Graphical abstract
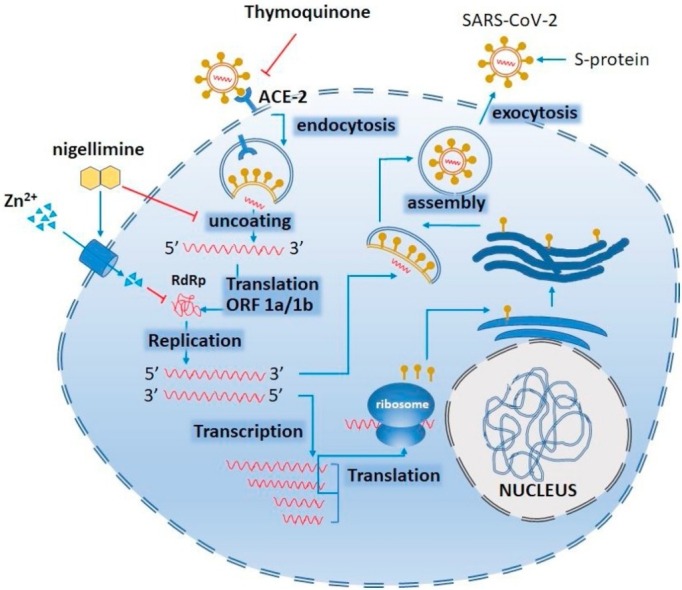
The SARS-CoV binds its spike (S) proteins to host angiotensin-converting enzyme 2 (ACE2). Following entry, the viral RNA is released in the cytoplasm - a potential site of thymoquinone to stop the release. ORF1a and ORF1ab are translated to RdRP which eventually synthesizes mediate both replication and transcription. The Zn2+ can stop viral replication by inactivating (―|) RdRP. Another bioactive component of black seed i.e., nigellimine might have dual actions working as ionophore (→) to enhance Zn entry into the infected cells as well as to inhibit (―|) the uncoating of the virus inside the infected cells. However, if allowed to continue, replication, full-length (−) RNA copies of the genome are produced and used as templates for full-length (+) RNA genomes. During transcription, sub-genomic RNAs are produced through discontinuous transcription which are eventually translated to synthesize viral proteins. Generally, viral nucleocapsids are assembled from genomic RNA and N protein in the cytoplasm, followed by budding into the lumen of the endoplasmic reticulum–Golgi intermediate compartment. Virions are then released from the infected cell through exocytosis. (→, activation or stimulation; ―|, inhibition) (modified from (Rahman and Idid, 2020).
Keywords: Chloroquine, SARS-CoV-2, RNA dependent RNA polymerase, Thymoquinone, Zinc transporter, Pneumocytes
Highlights
-
•
The COVID-19 has been declared a pandemic while there is no specific medicine against its causative agent SARS-CoV-2.
-
•
As an complementary medicine Nigella sativa (black seed) could be considered for its bioactive components such as thymoquinone which was proven to have anti-viral activity.
-
•
Further benefits to use N. sativa could be augmented by Zn supplement. Notably, Zn has been proven to improve innate and adaptive immunity in course of microbial infection.
-
•
The effectiveness of the Zn salt supplement can be enhanced with N. sativa as its major bioactive component might work as ionophore to allow Zn2+ to enter pneumocytes and inhibit SARS-CoV-2 replication by stopping its replicase enzyme system.
Abstract
An effective vaccine to prevent the SARS-CoV-2 causing COVID-19 is yet to be approved. Further there is no drug that is specific to treat COVID-19. A number of antiviral drugs such as Ribavirin, Remdesivir, Lopinavir/ritonavir, Azithromycin and Doxycycline have been recommended or are being used to treat COVID-19 patients. In addition to these drugs, rationale and evidence have been presented to use chloroquine to treat COVID-19, arguably with certain precautions and criticism. In line with the proposed use of chloroquine, Nigella sativa (black seed) could be considered as a natural substitute that contains a number of bioactive components such as thymoquinone, dithymoquinone, thymohydroquinone, and nigellimine. Further benefits to use N. sativa could be augmented by Zn supplement. Notably, Zn has been proven to improve innate and adaptive immunity in the course of any infection, be it by pathogenic virus or bacteria. The effectiveness of the Zn salt supplement could also be enhanced with N. sativa as its major bioactive component might work as ionophore to allow Zn2+ to enter pneumocytes – the target cell for SARSCoV-2. Given those benefits, this review paper describes how N. sativa in combination with Zn could be useful as a complement to COVID-19 treatment.
1. Introduction
Coronaviruses (CoVs) are so called because of their crown-like appearances under an electron microscope. In the last few decades, two major groups of CoVs namely severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) have caused epidemics with high mortality (Hui et al., 2014). Another member of the coronaviridae family - SARS-CoV-2 is responsible for the ongoing pandemic Coronavirus Disease 2019 (COVID-19). On March 11, 2020, World Health Organization (WHO) declared the COVID-19 outbreak as a pandemic.
The SARS-CoV-2 primarily infects cells of the small air sacs known as alveoli, consisting of alveolar cells (also known as pneumocytes) and alveolar macrophages. Infection by the SARS-CoV-2 augments inflammatory conditions in the lungs, causing pneumonia with symptoms like dry cough, chest pain, fever, and difficulty in breathing (Huang et al., 2020; Lescure et al., 2020). The pneumonic condition in COVID-19 is severe and is associated with its high mortality rate (Mallapaty, 2020).
The critical challenges to manage the current COVID-19 pandemic are due to a lack of a preventive vaccine as well as an effective drug against the SARS-CoV-2. Furthermore, there is an unprecedented rate of spread of the virus and mortality on a global scale. Researchers and clinicians around the world are competing to find an effective treatment for COVID-19. The current opinion paper will highlight the potential of using Nigella sativa (commonly known as black seed) and Zn salt as a supplement to treat COVID-19 patients.
2. The SARS-COV-2 Virus and its replication
The SARS-CoV-2 is one of the seven types of coronavirus that are known to infect humans (Zhu et al., 2020). There are four genera of coronaviruses namely α-CoV, β-CoV, γ-CoV, and δ-CoV (Su et al., 2016) and the SARS-CoV-2 belongs to β-CoV (Zhu et al., 2020). The SARS-CoV-2 is an enveloped virus with a single strand, positive-sense RNA genome (+RNA) (Forni et al., 2017). Along with 16 non-structural proteins and four major structural proteins namely spike (S), envelope (E), membrane (M), and nucleocapsid (N), SARS-CoV-2 contains eight accessory proteins (Wu et al., 2020).
The SARS-CoV2 enters human pneumocytes through a process called endocytosis using its spike glycoproteins (S-glycoprotein) which then bind angiotensin-converting enzyme 2 (ACE2) that are expressed on pneumocytes (Hamming et al., 2004; Li et al., 2003; Ou et al., 2020). Notably, ACE2 is widely expressed on the epithelial cells of alveoli, trachea, bronchi, bronchial serous glands (Liu et al., 2011), and alveolar monocytes and macrophages (Kuba et al., 2005). The fusion of the viral E proteins and endosomal phospholipidic membrane allows the release of the viral + RNA into the host cell cytoplasm.
Central to its replication, SARS-CoV-2 uses its own genome-encoded RNA-dependent RNA polymerase (RdRp). The RdRp which is integrated into a membrane-associated viral enzyme complex synthesizes the negative-strand RNA (−RNA) using the + RNA as the template. The negative RNA strand is then used as a template to synthesize viral mRNAs.
The polycistronic ribosome machinery of the infected cell synthesizes non-structural proteins (NSPs) of the SARS-COV-2 and assemble into the replicase-transcriptase complex. Following replication, the envelope proteins are translated and inserted into the endoplasmic reticulum of the host cells to finally enter into the Golgi compartment. Consequently, the viral genomic RNA is packaged into the nucleocapsid and then envelope proteins are incorporated during the budding step to form mature virions For detail please see review articles (Devaux et al., 2020; Fehr and Perlman, 2015).
3. Immunological profile of COVID-19 patients
Changes in the immunological profile of COVID-19 patients have more or less similar patterns. This may however, vary depending on the demographic and other clinical conditions of the patients. Generally, a decreased count of CD4+ and CD8+ lymphocytes, monocytes and platelets with increased count of neutrophils were recorded (Wang et al., 2020a). Furthermore, a high level of lymphocytes was found as a predictive better outcome (OR = 0.10, P < 0.001) for the patients who recovered compared with those who died (Chen et al., 2020b; Wang et al., 2020a). It is important to note that the number of CD4+ and CD8+ T cells are critical in antiviral immunity (Jansen et al., 2019; Whitmire and Ahmed, 2000). In other words, the CD4+ and CD8+ T cell responses to a viral infection require distinct costimulatory pathways for activation that in turn play an important role in determining the number of effector T cells that survive to become memory T cells (Whitmire and Ahmed, 2000). Again, specific T cell responses against influenza virus were attributed to the protective immunity provided by the cells by limiting duration and severity of the disease (Jansen et al., 2019).
An increased concentration of C-reactive protein, interleukin-6 (IL-6), Serum ferritin, and erythrocyte sedimentation rate were also recorded in COVID-19 patients (Chen et al., 2020b). Similar phenomena were observed in cytokine storms, with an overproduction of IL-7, IL-10, GCSF, IP10, MCP1, MIP1A, and TNF-α (Huang et al., 2020; Lescure et al., 2020; Wang et al., 2020c).
4. Distribution, exchange, and requirement of Zn
In humans, virtually all Zn is present in intracellular compartments. Zn distribution in different organelles was estimated as follows: nucleus (30–40 %), cytosol and other organelles and specialized vesicles (50 %), and the rest is bound with cell membrane proteins (Vallee and Falchuk, 1993). In humans, plasma Zn level ranges between 10–18 mol/L representing 0.1 % of total body Zn (Foster and Samman, 2012). Earlier it was reported that the in vitro Zn requirement for typical fibroblast-like cells is about 0.25 fmol per cell or 200 μM in ordinary culture media. However, in vitro growth of the cells stops at cellular Zn levels below ∼0.2 fmol per cell (Palmiter and Findley, 1995).
Above the level that is required for growth and survival requirement however, free Zn ions (Zn2+) can be toxic to the cells by inhibiting cytoplasmic enzymes such as adenylate cyclase (Klein et al., 2004). Therefore, intracellular homeostasis of Zn as well as exchange of Zn in and out of the cells is critical and is controlled by two Zn transporter protein families namely, SLC39A (Zn importer protein i.e., ZIP and ZRT/IRT-related protein, 14 ZIP) and SLC30A (Zn Transporter i.e., ZnT, 10 ZnTs) (Cousins et al., 2006; Lichten and Cousins, 2009). At the same time, metallothioneins – a cysteine rich of low molecular weight group of proteins act as a reservoir of the intracellular free Zn2+ (Chasapis et al., 2012; Lynes et al., 2006; Stefanidou et al., 2006). ZnTs transport Zn2+ out of the cytosol and ZIPs import them from cellular compartments into the cytosol (Cousins et al., 2006, 1986). Intracellular compartments, such as endosomes, Golgi, or endoplasmic reticulum mostly express ZnTs (Palmiter and Findley, 1995). However, ZIPs are expressed on plasma membrane (Zip7 is located at the Golgi apparatus) (Huang et al., 2005).
5. Zinc boosts immune responses during viral infection
Zn is involved in a number of immunome activation pathways such as NF-κB signalling pathway which influences the expression of cytokines (such as IL-1b, IL-6, IL-8, TNF-α, and MCP-1), chemokines, acute phase proteins (CRP and fibrinogen), matrix metalloproteinases, adhesion molecules, growth factors, and other factors involved in the inflammatory response, such as COX-2 and iNOS (Hayden and Ghosh, 2014; Lawrence, 2009). In vitro Zn addition was shown to stimulate autophagy in human hepatoma cells VL-17A. However, Zn depletion can cause a significant suppression of autophagy (Liuzzi and Yoo, 2013). Free intracellular Zn2+ is essential in extravasation of neutrophils to the site of the infection to uptake and kill the infectious microorganisms (Hasan et al., 2016). For more detailed functions of Zn in immunity, please see the review articles (Bonaventura et al., 2015; Rahman and Karim, 2018; Shankar and Prasad, 1998).
It is important to note that autophagy plays an important protective role as host defence mechanism (Jiang and Mizushima, 2014; Levine and Kroemer, 2019; Meijer and Codogno, 2009; Mizushima and Levine, 2010). In autophagy, intracellular components such as protein aggregates and damaged organelles are engulfed into a double-membrane structure called an autophagosome and fused with a lysosome (Levine and Kroemer, 2019; Mizushima, 2018). A lysosome contains more than 50 enzymes, including proteases, peptidases, phosphatases, nucleases, glycosidases, sulfatases, and lipases (Lubke et al., 2009). Essentially, structural and functional integrity of many of these enzymes depends on Zn (Andreini et al., 2008).
In vitro Zn administration was also shown to induce a subset of T cells (CD4+CD25+Foxp3+ and CD4+CD25+CTLA-4+) which are important in eliciting immune response in the state of infections (Maywald and Rink, 2017; Rosenkranz et al., 2016).
In addition to these host immune responses that are directly or indirectly triggered by the Zn, the same element also acts directly on the infectious pathogens more specifically for a number of viruses. In vitro studies suggested that intracellular Zn2+ inhibit the replicative cycle of a number of viruses including influenza virus (Uchide et al., 2002), respiratory syncytial virus (Suara and Crowe, 2004), and several picornaviruses (Korant et al., 1974; Krenn et al., 2009; Lanke et al., 2007). More particularly, Zn2+ was shown to inhibit polyprotein processing in cells infected with human rhinovirus and coxsackievirus B3 (Krenn et al., 2009). Replication of other viruses such as HIV, HSV, and vaccinia virus as well as SARS-CoVs were also known to be inhibited by Zn salts. In those viruses, Zn is known to inhibit the viral entry, blocking of polyprotein processing, or inhibition of viral RdRp activity (Haraguchi et al., 1999; Katz and Margalith, 1981; Kaushik et al., 2017; te Velthuis et al., 2010).
6. Hydroxy/chloroquine: among the suggested treatments for COVID-19
Lack of specific drugs for COVID-19 compels clinicians to depend on an array of treatment strategies which have been used to treat other viral infections such as: (i) convalescent plasma (Casadevall and Pirofski, 2020; Chen et al., 2020a; Wong and Lee, 2020), (ii) Ribavirin a nucleoside analogue that inhibits MERS-CoV replication (Falzarano et al., 2013), (iii) Lopinavir/Ritonavir - a combination of protease inhibitors that are used to treat HIV infection, (iv) Remdesivir, a nucleotide analogue that inhibits RNA polymerase of human and zoonotic coronavirus (Gordon et al., 2020; Wang et al., 2020b), (v) Favipiravir, known to inhibit RNA polymerase of pathogenic virus (Furuta et al., 2009), (v) azithromycin and doxycycline - commonly used antibiotics to inhibit viral replication and IL-6 production (Sargiacomo et al., 2020), (vi) drugs that suppress IL-1 or IL-1R (Conti et al., 2020).
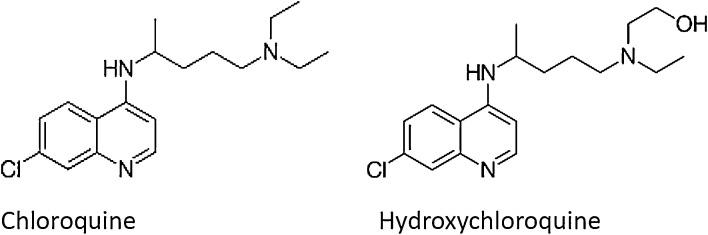
In addition to these treatment strategies, chloroquine and hydroxychloroquine (Fig. 1 ) have been recommended as potential candidates to treat COVID-19 (Touret and de Lamballerie, 2020). In a systematic review, Cortegiani et al. (Cortegiani et al., 2020) argued that “there is sufficient pre-clinical rationale and evidence regarding the effectiveness of chloroquine for treatment of COVID-19 as well as evidence of safety from long-time use in clinical practice for other indications.” Use of chloroquine to treat COVID-19 patients in China also showed clinical and virologic benefit (Gao et al., 2020). Chloroquine administered transplacentally or via maternal milk was shown to successfully treat lethal HCoV-OC43 infection in newborn C57BL/6 mice (Keyaerts et al., 2009). Chloroquine at a reasonably low concentration (EC90 = 6.90 μM) was shown to inhibit in vitro replication of SARS-CoV-2 in Vero E6 cells. Note that this concentration can be easily achievable in cells with standard oral dosing (Wang et al., 2020b). However, hydroxychloroquine having relatively higher potency against SARS-CoV-2 (Colson et al., 2020; Wang et al., 2020b; Yao et al., 2020). By analysing the potential benefits, based on a systematic review, both chloroquine and hydroxychloroquine were suggested to treat COVID-19 patients with or without diabetes (Singh et al., 2020).
Fig. 1.
Structure of chloroquine and hydroxychloroquine.
Chloroquine was further hypothesized to interfere with SARS-CoV-2′s abiity to bind to ACE2 receptor and prevents its entry into pneumocytes as it might inhibit sialic acids biosynthesis to limit cell surface binding of SARS-CoV-2. Among the other possible mode of protection, chloroquine has been hypothesized to modulate the acidification of endosomes thereby inhibiting formation of the autophagosome. Through reduction of cellular mitogen-activated protein (MAP) kinase activation, chloroquine may also inhibit virus replication. Moreover, chloroquine could alter M protein maturation and interfere with virion assembly and budding (Devaux et al., 2020).
7. Black seed from Nigella sativa: a natural alternative to Chloroquine
Black seed from an annual flowering plant Nigella sativa of Ranunculaceae family has been reported for its range of medicinal applications. The use of black seeds and its oil has been recommended for rheumatoid arthritis, asthma, inflammatory diseases, diabetes and digestive diseases (Ahmad et al., 2013; Butt and Sultan, 2010; Ijaz et al., 2017; Kooti et al., 2016; Padhye et al., 2008).
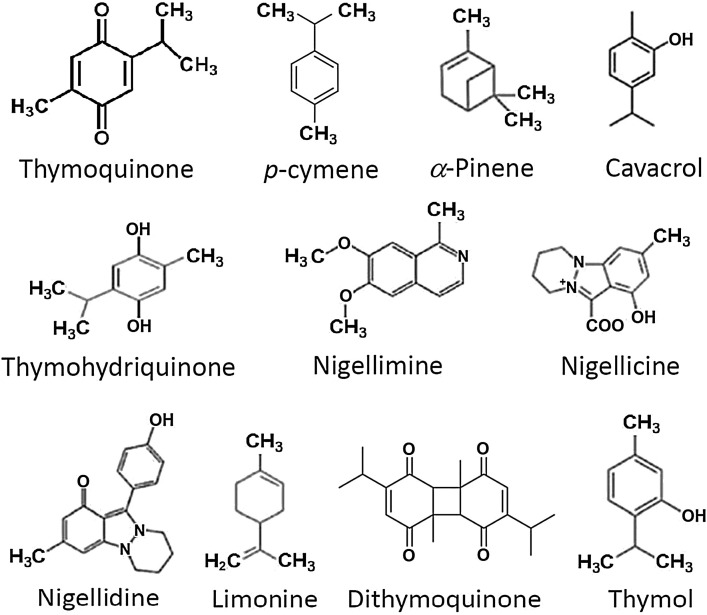
N. sativa seeds contain unsaturated fatty acids (26 %–38 %), proteins, alkaloids, saponins (melanin), and essential oil (0.4 %–2.5 %). A GC–MS analysis has revealed a mixture of eight fatty acids and 32 volatile terpenes in the seed extract (Nickavar et al., 2003). Thymoquinone, dithymoquinone (nigellone), thymohydroquinone, and thymol are considered the main active constituents. Thymoquinone is the major component (28 %–57 %) of the volatile essential oil (Kiralan, 2012; Liu et al., 2012). The major alkaloids that have been isolated from N. sativa seeds are nigellicine, nigellidine (indazoles), nigellimine and nigellimine N-oxide (isoquinolines) (Fig. 2 ). Other constituents include palmitic, glutamic, ascorbic, and stearic acids; arginine; methionine; lysine; glycine; leucine; and phytosterols (Avula et al., 2010). It can be noted that a number of bioactive components such as nigellimine share structural similarities with chloroquine and hydroxychloroquine.
Fig. 2.
Major bioactive terpenes of black seed oil extract.
Among the active medicinally bioactive constituents from N. sativa, thymoquinone has been given more emphasis (Gholamnezhad et al., 2016; Houghton et al., 1995). For example, N. sativa oil and thymoquinone were found to produce antinociceptive effects through indirect activation of the supraspinal μ1- and κ-opioid receptor subtypes (Abdel-Fattah et al., 2000). Furthermore, brain endogenous angiotensin II was suggested to be involved in central nociceptive mechanisms by its antagonistic interaction with the endogenous opioid system (Takai et al., 1996). In addition, opioid active peptides such as hemorphins were shown to have inhibitory effect on ACE (Lantz et al., 1991). These lines of evidence suggest that opioid receptors and ACE share similar inhibitory molecules. Hence it is not impossible that thymoquinone might also block ACE2. In other words, thymoquinone may block the SARS-CoV-2 entry via ACE2 in pneumocytes.
Therefore, both nigellimine and thymoquinone from N. sativa might be considered as potential medicinally bioactive components to treat COVID-19 patients.
8. Why a combination of Zn and black seed could be a natural alternative for COVID-19 treatment
It has been discussed in the preceding section that Zn is involved in boosting the immune response against viral infection including SARS-CoV-2. Immune-boosting activities of Zn include proliferation and activation of neutrophils, NK cells, macrophages, and T and B cells as well as cytokine production by the immune cells. Zinc also mediates protection from the adverse effect of ROS that are generally produced during inflammatory processes. In addition, Zn2+ was shown to stop recombinant SARS-CoV RdRp activity by inhibiting elongation and template binding (te Velthuis et al., 2010). Earlier it was also shown that Zn2+ inhibits the proteolytic processing of replicase polyproteins (Denison et al., 1992; Denison and Perlman, 1986).
Therefore, cellular availability of Zn2+ access to SARS-CoV-2 infected pneumocytes is crucial to fight back the viral pathogenesis. However, oral supplement of Zn alone may not make sufficient availability of Zn in pneumocytes. Earlier it was proven that chloroquine can enhance the uptake of Zn by lysosomes - a cellular organelle important for SARS-CoV-2 replication (Xue et al., 2014). In an in vitro condition, A2780 cells treated with 100–300 μM chloroquine showed increased uptake of ZnCl2 by doubling intracellular Zn levels in a dose dependent manner (Xue et al., 2014). In other words, chloroquine can act as ionophore for Zn to enter in pneumocytes. Given the similar chemical structure of a number of terpenes present in black seed such as nigellimine, they might provide similar ionophore functions to aid Zn entry to pneumocytes. While the other component, thymoquinone, might inhibit the binding of the virus with ACE2 on the pneumocytes.
9. Conclusion
Having a range of bioactive components such as thymoquinone and nigellimine, black seed might offer a number of benefits to treat COVID-19 such as (i) blocking the entry of the virus into pneumocytes and (ii) providing ionophore for enhanced uptake of Zn2+ which in turn can enhance host immune response against SARS-CoV-2 as well as inhibit its replication by blocking the viral RdRp. However, it is important to identify the right doses for both black seed or its derivatives such as oil, as well as for Zn. It can be noted that black seed oil has been used at doses of between 40−80 mg/kg/day as adjunctive therapy without any side effects. On the other hand, Zn intake above its recommended daily allowance (RDA) might be harmful which varies according to age, sex and other health conditions. For example, the RDA varies for children 1–8 years old (3−5 mg), males 9–13 years (8 mg), males > 14 years (11 mg), females > 18 years (8 mg), and females 14–18 years (9 mg). Individuals with health conditions such as with liver and kidney diseases as well as pregnant women must consult the physicians before deciding to take any self-prescribed Zn supplement.
Declaration of Competing Interest
The author declare that he has no competing interests.
References
- Abdel-Fattah A.M., Matsumoto K., Watanabe H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur. J. Pharmacol. 2000;400:89–97. doi: 10.1016/s0014-2999(00)00340-x. [DOI] [PubMed] [Google Scholar]
- Ahmad A., Husain A., Mujeeb M., Khan S.A., Najmi A.K., Siddique N.A., Damanhouri Z.A., Anwar F. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C., Bertini I., Cavallaro G., Holliday G.L., Thornton J.M. Metal ions in biological catalysis: from enzyme databases to general principles. J. Biol. Inorg. Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- Avula B., Wang Y.-H., Ali Z., Khan I.A. Quantitative determination of chemical constituents from seeds of Nigella sativa L. using HPLC-UV and identification by LC-ESI-TOF. J. AOAC Int. 2010;93:1778–1787. [PubMed] [Google Scholar]
- Bonaventura P., Benedetti G., Albarede F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Butt M.S., Sultan M.T. Nigella sativa: reduces the risk of various maladies. Crit. Rev. Food Sci. Nutr. 2010;50:654–665. doi: 10.1080/10408390902768797. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Pirofski L.-A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasapis C.T., Loutsidou A.C., Spiliopoulou C.A., Stefanidou M.E. Zinc and human health: an update. Arch. Toxicol. 2012;86:521–534. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Gallenga C.E., Tete G., Caraffa A., Ronconi G., Younes A., Toniato E., Ross R., Kritas S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020 doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R.J., Dunn M.A., Leinart A.S., Yedinak K.C., DiSilvestro R.A. Coordinate regulation of zinc metabolism and metallothionein gene expression in rats. Am. J. Physiol. 1986;251:E688–94. doi: 10.1152/ajpendo.1986.251.6.E688. [DOI] [PubMed] [Google Scholar]
- Cousins R.J., Liuzzi J.P., Lichten L.A. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 2006;281:24085–24089. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- Denison M.R., Perlman S. Translation and processing of mouse hepatitis virus virion RNA in a cell-free system. J. Virol. 1986;60:12–18. doi: 10.1128/jvi.60.1.12-18.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M.R., Zoltick P.W., Hughes S.A., Giangreco B., Olson A.L., Perlman S., Leibowitz J.L., Weiss S.R. Intracellular processing of the N-terminal ORF 1a proteins of the coronavirus MHV-A59 requires multiple proteolytic events. Virology. 1992;189:274–284. doi: 10.1016/0042-6822(92)90703-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M., Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4:676–694. doi: 10.3390/nu4070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L., Gowen B.B., Julander J.G., Morrey J.D. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gholamnezhad Z., Havakhah S., Boskabady M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: a review. J. Ethnopharmacol. 2016;190:372–386. doi: 10.1016/j.jep.2016.06.061. [DOI] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020 doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y., Sakurai H., Hussain S., Anner B.M., Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43:123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Hasan R., Rink L., Haase H. Chelation of free Zn(2)(+) impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol. Trace Elem. Res. 2016;171:79–88. doi: 10.1007/s12011-015-0515-0. [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014;26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton P.J., Zarka R., de las Heras B., Hoult J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- Huang L., Kirschke C.P., Zhang Y., Yu Y.Y. The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Memish Z.A., Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr. Opin. Pulm. Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- Ijaz H., Tulain U.R., Qureshi J., Danish Z., Musayab S., Akhtar M.F., Saleem A., Khan K.K., Zaman M., Waheed I., Khan I., Abdel-Daim M. Review: nigella sativa (Prophetic medicine): a review. Pak. J. Pharm. Sci. 2017;30:229–234. [PubMed] [Google Scholar]
- Jansen J.M., Gerlach T., Elbahesh H., Rimmelzwaan G.F., Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J. Clin. Virol. 2019;119:44–52. doi: 10.1016/j.jcv.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Jiang P., Mizushima N. Autophagy and human diseases. Cell Res. 2014;24:69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Margalith E. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob. Agents Chemother. 1981;19:213–217. doi: 10.1128/aac.19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N., Subramani C., Anang S., Muthumohan R., Shalimar, Nayak B., Ranjith-Kumar C.T., Surjit M. Zinc salts block hepatitis e virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol. 2017:91. doi: 10.1128/JVI.00754-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts E., Li S., Vijgen L., Rysman E., Verbeeck J., Van Ranst M., Maes P. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiralan M. Volatile compounds of black cumin seeds (Nigella sativa L.) from microwave-heating and conventional roasting. J. Food Sci. 2012;77:C481–4. doi: 10.1111/j.1750-3841.2012.02638.x. [DOI] [PubMed] [Google Scholar]
- Klein C., Heyduk T., Sunahara R.K. Zinc inhibition of adenylyl cyclase correlates with conformational changes in the enzyme. Cell. Signal. 2004;16:1177–1185. doi: 10.1016/j.cellsig.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kooti W., Hasanzadeh-Noohi Z., Sharafi-Ahvazi N., Asadi-Samani M., Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin. J. Nat. Med. 2016;14:732–745. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- Korant B.D., Kauer J.C., Butterworth B.E. Zinc ions inhibit replication of rhinoviruses. Nature. 1974;248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- Krenn B.M., Gaudernak E., Holzer B., Lanke K., Van Kuppeveld F.J.M., Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J. Virol. 2009;83:58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanke K., Krenn B.M., Melchers W.J.G., Seipelt J., van Kuppeveld F.J.M. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J. Gen. Virol. 2007;88:1206–1217. doi: 10.1099/vir.0.82634-0. [DOI] [PubMed] [Google Scholar]
- Lantz I., Glamsta E.L., Talback L., Nyberg F. Hemorphins derived from hemoglobin have an inhibitory action on angiotensin converting enzyme activity. FEBS Lett. 1991;287:39–41. doi: 10.1016/0014-5793(91)80011-q. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., Gaymard A., Bouscambert-Duchamp M., Donati F., Le Hingrat Q., Enouf V., Houhou-Fidouh N., Valette M., Mailles A., Lucet J.-C., Mentre F., Duval X., Descamps D., Malvy D., Timsit J.-F., Lina B., van-der-Werf S., Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten L.A., Cousins R.J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H., Jiang H., Zhou J., Lam P., Zhang L., Lackner A., Qin C., Chen Z. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011;85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., El-Aty Abd, A.M Cho, S.-K Yang, A Park, J.-H Shim, J.-H Characterization of secondary volatile profiles in Nigella sativa seeds from two different origins using accelerated solvent extraction and gas chromatography-mass spectrometry. Biomed. Chromatogr. 2012;26:1157–1162. doi: 10.1002/bmc.2671. [DOI] [PubMed] [Google Scholar]
- Liuzzi J.P., Yoo C. Role of zinc in the regulation of autophagy during ethanol exposure in human hepatoma cells. Biol. Trace Elem. Res. 2013;156:350–356. doi: 10.1007/s12011-013-9816-3. [DOI] [PubMed] [Google Scholar]
- Lubke T., Lobel P., Sleat D.E. Proteomics of the lysosome. Biochim. Biophys. Acta. 2009;1793:625–635. doi: 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes M.A., Zaffuto K., Unfricht D.W., Marusov G., Samson J.S., Yin X. The physiological roles of extracellular metallothionein. Exp. Biol. Med. 2006;231:1548–1554. doi: 10.1177/153537020623100915. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. Why does the coronavirus spread so easily between people? Nature. 2020 doi: 10.1038/d41586-020-00660-x. [DOI] [PubMed] [Google Scholar]
- Maywald M., Rink L. Zinc supplementation induces CD4(+)CD25(+)Foxp3(+) antigen-specific regulatory T cells and suppresses IFN-gamma production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. Eur. J. Nutr. 2017;56:1859–1869. doi: 10.1007/s00394-016-1228-7. [DOI] [PubMed] [Google Scholar]
- Meijer A.J., Codogno P. Autophagy: regulation and role in disease. Crit. Rev. Clin. Lab. Sci. 2009;46:210–240. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018;20:521–527. doi: 10.1038/s41556-018-0092-5. [DOI] [PubMed] [Google Scholar]
- Mizushima N., Levine B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickavar B., Mojab F., Javidnia K., Amoli M.A.R. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z. Naturforsch. C. 2003;58:629–631. doi: 10.1515/znc-2003-9-1004. [DOI] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhye S., Banerjee S., Ahmad A., Mohammad R., Sarkar F.H. From here to eternity - the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- Palmiter R.D., Findley S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639–649. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.T., Idid S.Z. Can Zn be a critical element in COVID-19 treatment? Biol. Trace Elem. Res. 2020 doi: 10.1007/s12011-020-02194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.T., Karim M.M. Metallothionein: a potential link in the regulation of zinc in nutritional immunity. Biol. Trace Elem. Res. 2018;182:1–13. doi: 10.1007/s12011-017-1061-8. [DOI] [PubMed] [Google Scholar]
- Rosenkranz E., Metz C.H.D., Maywald M., Hilgers R.-D., Wessels I., Senff T., Haase H., Jager M., Ott M., Aspinall R., Plumakers B., Rink L. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol. Nutr. Food Res. 2016;60:661–671. doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- Sargiacomo C., Sotgia F., Lisanti M.P. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany. NY) 2020 doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidou M., Maravelias C., Dona A., Spiliopoulou C. Zinc: a multipurpose trace element. Arch. Toxicol. 2006;80:1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suara R.O., Crowe J.E.J. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004;48:783–790. doi: 10.1128/aac.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Song K., Tanaka T., Okunishi H., Miyazaki M. Antinociceptive effects of angiotensin-converting enzyme inhibitors and an angiotensin II receptor antagonist in mice. Life Sci. 1996;59:PL331–336. doi: 10.1016/0024-3205(96)00527-9. [DOI] [PubMed] [Google Scholar]
- te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchide N., Ohyama K., Bessho T., Yuan B., Yamakawa T. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002;56:207–217. doi: 10.1016/s0166-3542(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire J.K., Ahmed R. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr. Opin. Immunol. 2000;12:448–455. doi: 10.1016/s0952-7915(00)00119-9. [DOI] [PubMed] [Google Scholar]
- Wong H.K., Lee C.K. Pivotal role of convalescent plasma in managing emerging infectious diseases. Vox Sang. 2020 doi: 10.1111/vox.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.-Q. Chloroquine is a zinc ionophore. PLoS One. 2014;9:e109180. doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]