Highlights
-
•
One of the routine essential health services that is being disrupted by coronavirus disease 2019 (COVID-19) in Africa is childhood immunization.
-
•
Experiences from previous outbreaks on the continent indicate that any disruption of immunization services could result in epidemics of childhood vaccine-preventable diseases, which will invariably increase child mortality.
-
•
Contextualized strategies are needed to mitigate the impact of COVID-19 on access to and utilization of immunization services.
-
•
Systems thinking could advance the understanding of the interaction between COVID-19 and immunization by explicitly elucidating the non-linear and dynamic relationships that exist between all elements of the system.
-
•
Implementation science models could be used to fast-track the use of evidence-based innovations to re-design local systems, such that they enhance access and utilization of immunization services despite the COVID-19 outbreak.
Keywords: Implementation science, Systems thinking, Immunization, Africa, COVID-19
Abstract
One of the routine health services that is being disrupted by coronavirus disease 2019 (COVID-19) in Africa is childhood immunization. This is because the immunization system relies on functioning health facilities and stable communities to be effective. Its disruption increases the risk of epidemics of vaccine-preventable diseases, which could increase child mortality. Therefore, policymakers must quickly identify robust and context-specific strategies to rapidly scale-up routine immunization in order to mitigate the impact of COVID-19 on their national immunization performance. To achieve this, we propose a paradigm shift towards systems thinking and use of implementation science in immunization decision-making. Systems thinking can inform a more nuanced and holistic understanding of the interrelationship between COVID-19, its control strategies, and childhood immunization. Tools like causal loop diagrams can be used to explicitly illustrate the systems structure by identifying feedback loops. Once mapped and leverage points for interventions have been identified, implementation science can be used to guide the rapid uptake and utilization of multifaceted evidence-based innovations in complex practice settings. As Africa re-strategizes for the post-2020 era, these emerging fields could contribute significantly in accelerating progress towards universal access to vaccines for all children on the continent despite COVID-19.
Coronavirus disease 2019 (COVID-19) is a respiratory disease caused by a novel virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Gorbalenya et al., 2020). The outbreak of this disease was first reported in December 2019 in Wuhan, China (Lu et al., 2020). The virus can spread from human to human through droplets or contaminated surfaces (Lai et al., 2020). It has an incubation period of 2–14 days and viral shedding has been reported in pre-symptomatic and asymptomatic carriers (He et al., 2020, Lai et al., 2020). Compared to the coronavirus causing severe acute respiratory syndrome (SARS), the transmissibility of this virus is higher, with an estimated basic reproductive number of 2.24–3.58 depending on the setting (Lai et al., 2020). COVID-19 was declared a public health emergency of international concern (PHEIC) on January 30, 2020 (World Health Organization 2020b).
The disease has now spread to most countries in Africa (Nkengasong and Mankoula, 2020, World Health Organization, 2020c). This is partly because of air travel interconnectivity and volume and the transmission efficacy of the virus itself (Gilbert et al., 2020, Lai et al., 2020). Since the introduction of the first case in February 2020, the number of cases on the continent has grown to more than 100 000 (World Health Organization, 2020c). A modelling study conducted by the World Health Organization (WHO) predicted that with widespread community transmission, an estimated 223 281 401 (representing 22% of the continent’s population) will become infected within the next 1 year (Cabore et al., 2020). The highest number of cases are expected in Nigeria, Algeria, and South Africa (Cabore et al., 2020). Out of the total cases, an estimated 36 967 532 will develop symptoms and 150 078 will die (Cabore et al., 2020). Based on this scenario, 4 637 240 Africans will require hospital admission, out of which 139 521 will require oxygen therapy and 89 043 will require ventilatory support (Cabore et al., 2020). A surge in COVID-19 cases will place enormous strain on the continent’s health systems, diminishing healthcare resources as already seen in other places (Emanuel et al., 2020), as well as funds and health worker time among others. The magnitude of this shock could cause the performance of health programmes to drastically decline.
To mitigate widespread transmission, African governments, like their counterparts in other parts of the world, have rolled out several COVID-19 control strategies (Nkengasong and Mankoula, 2020). Based on lessons learnt from countries that are at advanced stages of the epidemic (Lau et al., 2020, Prem et al., 2020), physical distancing is one of the key interventions that is being prioritized across the continent, in addition to strategies such as hand hygiene and the use of facemasks among others. The goal of physical distancing is to limit interaction between people, as the disease can be transmitted from person to person (Chen 2020). To enforce physical distancing, lockdowns are being imposed at the national or sub-national level (Hamzelou, 2020). In some countries, these lockdown orders have been accompanied by roadblocks to restrict the movement of people and vehicles. These can have unintended negative consequences on access to routine essential health services.
One of the routine health services that is being disrupted by COVID-19 is childhood immunization. According to the World Health Organization, an estimated 80 million children in 68 countries are at risk of developing vaccine-preventable diseases such as measles, diphtheria, and polio, because of the disruption of routine immunization services (World Health Organization, 2020a). This is not surprising, as the immunization system relies on functioning health facilities and stable communities to be effective. In fact, the WHO recommended the suspension of mass vaccination campaigns to prevent the worsening of community transmission of COVID-19 (World Health Organization, 2020b) and this could have an immediate effect on immunization coverage, especially in rural and underserved communities.
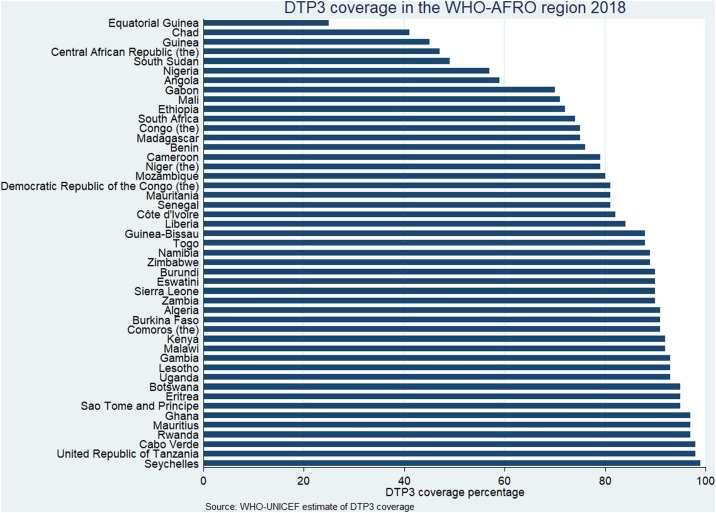
Africa is of particular concern because the performance of immunization programmes on the continent even in the pre-COVID-19 era was largely sub-optimal (World Health Organization, 2019b). In the WHO African region, coverage with the third dose of diphtheria–tetanus–pertussis-containing vaccine (DTP3) has stagnated at 76%, a level it attained in 2016 (World Health Organization, 2019a). According to WHO–UNICEF estimates of immunization coverage, the national DTP3 coverage level was less than 90% in 26 out of the 47 countries in the region in 2018 (World Health Organization, 2019c). The coverage level for these countries is shown in Figure 1 . Nigeria and South Africa are among the countries that are expected to have the highest numbers of COVID-19 cases, and the DTP3 coverage level in these countries was recently reported as 57% and 74%, respectively (World Health Organization, 2019c). Countries like Equatorial Guinea, Chad, and Guinea have DTP3 coverage as low as 25%, 41%, and 45%, respectively (World Health Organization, 2019c). Single-dose measles-containing vaccine (MCV1) coverage for the region is at 74%, and only eight countries have attained the recommended 95% MCV1 coverage level (World Health Organization, 2019b). At this rate, most countries are unlikely to meet the Global Vaccine Action Plan (GVAP) targets for DTP3 or measles elimination (World Health Organization, 2013). The impact of the COVID-19 outbreak could cause immunization performance to decline even further.
Figure 1.
DTP3 coverage for countries in the WHO-AFRO region.
Historical evidence from previous epidemics like the Ebola outbreak in West Africa, has shown that the indirect effect of such events exacerbates morbidity and mortality (Elston et al., 2017). This is because overall healthcare utilization declines (Wilhelm and Helleringer, 2019). Suk and colleagues (Suk et al., 2016) found that multiple epidemics of measles occurred in 2015 in Guinea because of the breakdown in public health systems, particularly immunization, on account of Ebola. If COVID-19 is allowed to trigger a similar breakdown of immunization systems, child mortality on the continent caused by vaccine-preventable diseases could increase significantly. The recently released World Health Statistics 2020 shows that African countries are already lagging behind in their progress towards achieving the child mortality targets for the sustainable development goals (World Health Organization, 2020d).
In a recent study, scientists showed that the benefit of sustaining routine immunization in Africa is greater than the risk of COVID-19 deaths that could result from visiting health services for immunization (CMMID nCov working group, 2020). This evidence underscores the value of immunization during COVID-19 and provides justifications for rapid action from governments and their partners. Hence, policymakers must quickly identify robust and context-specific strategies to rapidly scale up routine immunization in order to mitigate the impact of COVID-19 on their national immunization performance.
Typically, in public health, efforts to explore population-based problems often focus on specific areas without giving due recognition to the patterns that exist within systems (Carey et al., 2015). Nonetheless, the real world continues to function as complex adaptive systems (CAS) (Peters, 2014). For this reason, we propose an alternative viewpoint that departs from this reductionist philosophy to systems science in exploring the interaction between COVID-19 and immunization (Luke and Stamatakis, 2012). This will inform a more nuanced and holistic understanding of how they interrelate. So, instead of studying individual components of the immunization system (e.g., vaccine supply and logistics), a systems-based approach that conceptualizes all components of the system as interrelated entities should be adopted. Although complex, this approach will explicitly expose the non-linear and dynamic relationships that exist between all elements of the system (Luke and Stamatakis, 2012).
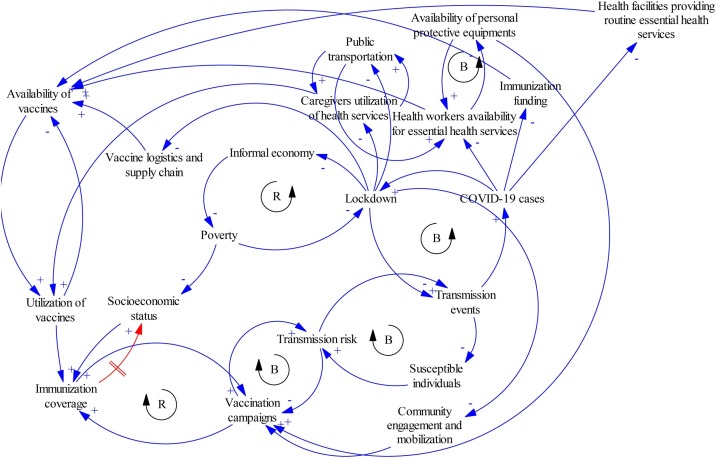
A range of methodologies for understanding complexity exist, one of which is systems dynamic modelling (Peters, 2014). This modelling technique can be used to analyse multiple, simultaneous, and complex interactions over time (Peters, 2014). A commonly used tool in systems dynamic modelling is the causal loop diagram (CLD) (Peters, 2014). It provides qualitative illustrations of the causal linkages between elements of a system, as well as the feedback loops (balancing and reinforcing) that exist between them (Peters, 2014).
As an example, the CLD in Figure 2 was constructed using Vensim PLE version 8.0.6 to illustrate the relationship between COVID-19, its control strategies, and immunization. The model revealed the linkage between COVID-19 cases and lockdown as a balancing loop. This means that an increase in the number of COVID-19 cases will prompt the government to impose lockdown as a containment strategy. This lockdown decreases transmission events, which will then cause the number of COVID-19 cases to decline. If the number of COVID-19 cases should surge, there will be a decrease in the number of health workers that are available to provide routine immunization services. This is because they would be redeployed to COVID-19-related tasks. Similarly, it will decrease available health facilities, as hospital and clinics will be converted to COVID-19 treatment and isolation centres. In addition, immunization funding will reduce, as funds will be diverted to the COVID-19 response. All of these will contribute to a decrease in the availability of immunization services.
Figure 2.
A causal loop diagram showing the relationship between COVID-19 and the immunization system (B = balancing loop; R = reinforcing loop).
Lockdown can directly affect immunization services by constraining access to vaccines. The presence of roadblocks dissuades caregivers from visiting health facilities for routine immunization. Vaccine logistics and the supply chain at the first and last mile are interrupted. Furthermore, public transportation services that facilitate the movement of caregivers and health workers to health facilities are stopped. An indirect effect of the lockdown on immunization is through its vicious cycle relationship with the economy. Its restrictive nature can be detrimental to vulnerable individuals whose source of livelihood depends on informal economic activities. The resultant poverty can further widen socioeconomic inequalities and this has been shown to affect immunization coverage (Ataguba et al., 2016).
Community mobilization activities would decrease with a resultant decrease in mass vaccination campaigns. This is because of the balancing loop that exists between vaccination campaigns and transmission risk. Conducting vaccination campaigns during the COVID-19 pandemic increases the contact rate in communities, which accentuates the transmission risk.
This simple CLD demonstrated that many elements are interacting with the immunization systems and that a change in one part of the system causes a cascade of changes in other parts. The advantage of presenting the structure of a system in this manner is that it explicitly depicts dynamic relationships. Using this to guide planning and the prioritization of areas for intervention will pave the way for system re-design and improvement.
Implementation science concepts can support immunization system re-design to adjust it to the pressures of COVID-19 by accelerating the uptake and utilization of multifaceted evidence-based strategies in policies and practice settings to improve system performance (Eccles and Mittman, 2006). For example, the risk of health worker infection is a challenge in the practice setting that could be minimized by using personal protective equipment (PPE). Tailored implementation strategies could be used to improve the adoption, implementation, and scale-up of this PPE in immunization clinics and other facility service delivery points (Powell et al., 2012). In some settings, rapid improvements in PPE use can even be engineered using quality improvement (McLaughlin and Kaluzny, 2004).
Implementation science can be used to improve strategy ‘fit’ by ensuring critical consideration of context in COVID-19-related policies (Moullin et al., 2019). This will change how policymakers and public health experts approach the outbreak vis-à-vis the health system. It could encourage the use of differentiated models for communities, population groups, and socioeconomic strata, among others. For example, existing structures like patent medicine vendors can be engaged to provide immunization services in slums and hard-to-reach areas when health facilities are overwhelmed (Adamu et al., 2020). A common constraint is the inability of health workers in some settings to reach health facilities because of disruption to the public transport system caused by lockdown. This is likely to be more prominent in underserved communities where alternative options might be limited. To address this, a special transportation scheme could be introduced to improve the mobility of health workers. To ease caregiver movement through roadblocks and promote adherence to the immunization schedule, the child home-based record could be regarded as a ‘pass’.
Implementation science also emphasizes the need to tailor information needs to the demand of different stakeholders. Community members are prone to misinformation about COVID-19. When coupled with policies like lockdown, they could potentially increase vaccine hesitancy among caregivers. To address this, appropriately tailored information about the novel disease, including recommended preventive strategies like use of a facemask when in public, could be communicated with community members. In addition, the importance of infant immunization could be re-emphasized to motivate caregivers to continue scheduled immunization visits.
In conclusion, integrating systems thinking and implementation science in health planning and decision-making could help African countries gain a better understanding of the influence of COVID-19 on health programmes, such as childhood immunization, and facilitate the implementation of multifaceted evidence-based strategies in complex practice settings. As Africa re-strategizes for the post-2020 era, these emerging fields could contribute significantly in accelerating progress towards universal access to vaccines for all children on the continent despite COVID-19.
Funding
No funding was received for this manuscript.
Ethical approval
Not applicable.
Author contributions
AAA conceptualized the manuscript and developed the first draft. AAA, RIJ, DH, and CSW contributed to writing, reviewing, and finalizing the manuscript. All authors approved the final draft.
Conflict of interest
None declared.
References
- Adamu Aa, Gadanya Ma, Jalo Ri, Uthman Oa, Nnaji Ca, Bello Iw. Assessing readiness to implement routine immunization among patent and proprietary medicine vendors in Kano, Nigeria: a theory-informed cross-sectional study. Expert Rev Vaccines [Internet] 2020;19(4):395–405. doi: 10.1080/14760584.2020.1750379. Apr 2 [cited 2020 May 28] Available from: [DOI] [PubMed] [Google Scholar]
- Ataguba J.E., Ojo K.O., Ichoku H.E. Explaining socio-economic inequalities in immunization coverage in Nigeria. Health Policy plan. 2016;31(9):1212–1224. doi: 10.1093/heapol/czw053. [DOI] [PubMed] [Google Scholar]
- Cabore J.W., Karamagi H.C., Kipruto H., Asamani J.A., Droti B., Seydi A.B.W. The potential effects of widespread community transmission of SARS-CoV-2 infection in the World Health Organization African Region: a predictive model. BMJ Global Health [Internet] 2020;5(5):e002647. doi: 10.1136/bmjgh-2020-002647. https://gh.bmj.com/content/5/5/e002647 May 1 [cited 2020 May 27] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey G., Malbon E., Carey N., Joyce A., Crammond B., Carey A. Systems science and systems thinking for public health: a systematic review of the field. BMJ Open. 2015;5(12) doi: 10.1136/bmjopen-2015-009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Pathogenicity and transmissibility of 2019-nCoV—A quick overview and comparison with other emerging viruses. Microbes Infect [Internet] 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. http://www.sciencedirect.com/science/article/pii/S1286457920300265 Mar 1 [cited 2020 May 27] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMMID nCov working group Benefit-risk analysis of health benefits of routine childhood immunisation against the excess risk of SARS-CoV-2 infections during the Covid-19 pandemic in Africa [Internet] Centre Math Model Infect Dis. 2020 doi: 10.1016/S2214-109X(20)30308-9. https://cmmid.github.io/topics/covid19/EPI-suspension.html [cited 2020 May 25]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles M.P., Mittman B.S. Springer; 2006. Welcome to implementation science. [Google Scholar]
- Elston J.W.T., Cartwright C., Ndumbi P., Wright J. The health impact of the 2014–15 Ebola outbreak. Public Health [Internet] 2017;143:60–70. doi: 10.1016/j.puhe.2016.10.020. http://www.sciencedirect.com/science/article/pii/S0033350616303225 Feb 1 [cited 2020 Jun 17] Available from: [DOI] [PubMed] [Google Scholar]
- Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A. Fair allocation of scarce medical resources in the time of Covid-19. Mass Med Soc. 2020 doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- Gilbert M., Pullano G., Pinotti F., Valdano E., Poletto C., Boëlle P.-Y.-Y. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet [Internet] 2020;395(10227):871–877. doi: 10.1016/S0140-6736(20)30411-6. http://www.sciencedirect.com/science/article/pii/S0140673620304116 2020 Mar 14 [cited 2020 Jun 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol [Internet] 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. https://www.nature.com/articles/s41564-020-0695-z 2020 Apr [cited 2020 Jun 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzelou J. World in lockdown. New Sci [Internet] 2020;245(3275):7. doi: 10.1016/S0262-4079(20)30611-4. http://www.sciencedirect.com/science/article/pii/S0262407920306114 2020Mar 28 [cited 2020 May 25] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med [Internet] 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. https://www.nature.com/articles/s41591-020-0869-5 2020May [cited 2020 Jun 17] Available from: [DOI] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents [Internet] 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. http://www.sciencedirect.com/science/article/pii/S0924857920300674 2020Mar 1 [cited 2020 Jun 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Schubert J., Bania J. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med. 2020;27(3) doi: 10.1093/jtm/taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol [Internet] 2020;92(4):401–402. doi: 10.1002/jmv.25678. [cited 2020 Jun 17]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke D.A., Stamatakis K.A. Systems science methods in public health: dynamics, networks, and agents. Annu Rev Pub Health. 2012;33:357–376. doi: 10.1146/annurev-publhealth-031210-101222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C.P., Kaluzny A.D. Continuous quality improvement in health care: theory, implementation, and applications. Jones Bartlett Learn. 2004 [Google Scholar]
- Moullin J.C., Dickson K.S., Stadnick N.A., Rabin B., Aarons G.A. Systematic review of the exploration, preparation, implementation, sustainment (EPIS) framework. Implementation Sci. 2019;14(1):1. doi: 10.1186/s13012-018-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J.N., Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet [Internet] 2020;395(10227):841–842. doi: 10.1016/S0140-6736(20)30464-5. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30464-5/abstract 2020Mar 14 [cited 2020 May 4] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D.H. The application of systems thinking in health: why use systems thinking? Health Res Policy Syst. 2014;12(1):51. doi: 10.1186/1478-4505-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell B.J., McMillen J.C., Proctor E.K., Carpenter C.R., Griffey R.T., Bunger A.C. A Compilation of Strategies for Implementing Clinical Innovations in Health and Mental Health. Med Care Res Rev [Internet] 2012;69(2):123–157. doi: 10.1177/1077558711430690. 2012Apr 1 [cited 2020 May 28] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem K., Liu Y., Russell T.W., Kucharski A.J., Eggo R.M., Davies N. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Pub Health [Internet] 2020;5(5):e261–270. doi: 10.1016/S2468-2667(20)30073-6. http://www.sciencedirect.com/science/article/pii/S2468266720300736 2020May 1 [cited 2020 May 27] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J.E., Jimenez A.P., Kourouma M., Derrough T., Baldé M., Honomou P. Post-Ebola Measles Outbreak in Lola, Guinea, January–June 20151. Emerg Infect Dis [Internet] 2016;22(6):1106–1108. doi: 10.3201/eid2206.151652. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4880080/ 2016Jun [cited 2020 May 27] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J.A., Helleringer S. Utilization of non-Ebola health care services during Ebola outbreaks: a systematic review and meta-analysis. J Glob Health [Internet] 2019;9(1) doi: 10.7189/jogh.09.010406. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6344071/ [cited 2020 Jun 17] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2013. Global vaccine action plan 2011-2020 [Internet]. World Health Organization.https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ [cited 2020 May 24]. Available from: [Google Scholar]
- World Health Organization 541 Meeting of the Strategic Advisory Group of Experts on Immunization, October 2019: conclusions and recommendations. Weekly Epidemiolo Record [Internet] 2019;94(47):541–560. http://www.who.int/wer/2019/wer9447/en/ 2019a Nov 22 [cited 2020 May 18] Available from: [Google Scholar]
- World Health Organization . World Health Organization; 2019. Global Vaccine Action Plan: 2019 regional reports on progress towards to the GVAP-RVAP goals [Internet]https://www.who.int/immunization/global_vaccine_action_plan/GVAP2019-RegionalReports-web.pdf?ua=1 [cited 2020 May 19]. Available from: [Google Scholar]
- World Health Organization . Time Series: DTP3 [Internet]; 2019. WHO UNICEF coverage estimates. Vaccine preventable diseases monitoring system 2019 Global Summary Reference.http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html [cited 2020 Jan 28]. Available from: [Google Scholar]
- World Health Organization . World Health Organization; 2020. Guiding principles for immunization activities during the COVID-19 pandemic [Internet]https://apps.who.int/iris/bitstream/handle/10665/331590/WHO-2019-nCoV-immunization_services-2020.1-eng.pdf [cited 2020 May 23]. Available from: [Google Scholar]
- World Health Organization . 2020. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV) [Internet]https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [cited 2020 May 3]. Available from: [Google Scholar]
- World Health Organization . 2020. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]https://covid19.who.int/ [cited 2020 May 27]. Available from: [Google Scholar]
- World Health Organization . 2020. World health statistics 2020: monitoring health for the SDGs, sustainable development goals [Internet]https://apps.who.int/iris/bitstream/handle/10665/332070/9789240005105-eng.pdf?ua=1 [cited 2020 May 18]. Available from: [Google Scholar]