 years of age receiving under-studied drugs of interest per SoC as prescribed by their treating provider for different diseases including COVID-19 | NA |
|
NCT04273321 Suspended |
NA | Randomized Open label Multicenter |
Experimental Arm: MP Standard Arm: Optimized SoC |
MP 1mg/kg/day IV or GTT for 7 days | Mild/Moderate cases of COVID-19 | Efficacy of MP vs SoC according to the pre-specified outcome measures | Incidence of treatment failure in 14 days:
|
COVID-19 positive adult pts admitted in the general wards for mild/moderate symptoms | NA |
|
NCT04263402 Recruiting |
Phase 4 | Randomized Single-blind Single center |
Experimental Arm (A): MP Experimental Arm (B): MP |
Arm (A): MP (<40 mg/d IV for 7 days). Arm (B): MP (40∼80 mg/d IV for 7 days) |
Moderate and Severe cases of COVID-19 | Efficacy of different doses of MP compared to each other according to the pre-specified outcome measures | Rate of disease remission
|
COVID-19 positive adult pts with any of the following requirements:
|
NA |
|
NCT04244591 Completed |
Phase 2 Phase 3 |
Randomized Open label Multicenter |
Experimental Arm: MP Standard Arm: Optimized SoC |
MP 40 mg IV every 12 h for 5 days | Severe cases of COVID-19 | Efficacy and Safety of MP vs SoC according to the pre-specified outcome measures | Lower Murray lung injury score The Murray scoring system range from 0 to 16 according to the severity of the condition. [Time Frame: 7 days after randomization] |
COVID-19 positive adult pts with the following requirements:
|
NA |
|
NCT03852537 Recruiting |
Phase 2 | Randomized Double-blind Single center |
Experimental Arm: MP Standard Arm: Optimized SoC |
MP will be administered based on CRP-guided protocol outlined under 'Biomarker-adjusted steroid dosing':
|
Severe cases of COVID-19 | Feasibility of the timely initiation of GC and implementation of biomarker-titrated GC dosing | Percentage of eligible pts adhered to the timely initiation (within 12 h of emergency room admission) and daily GC treatment according to ESICM/SCCM clinical practice guideline (control group) or biomarker concordance (intervention group) [Time Frame: Within 30 days of enrollment in study] |
COVID-19 positive adult pts with the following requirements:
|
NA |
|
NCT04377711 Not yet recruiting |
Phase 3 | Randomized Double-blind Placebo-controlled Multicenter |
Experimental Arm: CIC Standard Arm: Placebo |
CIC 160 μg Inhaler | Mild/Moderate cases of COVID-19 | Efficacy and Safety of CIC vs Placebo according to the pre-specified outcome measures | Percentage of pts with subsequent emergency department visit or hospital admission for reasons attributable to COVID 19 by day 30 [Time Frame: Day 30] |
COVID-19 positive child, young and adult pts not currently hospitalized or under immediate consideration for hospitalization at the time of enrollment | NA |
|
NCT04381364 Not yet recruiting |
Phase 2 | Randomized Open label Multicenter |
Experimental Arm: CIC Standard Arm: Optimized SoC |
CIC 320 μg twice daily for 14 days | Mild/Moderate cases of COVID-19 | Efficacy of CIC vs SoC according to the pre-specified outcome measures | Duration of received supplemental O2 therapy Time (in days) of received supplemental O2 therapy (defined as being alive and discharged from hospital to home or at least 48 h of not receiving O2 therapy during hospitalization). [Time Frame: 30 days after study inclusion] |
COVID-19 positive adult pts that are hospitalized and require O2 therapy | NA |
|
NCT04377503 Not yet recruiting |
Phase 2 | Randomized Open label Single center |
Experimental Arm: Tocilizumab Standard Arm: MP |
MP 1.5 mg/kg/day IV divided into 2 daily doses for 7 days, followed by MP 1 mg/kg/day IV for another 7 days. Finally, MP 0.5 mg/kg/day IV until 21 days of use | Mild/Moderate and Severe cases of COVID-19 | Efficacy and Safety of Tocilizumab vs MP in preventing the cytokine release syndrome | Pts clinical status 15 days after randomization A seven-category ordinal scale consisting of: 1) Death; 2) Hospitalized on invasive mechanical ventilation or ECMO; 3) Hospitalized on non-invasive ventilation or high flow O2 devices; 4) Hospitalized, requiring supplemental O2; 5) Hospitalized, not requiring supplemental O2 - requiring ongoing medical care (COVID-19 related or otherwise); 6) Hospitalized, not requiring supplemental O2 - no longer requires ongoing medical care; 7) Not hospitalized, limitation on activities and/or requiring home O2; 8) Not hospitalized, no limitations on activities. [Time Frame: 15 days after randomization] |
COVID-19 positive adult pts | NA |
|
NCT04374071 Completed |
Observational | Retrospective Multicenter |
Experimental Arm: MP Control group: Optimized SoC without use of GC |
MP 0.5 to 1 mg/kg/day IV in 2 divided doses for 3 days | Moderate and Severe cases of COVID-19 | Efficacy and Safety of MP vs Pre-GC protocol according to the pre-specified outcome measures | Number of pts transferred to ICU in each group [Time Frame: 14 days follow-up for every pt in each group] Number of pts needed mechanical ventilation in each group [Time Frame: 14 days follow-up for every pt in each group] Number of pts who died in each group [Time Frame: 14 days follow-up for every pt in each group] |
COVID-19 positive adult pts with O2 requirement by nasal cannula, HFNC or mechanical ventilation | NA |
|
NCT04374474 Not yet recruiting |
Phase 4 | Randomized Open label Single center |
Experimental Arm: BUD Standard Arm: Optimized SoC for olfactory retraining |
BUD twice a day | Mild cases of COVID-19 | Efficacy of BUD vs SoC in anosmia rehabilitation for post-COVID-19 pts according to the pre-specified outcome measures | Change from S&ST Test at 3 and 6 months [Time Frame: 3and 6 months] Change from baseline SIT at 3 and 6 months [Time Frame: 3and 6 months] |
COVID-19 positive adult pts with hyposmia/anosmia of onset immediately after an upper respiratory viral illness confirmed on S&ST testing | NA |
Legend:
ARDS: adult respiratory distress syndrome; AUC: Area under the curve; BID: Bis in die; BUD: Budesonide; CIC: Ciclesonide; CL: Clearance; CL/F: apparent oral clearance; Cmax: Maximum concentration; COVID-19: Coronavirus disease 2019; CPAP: Continuous positive airway pressure; CRP: C-Reactive Protein; CT: Computed Tomography; DX: dexamethasone; ECMO: Extracorporeal Membrane Oxygenation; ESICM/SCCM: European Society of Intensive Care Medicine alongside the Society of Critical Care Medicine; FMTVDM: Fleming Method for Tissue and Vascular Differentiation and Metabolism; GC: glucocorticoids; GTT: guttae; h: hours; HCT: Hydrocortisone; HFNC: High Flow Nasal Cannula; ICU: Intensive Care Unit; IL: Interleukin; IV: intravenous; MP: methylprednisolone; NA: not available; NEWS: National Early Warning Score; O2: Oxygen; PCR: Polymerase Chain Reaction; PD: prednisone; PEEP: Positive end-expiratory pressure; P/F: PaO2/FIO2 ratio; PK/PD: Pharmacokinetics and Pharmacodynamics; PO: per os; pts: patients; RP2D: Recommended Phase 2 Dose; RR: Respiratory Rate; SARS-CoV-2: Severe Acute Respiratory Syndrome-Coronavirus-2; S&ST: Snap and Sniff Threshold Test; SoC: Standard of Care; SOFA: Sequential Organ Failure Assessment; SIT: Smell Identification Test; Tmax: Time to achieve maximum concentration; V: Volume of distribution; V/F: apparent oral volume of distribution; WHO: Word Health Organization.
Considering the 27 studies listed in Table 2: 13 trials are currently recruiting, 10 are not yet recruiting, 2 are completed, 1 is suspended, and 1 is enrolling by invitation. Most of these studies are phase II ( = 10), followed by 7 phase III, 4 phase IV, 2 phase II/III, 2 observational and 1 phase I/II trials. No classification was provided for one study (NCT04344730). Almost all of the trials cited in Table 2 are randomized except for the 2 aforementioned observational studies (NCT04278404, NCT04374071) and 2 non-randomized trials (NCT04355247, NCT04323592); only 1 study is retrospective (NCT04374071). Monotherapy with GC was tested as an experimental arm in 23 trials while GCs were used as standard control arm in the remaining 4 studies.
The most commonly used GCs are: intravenous (IV) MP, followed by IV DX, IV hydrocortisone and prednisone administered per os. Interestingly, inhaled steroids such as budesonide and ciclesonide are also tested in 4 and 3 trials respectively.
Specifically, IV GCs are being tested mainly in moderate or severe cases of COVID-19 (i.e. young and adult patients requiring non-invasive ventilation, O2-dependent disease, subjects needing invasive intubation with mechanical ventilation for moderate/severe ARDS or immediately life-threatening patients admitted to ICU for acute hypoxemic respiratory failure and at high risk for cytokine/chemokine storm complications), while inhaled steroids are tested in cases of mild disease, mainly for rehabilitation of hyposmia/anosmia that arose immediately after the upper respiratory COVID-19 infection or in patients with moderate symptoms, with the aim to reduce the need for O2 supplementation.
The remaining studies that have not been included in Table 2 evaluate GCs in association with other drugs as combinational therapies for the treatment of patients with SARS-CoV-2 infection. NCT04371601 is an early phase I randomized trial which explores the addition of umbilical cord mesenchymal stem cells to conventional treatments such as anti-viral, GCs and O2 therapy versus the above-mentioned symptomatic therapies for patients with severe COVID-19 pneumonia. A French multicenter, randomized phase III trial (NCT04347980) is evaluating DX plus hydroxychloroquine in comparison to hydroxychloroquine alone for the treatment of SARS induced by COVID-19.
Intriguingly, the COPERNICO study, a prospective, multicenter, randomized, open-label, phase II trial (NCT04335305), is assessing the efficacy of tocilizumab combined with pembrolizumab compared to standard care with GC, tocilizumab, virally targeted agents, chloroquine or hydroxychloroquine and supplemental O2 in adult patients with COVID-19 pneumonia and bad prognostic factors who are non-responsive to frontline therapy within 48 h from treatment initiation.
The inhaled steroid budesonide in combination with the β2 agonist formoterol is under investigation in 2 randomized phase III trials (NCT04331470 and NCT04331054) with the aim to prevent an excessive local immune reaction in the respiratory system.
MP in association with immunosuppressive drugs such as tacrolimus and thalidomide is being tested in a phase III (NCT04341038) and a phase II (NCT04273581) trial, respectively, in patients at high risk for cytokine storm development.
Finally, the REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform Trial for CAP) is an adaptive research platform for the evaluation of multiple treatment modalities in the event of a respiratory pandemic resulting in critical illness, that has set-up a sub-platform called “REMAP−COVID” (NCT02735707) for the evaluation of specific treatments for COVID-19. In particular, the aim of this study is to assess the effect of a range of interventions including hydrocortisone to improve the outcome of patients with SARS-CoV-2 infection admitted to ICU.
6. Conclusions
Synthetic GCs represent a well-known, cheap, widely available and easily manageable class of drugs used in a variety of settings, particularly for their immunosuppressive activities. In this review, we have synthesized their principal mechanisms of action and their putative roles in limiting an exaggerated immune response starting in the lung alveoli of 20 % of patients that develop COVID-19.
Despite a previous lack of evidence of a clear benefit for patient survival outcomes, GCs have been used in clinical practice for the management of COVID-19 hospitalized patients, for their hypothetical benefit on respiratory function and consequently their potential effect on reducing the number of patients requiring invasive procedures (i.e., ventilation or intubation). Indeed, in such a health emergency situation, clinicians have employed GCs as an “accepted” therapy, for their reasonable expectation of success in the treatment of individual patients[k]. Thus, GCs have been administered according to physician’s judgment, based on the patient’s best interest. To this end, the use of these immunosuppressive drugs has been considered reasonable despite the risk of developing adverse events and that of delaying the clearance of the virus, the resolution of the respiratory failure being the biggest issue in the phases IIb and III of COVID-19 patients. This intervention was reasonable when treating an individual patient, particularly when facing with the lack of effective drugs that had been proven to be effective in this disease at the beginning of the Public Health Emergency. Thus physicians were ethically allowed to use an unproven intervention if they judged it helpful for saving lives, re-establishing health and alleviating suffering (Declaration of Helsinki[l]). However, this approach does not apply to the main purpose of research, which requires testing a hypothesis, giving rise to new generalizable knowledge[k]. A step forward would be represented by the publication of the results of the RECOVERY RCT[b], revealing a death risk reduction for patients on ventilators and on O2 therapy treated with low dose DX.
Further, considering that in most of the institutions, GCs have been given in association with other complementary compounds, such as anti-viral, anti-malarial and anti-rheumatic agents, serine protease inhibitors, IL blockers, and LMWH, it is difficult to evaluate whether the benefits for patients are associated with single versus the combination of all these drugs.
In a situation of Public Health Emergency with such a severe life-threatening infection, it had been very hard to plan and wait for results from RCT (i.e., will it be ethically correct to exclude patients from GC treatment?), rendering it a priority to employ drugs that support the vital functions of sick people rapidly. Other issues related to starting clinical trials in this context we acknowledge: 1) the choice of the treatment (old and manageable drugs with well known safety profiles are preferably used with respect to new treatments; but will these old drugs will be administered at the same doses? Or at different doses and treatment schedules?); 2) the selection of patients (at which stage of the disease? In seriously ill versus those with low/mild symptoms? Will seriously ill patients be able to provide an informed consent? Will relatives be allowed to do that, also considering the rules of prevention of the spread of this infection?); 3) clinical trial design: the ideal setting would be double blinded RCT, but is there time to produce an adequate placebo for the standard treatment arm? Will the design of the trial be feasible once the number of cases will diminish during the course of the epidemic[l]?
Moving to clinical practice, hopefully in the near future, when the incidence and prevalence of the disease decreases, the diagnosis of SARS-CoV-2 infection would be performed at earlier stages, the expertise in the management of the disease will be increased, observations from retrospective analyses and results from clinical trials will be much more numerous, researchers will have clearer ideas on which topics and questions still need to be more clearly addressed.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
NP reports personal fees from SHIONOGI Ltd, personal fees from MSD, personal fees from Becton & Dickinson, personal fees from Pfizer, personal fees from Cepheid, outside the submitted work.
All other authors (CS, LP, MA, and EM) have no conflict of interest to declare.
Acknowledgments
Authors thank Dr. David Gray for assistance in writing in English.
Biographies

Doctor Cinzia Solinas is a Medical Oncologist who earned her PhD in Medical Sciences (topic: Immuno-Oncology in Breast Cancer) in January 2019 at the Universitè Libre de Bruxelles (Belgium), after her four-year research thesis performed at the Institut Jules Bordet (Bruxelles, Belgium; mentors: Profs. Martine Piccart and Christos Sotiriou). Currently she works at the Hospital Antonio Segni, Ozieri (SS), Italy as a clinician. She graduated in Medicine at the University of Cagliari (Italy). Afterwards she became an Oncologist at the school of Medical Oncology at the University of Cagliari (Italy) and she spent the last 9 months of her specialty at the Medical Oncology Department,Institut Jules Bordet, Bruxelles (Belgium). In October 2014 she started her PhD at the Molecular Immunology Unit (Head: Dr Willard-Gallo) of the same institute. Cinzia's main areas of research have been the characterization of the immune response, of immune checkpoint molecules in breast cancer, and of different clinical aspects related to the use of cancer immunotherapy (i.e., radiological, adverse events, etc). Cinzia is a member of the International Immuno-Onvcology Biomarker Working Group.

Doctor Laura Perra is an Endocrinologist and Diabetes specialist. She takes care of around 4.000 patients/year and currently works at the Azienda Tutela della Salute, Sardegna (Italy). She graduated in Medicine at the University of Cagliari (Italy) and afterwards she became an Endocrinologist and Diabetes specialist at the University of Cagliari (Italy) under the supervision of Prof. Marco Baroni and Stefano Mariotti. In addition, Doctor Perra is the Medical Director of the “Centro Medico HB” (in the city of Quartu Sant’Elena, Sardinia) a multi-specialized center where she contributed to developing the protocols for the prevention and management of the spread of SARS-CoV-2 infection.

Doctor Marco Aiello is a Medical Oncologist who earned his second Level Master Degree in Immuno-Oncology in March 2019 at the Università degli Studi "La Sapienza" of Rome (Italy). Currently he works at the Medical Oncology Unit of the Azienda Ospedaliero Universitaria “Policlinico - San Marco” of Catania as a clinician and a researcher. He graduated in Medicine at the University of Catania (Italy). Afterwards he became a Medical Oncologist at the school of Medical Oncology at the University of Catania (Italy) under the supervision of Prof. Hector Soto Parra, and he spent the last 6 months of his residency at the “New Drugs Development and Clinical Research Unit” of the Oncology Institute of Southern Switzerland (IOSI) in Bellinzona, Switzerland. Fully devoted to the oncological clinical practice and clinical research with main interests focused on lung cancer patients treated with cancer immunotherapies and the development of new anti-cancer drugs, Doctor Aiello has served as sub-investigator for several phase I, II and III clinical trials, focusing on new drugs and immunotherapy development, especially in lung cancer. Currently, in his institution, he is involved in the screening of cancer patients that developed an infection from SARS-CoV-2.

Doctor Edoardo Migliori is a postdoctoral research scientist at the Columbia Center for Translational Immunology (CCTI), New York City. He earned his PhD in Biomedical and Pharmaceutical Sciences in October 2017 at the Universitè Libre de Bruxelles (Belgium), under the supervision of Prof. Martine Piccart and Dr. Karen Willard-Gallo. His thesis focused on the importance of CD4+ follicular helper T cells and tertiary lymphoid structures in the anti-tumor immune response to breast cancer, performed at the Institut Jules Bordet (Brussels, Belgium). He joined CCTI in February 2018, to work with Dr. Pawel J. Muranski on basic and translational tumor immunology with a focus on T cell biology and adoptive immunotherapy of cancer and viral diseases, with the goal of developing new treatments for patients. His project on COVID-19 focuses on generation and manipulation of SARS-CoV-2-specific T cells, aiming to obtain a rapid reconstitution of the adaptive T cell immunity, which might prevent the development of severe COVID-19 disease in stem cell transplantation (SCT) recipients and other vulnerable patients by providing specific self-renewing immune repertoire.

Doctor Nicola Petrosillo has a degree in Medicine (1977), and specialization in Infectious Diseases (1981), Internal Medicine (1985), and Civil Protection (1986). Currently he is the Director of the Clinical and Research Infectious Disease Department at the National Institute for Infectious Diseases, “Lazzaro Spallanzani,” in Rome. He his Researcher at Clinical Microbiology and Infection Prevention, University Medical Center of Groningen University, Groningen, the Netherlands; he is an Extraordinary Professor at the Unicamillus University of Rome, Italy. Since 2018 he is Chair of the International Affairs SubCommittee of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID); furthermore, he is co-leader of the ESCMID Emerging Infection Taskforce. He is also President of the Multidisciplinary Joint Committee - Infection Control of European Union of Medical Specialists (UEMS), and Vice Chair of the European Board of Infectious Diseases of the Infectious Disease Section of UEMS. He is Leader of the International Taskforce on HIV-associated Pulmonary Hypertension of the Pulmonary Vascular Research Institute. He was WHO clinical and Infection Prevention Consultant in Lagos, Nigeria for the 2014 Ebola epidemic, and Chief of the Crisis Unit of the National Institute “Spallanzani” during the 2014–2015 Ebola Epidemic, taking care of Ebola Virus infected patients. Currently, as Chief of the Clinical & Research Department, he is involved in the care of COVID-19 patients at the National Institute “L. Spallanzani”, in Rome, Italy. His clinical and research interests focus on emerging infections and on severe, healthcare acquired and systemic infections, in particular those caused by multidrug resistant organisms. He is author of 345 articles in peer reviewed journals (H-index 44; citations 9159; P 13,45 %; 23.9 %; PMA 855), and of several chapters on infectious disease books. He is editor-in-chief of Infectious Disease Reports.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cytogfr.2020.06.012.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J., Shu H., Xia Ja., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020;2020 02.10.20021832. [Google Scholar]
- 5.Wu C., Chen X., Cai Y., Ja. Xia, Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. Published online March 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun. Rev. 2020;19(5) doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuel S., Nguyen T., Choi H.A. Pharmacologic characteristics of corticosteroids. J Neurocrit Care. 2017;10(2):53–59. [Google Scholar]
- 8.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 9.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancer. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? ecancer. 2020;14 doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N.L.-S., Chan P.K.-S., Wong C.-K., To K.-F., Wu A.K.-L., Sung Y.-M., Hui D.S.-C., Sung J.J.-Y., Lam C.W.-K. Early enhanced expression of interferon-inducible Protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005;51(12):2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Solaiman O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Al Harthy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 13.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memish Z.A., Perlman S., Van Kerkhove M.D., Zumla A. Middle east respiratory syndrome. Lancet. 2020;395(10229):1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streeten D.H.P., Phil D., Corticosteroid Therapy I. Pharmacological properties and principles of corticosteroid use. Trends in Therapy. 1975:4. doi: 10.1001/jama.232.9.944. [DOI] [PubMed] [Google Scholar]
- 17.Hodgens A., Sharman T. StatPearls Publishing; Treasure Island (FL): 2020. Corticosteroids. [PubMed] [Google Scholar]
- 18.Ericson-Neilsen W., Kaye A.D. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14(2):5. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D., Ahmet A., Ward L., Krishnamoorthy P., Mandelcorn E.D., Leigh R., Brown J.P., Cohen A., Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013;9(1):30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., Martín-Rodríguez C., Díaz-Domínguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Añón J.M., Fernández R.L., González-Martín J.M., Aguilar G., Alba F., Álvarez J., Ambrós A., Añón J.M., Asensio M.J., Belda J., Blanco J., Blasco M., Cachafeiro L., del Campo R., Capilla L., Carbonell J.A., Carbonell N., Cariñena A., Carriedo D., Chico M., Conesa L.A., Corpas R., Cuervo J., Díaz-Domínguez F.J., Domínguez-Antelo C., Fernández L., Fernández R.L., Ferrando C., Ferreres J., Gamboa E., González-Higueras E., González-Luengo R.I., González-Martín J.M., Martínez D., Martín-Rodríguez C., Muñoz T., Ortiz Díaz-Miguel R., Pérez-González R., Prieto A.M., Prieto I., Rivas R., Rojas-Viguera L., Romera M.A., Sánchez-Ballesteros J., Segura J.M., Serna-Grande P., Serrano A., Solano R., Soler J.A., Soro M., Tallet A., Villar J. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 21.Nicastri E., Petrosillo N., Ippolito G., D’Offizi G., Marchioni L., Ascoli Bartoli T., Lepore L., Mondi A., Murachelli S., Antinori A. National institute for the infectious diseases “L. spallanzani” IRCCS. Recommendations for COVID-19 clinical management. Infect. Dis. Rep. 2020;12(1) doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Confalonieri M., Urbino R., Potena A., Piattella M., Parigi P., Puccio G., Della Porta R., Giorgio C., Blasi F., Umberger R., Meduri G.U. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am. J. Respir. Crit. Care Med. 2005;171(3):242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q., Shi J.X., Hu R., Li Q., Zhang C.Y., Li J.S. Effect of glucocorticoids on mortality in patients with acute respiratory distress syndrome: a meta‑analysis. Exp. Ther. Med. 2019;18(6):4913–4920. doi: 10.3892/etm.2019.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres A., Sibila O., Ferrer M., Mendendez R., Mensa J., Gabarrus A., Sellares J., Restrepo M., Anzueto A., Niederman N., Agusti C., Polverino E. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. Annual Congress 2015, European Respiratory Society. 2015:OA3267. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst. Rev. 2016;3:CD010406. doi: 10.1002/14651858.CD010406.pub2. [DOI] [PubMed] [Google Scholar]
- 26.H.N.C. on behalf of the Canadian Critical Care Trials Group, Delaney J.W., Pinto R., Long J., Lamontagne F., Adhikari N.K., Kumar A., Marshall J.C., Cook D.J., Jouvet P., Ferguson N.D., Griesdale D., Burry L.D., Burns K.E.A., Hutchison J., Mehta S., Menon K., Fowler R.A. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016;20(1):75. doi: 10.1186/s13054-016-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C., Edwards K.M., Gandhi R., Muller W.J., O’Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of america guidelines on the treatment and management of patients with COVID -19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa478. ciaa478. Accepted/In press - Apr 27 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Web References
https://www.bbc.com/news/health-53061281
https://www.who.int/teams/blueprint/covid-19
http://www.simit.org/IT/index.xhtml
http://www.siaarti.it/News/COVID19%20-%20documenti%20SIAARTI.aspx
https://www.simi.it/news/pharmacologic-treatments-for-coronavirus-disease
https://medicine.yale.edu/news-article/23611/
https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001113-21/GB
https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
https://www.alnap.org/help-library/handbook-of-covid-19-prevention-and-treatment
https://clinicaltrials.gov/ct2/show/NCT04323592?term=NCT04323592&draw=2&rank=1
https://www.recoverytrial.net/
https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001113-21/GB
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.