Abstract
Background
Immunomodulatory therapy (IMT) is often considered for systemic treatment of non-infectious uveitis (NIU). During the evolving coronavirus disease-2019 (COVID-19) pandemic, given the concerns related to IMT and the increased risk of infections, an urgent need for guidance on the management of IMT in patients with uveitis has emerged.
Methods
A cross-sectional survey of international uveitis experts was conducted. An expert steering committee identified clinical questions on the use of IMT in patients with NIU during the COVID-19 pandemic. Using an interactive online questionnaire, guided by background experience and knowledge, 139 global uveitis experts generated consensus statements for IMT. In total, 216 statements were developed around when to initiate, continue, decrease and stop systemic and local corticosteroids, conventional immunosuppressive agents and biologics in patients with NIU. Thirty-one additional questions were added, related to general recommendations, including the use of non-steroidal anti-inflammatory drugs (NSAIDs) and hydroxychloroquine.
Results
Highest consensus was achieved for not initiating IMT in patients who have suspected or confirmed COVID-19, and for using local over systemic corticosteroid therapy in patients who are at high-risk and very high-risk for severe or fatal COVID-19. While there was a consensus in starting or initiating NSAIDs for the treatment of scleritis in healthy patients, there was no consensus in starting hydroxychloroquine in any risk groups.
Conclusion
Consensus guidelines were proposed based on global expert opinion and practical experience to bridge the gap between clinical needs and the absence of medical evidence, to guide the treatment of patients with NIU during the COVID-19 pandemic.
Keywords: Choroid, Imaging, Inflammation, Telemedicine, Conjunctiva, Immunology, Macula, Retina, Infection, Iris, Treatment medical, Epidemiology, Cornea, Vitreous, Treatment lasers, Ciliary body, Drugs
INTRODUCTION
Non-infectious uveitis (NIU)is a group of sight-threatening inflammatory diseases, sometimes associated with systemic inflammatory disorders. Immunomodulatory therapy (IMT) is often considered for systemic treatment of NIU, and commonly selected drugs include corticosteroids (topical, peribulbar, intravitreal, oral and intravenous), with conventional immunosuppressive agents or biologics used when long-term treatment is required and a corticosteroid-sparing approach is necessary.1 2 It is well known that one of the most important concerns related to IMT is the increased risk of infections, as these drugs act by limiting the patient’s immune responses.3
On 11 March 2020, the WHO declared coronavirus disease-2019 (COVID-19) as a pandemic.4 While all persons are at risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), patients who are receiving IMT may be at additional risk of infection with the virus and/or a more severe course of, or even fatality from, COVID-19. Therefore, during the pandemic, there is an urgent need for guidance on the management of IMT in the patient population with uveitis. However, there is virtually no literature on this topic. At present, there is lack of robust data as to whether patients with systemic inflammatory diseases or those using IMT who contract COVID-19 are at increased risk of infection or worse outcomes, with few reports of COVID-19 in patients with rheumatic diseases.5– 8 However, extrapolation of this data to SARS-CoV-2 infection and also ocular inflammatory disease is a dilemma.
A survey-based clinical study was hence designed to synthesise the opinions of the international uveitis specialist community on the approach to the management of IMT in patients with uveitis during the COVID-19 pandemic. This study was supported by the International Uveitis Study Group (IUSG), the International Ocular Inflammation Society (IOIS), the Uveitis Society of India (USI) and the Foster Ocular Immunology Society (FOIS).
METHODS
A total of 216 statements related to the use of IMT in patients with NIU during the current COVID-19 pandemic were written with the input of an expert steering committee (online appendix 1). In addition, 31 questions related to general recommendations for managing patients with inflammatory eye disease at the present time were also generated. These 216 items and 31 questions were formatted into an interactive web-based survey (Cognito Form, Columbia, South Carolina, USA) and circulated to the members of IUSG, IOIS, USI and FOIS. Consensus for the management of IMT was achieved if more than 75% of the experts agreed on a proposed statement, and consensus for general recommendations was achieved if more than 75% of the experts gave the same answer to a proposed question. Ethics approval to conduct the survey based on experts’ opinions with no patient data was obtained (identifier, NK/6128/Study/499). The study was conducted according to the tenets of the Declaration of Helsinki.
For the statements related to using IMT in patients with NIU, scenarios were built around different categories and groups of patients. Experts were asked to provide their opinion on IMT in each scenario, using a yes/no response. Patients were presented as two broad categories, based on (1) the patient’s clinical signs and symptoms suggestive of COVID-19 and (2) the systemic risk factors, including the number of drugs given for IMT. The first category included four groups of patients with NIU based on signs or symptoms of COVID-19: (1) otherwise healthy patient, defined as a patient with no signs of any infections and no apparent contact history with confirmed COVID-19; (2) otherwise healthy patient who had close contact with an infected COVID-19 individual; (3) sick patient with COVID-19 suspected, defined as a patient having clinical features consistent with COVID-19 (including upper respiratory tract infection), but with diagnostic testing not done or not available; and (4) sick patient with COVID-19, defined as a patient having clinical features consistent with COVID-19 with a positive test for SARS-CoV-2 infection.
The second category was defined on the basis of systemic risk factors and the level of immunosuppression: (1) increased risk patients, defined as having NIU on immunosuppressive drugs (biologics excluded); (2) high-risk patients, defined as having NIU with one of the following risk factors: use of biologics; high doses of immunosuppressive drug; use of multiple immunosuppressive drugs; active systemic inflammatory disease associated with NIU; presence of other comorbidities or multisystem diseases involving heart, lung and/or kidney; neutropenia, smoking; pregnancy; age more than 60 years; or previous history of infection while on IMT; and (3) very high-risk patients, defined as having NIU with two or more risk factors. This risk stratification based on the British Society of Rheumatology also serves as a surrogate marker for the severity of uveitis.9
IMT included local and oral corticosteroids, intravenous methylprednisolone, conventional immunosuppressive drugs and biologics. Conventional immunosuppressive drugs included antimetabolites, such as methotrexate, azathioprine and mycophenolate mofetil; alkylating agents, such as cyclophosphamide; and T-cell inhibitors, such as cyclosporine and tacrolimus. We did not include corticosteroid as part of the conventional IMT to be able to differentiate the survey responses. Biologics included tumour necrosis factor (TNF)-alpha inhibitors, such as adalimumab, infliximab, golimumab and certolizumab; lymphocyte inhibitors, such as daclizumab, rituximab and abatacept; and specific receptor antagonists, including anakinra and tocilizumab. A separate set of questions was specifically created for tocilizumab as anecdotal data, and ongoing clinical trials have suggested beneficial effects of tocilizumab in controlling cytokine storm in patients with COVID-19.10 11
Various scenarios derived from the association of the different groups within the categories of patients were therefore formed. Experts were asked to provide their inputs on how to manage IMT in each specific scenario presented, using yes/no responses, based on their opinion and experience. A total of 139 global experts completed the survey in April 2020. Consensus for the management of IMT was achieved if more than 75% of the experts agreed on the proposed question (statement).
In addition, 31 questions related to general recommendations in patients with uveitis in times of COVID-19 were created. General recommendations also included questions related to the use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with scleritis, including non-selective COX inhibitors, namely acetates (diclofenac, indomethacin, sulindac), fenamates (mefenamic acid), oxicams (piroxicam), propionates (ibuprofen, ketoprofen, naproxen), pyrazolones (phenylbutazone), salicylates (aspirin, diflunisal), flurbiprofen; selective COX2 inhibitors, such as meloxicam, nimesulide, celecoxib, etoricoxib, valdecoxib; and the use of hydroxychloroquine. Consensus for general recommendations was achieved if more than 75% of the experts agreed on the proposed question (statements).
RESULTS
Description of participants
A total of 139 uveitis specialists participated in the survey in April 2020 (online appendix 2: the credit roster for the COVID-19 IMT for NIU Study Group in footnotes). Of these 139 experts, 42.4% (n=59/139) respondents had more than 15 years of experience in the management of patients with NIU and 38.84% (n=54/139) treated more than 300 patients with IMT annually. Geographical distribution of practice was as follows: Asia (n=44/139, 31.7%), Europe (n=43/139, 30.9%), North America (n=34/139, 24.5%), Oceania (n=8/139, 5.8%), South America (n=7/139, 5.0%) and Africa (n=3/139, 2.2%). The subgroup distributions based on expertise of uveitis specialists and their geographical location of practice are provided in online supplementary tables 1–6.
bjophthalmol-2020-316776s001.pdf (254.5KB, pdf)
bjophthalmol-2020-316776s002.pdf (83.3KB, pdf)
bjophthalmol-2020-316776s003.pdf (174.7KB, pdf)
bjophthalmol-2020-316776s004.pdf (218.5KB, pdf)
bjophthalmol-2020-316776s005.pdf (216.2KB, pdf)
bjophthalmol-2020-316776s006.pdf (103.1KB, pdf)
Consensus on increased risk patients with NIU
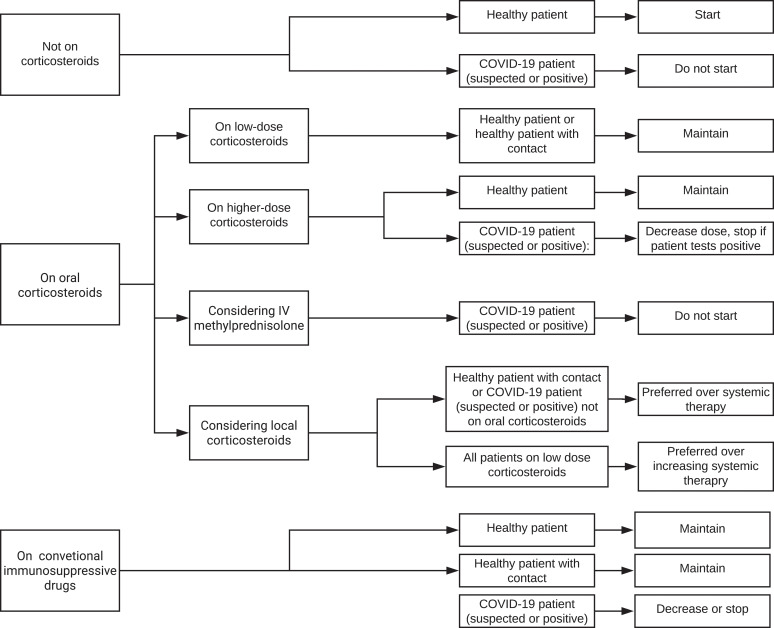
Consensus statements for the management of IMT in increased risk patients with NIU are presented in table 1, figure 1 and online supplementary tables 1A and 1B. For increased risk patients who are otherwise healthy and have a new diagnosis of NIU, there was agreement that these patients should be started on oral corticosteroids if needed. For healthy patients with inactive NIU who are taking low-dose oral corticosteroids, medications should be maintained, irrespective of a COVID-19 contact history. Higher-dose oral corticosteroids also should not be tapered or stopped, unless indicated for the NIU. When NIU is active, irrespective of a contact history, local corticosteroids are preferred to increasing the dose of systemic corticosteroid therapy, and in patients not on oral corticosteroids who had a contact history, local therapy is preferred to systemic therapy. With regard to conventional immunosuppressive drugs, otherwise healthy patients with active NIU should be started on or continue these medications. Even when these patients have a known COVID-19 contact, there was agreement that conventional immunosuppressive drugs should not be stopped.
Table 1.
Consensus guidelines on immunomodulatory therapy in times of COVID-19 in increased risk patients* (n=139)
| Healthy patients | Healthy patients with close contact | Sick patient: COVID-19 suspected | Sick patient: COVID-19 positive | |||
|---|---|---|---|---|---|---|
| Oral corticosteroids | Pt not on oral corticosteroids | To be started | 123 (88.5%) | 44 (31.7%) | 7 (5%) | 8 (5.8%) |
| Pt on low-dose oral corticosteroids | To be maintained | 132 (95.0%) | 112 (80.6%) | 57 (41.0%) | 38 (27.3%) | |
| To be decreased | 11 (7.9%) | 51 (36.7%) | 92 (66.2%) | 104 (74.8%) | ||
| To be tapered and stopped | 13 (9.4%) | 38 (27.3%) | 83 (59.7%) | 98 (70.5%) | ||
| Pt on higher-dose oral corticosteroids | To be maintained | 102 (73.4%) | 55 (39.6%) | 19 (13.7%) | 11 (7.9%) | |
| To be decreased | 38 (27.3%) | 80 (57.6%) | 117 (84.2%) | 125 (89.9%) | ||
| To be tapered and stopped | 21 (15.1%) | 52 (37.4%) | 99 (71.2%) | 107 (77%) | ||
| Intravenous methyl prednisolone | To consider | 103 (74.1%) | 32 (23%) | 6 (4.3%) | 5 (3.6%) | |
| Local corticosteroids | Pt not on oral corticosteroids | To be preferred to systemic therapy | 83 (59.7%) | 118 (84.9%) | 128 (92.1%) | 122 (87.8%) |
| Pt on low-dose oral corticosteroids | To be preferred to increasing the dose of systemic therapy | 106 (76.3%) | 121 (87.1%) | 126 (90.6%) | 122 (87.8%) | |
| Conventional IMT | To be started | 104 (74.8%) | 38 (27.3%) | 3 (2.2%) | 1 (0.7%) | |
| To be maintained | 135 (97.1%) | 95 (68.3%) | 26 (18.7%) | 13 (9.4%) | ||
| To be decreased | 9 (6.5%) | 51 (36.7%) | 113 (81.3%) | 123 (88.5%) | ||
| To be stopped | 5 (3.6%) | 32 (23%) | 107 (77%) | 122 (87.8%) | ||
Consensus for ‘No’
Consensus for ‘Yes’
*Increased risk category: patients with uveitis or rheumatologic disease on immunosuppressives (not on biologics).
MT, immunosuppressive therapy; Pt, patients.
Figure 1.
Flow chart of treatment recommendations for increased risk patients.
bjophthalmol-2020-316776s007.pdf (87.9KB, pdf)
bjophthalmol-2020-316776s008.pdf (92.3KB, pdf)
For increased risk patients with NIU who also are suspected of having COVID-19 or have tested positive for SARS-CoV-2 infection, there was consensus that these patients should not be started on oral corticosteroids or intravenous methylprednisolone. Higher-dose oral corticosteroids should be tapered to a lower safer dose. There was no consensus around whether to maintain, decrease or stop low-dose corticosteroids in these patients, but there was agreement that local corticosteroids are preferred to starting systemic therapy or increasing the dose of systemic therapy. In addition, in patients with suspected or confirmed COVID-19, conventional immunosuppressive drugs should not be started or maintained, rather it should be decreased or stopped.
Consensus on high-risk and very high-risk patients with NIU
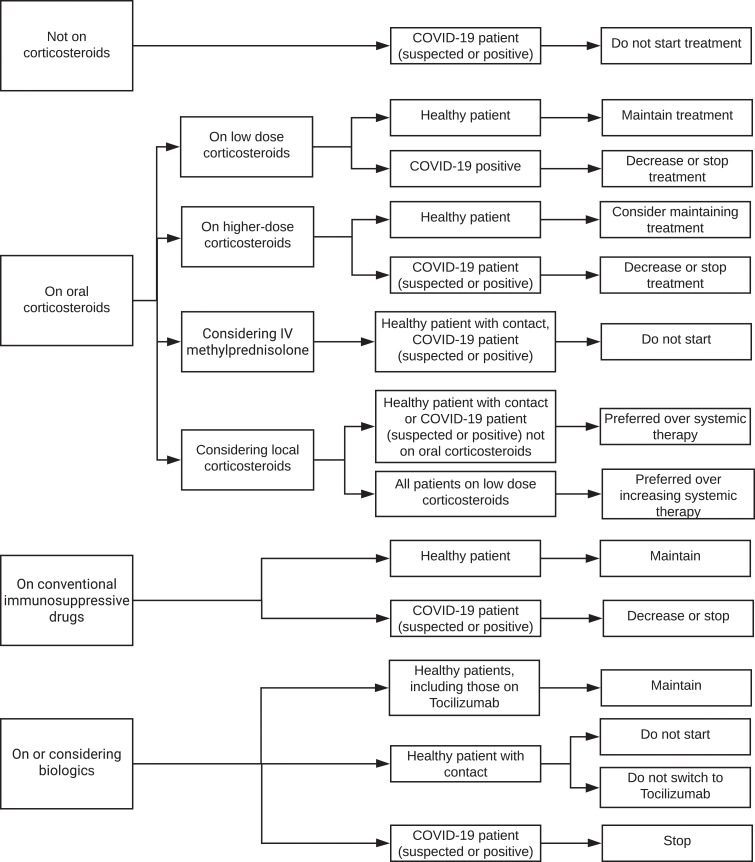
Consensus statements for the management of IMT in high-risk patients with NIU are presented in table 2, figure 2 and online supplementary tables 2A and 2B. In healthy patients with NIU who are otherwise healthy, low-dose corticosteroids should be maintained, with no need to decrease the dose or stop the medications. Irrespective of a COVID-19 contact history, local corticosteroid treatment is preferred to increasing the dose of systemic therapy, and in the case of a patient with a contact history who is not on oral corticosteroids, local therapy is preferred to starting systemic treatment. In healthy patients, higher-dose oral corticosteroids should not be tapered and stopped. In healthy patients with a contact history, intravenous methylprednisolone should be avoided. With regard to healthy patients and conventional immunosuppressive drugs and biologics, medications should be continued without reduction in dose. In the case of a patient with a contact history, biologics should not be started.
Table 2.
Consensus guidelines on immunomodulatory therapy in times of COVID-19 in high-risk patients* (n=139)
| Healthy patients | Healthy patients with contact | Sick patient—COVID-19 suspected | Sick patient—COVID-19 positive | |||
|---|---|---|---|---|---|---|
| Oral corticosteroids | Pt not on oral corticosteroids | To be started | 98 (70.5%) | 43 (30.9%) | 7 (5%) | 5 (3.6%) |
| Pt on low-dose oral corticosteroids | To be maintained | 129 (92.8%) | 77 (55.4%) | 36 (25.9%) | 28 (20.1%) | |
| To be decreased | 22 (15.8%) | 75 (54%) | 103 (74.1%) | 111 (79.9%) | ||
| To be tapered and stopped | 16 (11.5%) | 62 (44.6%) | 98 (70.5%) | 105 (75.5%) | ||
| Pt on higher-dose oral corticosteroids | To be maintained | 86 (61.9%) | 43 (30.9%) | 17 (12.2%) | 10 (7.2%) | |
| To be decreased | 63 (45.3%) | 96 (69.1%) | 123 (88.5%) | 128 (92.1%) | ||
| To be tapered and stopped | 34 (24.5%) | 70 (50.4%) | 110 (79.1%) | 118 (84.9%) | ||
| Intravenous methyl prednisolone | To be started | 73 (52.5%) | 25 (18%) | 7 (5%) | 6 (4.3%) | |
| Local corticosteroids | Pt not on oral corticosteroids | To be preferred to systemic therapy | 101 (72.7%) | 122 (87.8%) | 123 (88.5%) | 122 (87.8%) |
| Pt on low-dose oral corticosteroids | To be preferred to increasing the dose of systemic therapy | 106 (76.3%) | 121 (87.1%) | 126 (90.6%) | 122 (87.8%) | |
| Conventional IMT | To be started | 82 (59%) | 36 (25.9%) | 7 (5%) | 3 (2.2%) | |
| To be maintained | 125 (89.9%) | 72 (51.8%) | 18 (12.9%) | 11 (7.9%) | ||
| To be decreased | 18 (12.9%) | 71 (51.1%) | 116 (83.5%) | 121 (87.1%) | ||
| To be stopped | 12 (8.6%) | 49 (35.3%) | 112 (80.6%) | 123 (88.5%) | ||
| Biologics | To be started | 69 (49.6%) | 23 (16.5%) | 6 (4.3%) | 6 (4.3%) | |
| To be continued | 123 (88.5%) | 73 (52.5%) | 18 (12.9%) | 10 (7.2%) | ||
| To be stopped | 17 (12.2%) | 63 (45.3%) | 116 (83.5%) | 123 (88.5%) | ||
| Tocilizumab | To be started | 85 (61.2%) | 57 (41%) | 39 (28.1%) | 40 (28.8%) | |
| To be continued | 128 (92.1%) | 98 (70.5%) | 66 (47.5%) | 62 (44.6%) | ||
| To switch to | 44 (31.7%) | 32 (23%) | 41 (29.5%) | 45 (32.4%) | ||
Consensus for ‘No’
Consensus for ‘Yes’
*High-risk category: patients with uveitis or rheumatologic disease with one of the following risk factors: use of biologics; high doses or multiple immunosuppressives; active systemic disease associated with uveitis; presence of other co-morbidities or multisystem disease including heart, lung and/or renal involvement; neutropenia; smoking; pregnancy; older age; previous history of infection while on IMT.
IMT, immunosuppressive therapy; Pt, patients.
Figure 2.
Flow chart of treatment recommendations for high-risk patients.
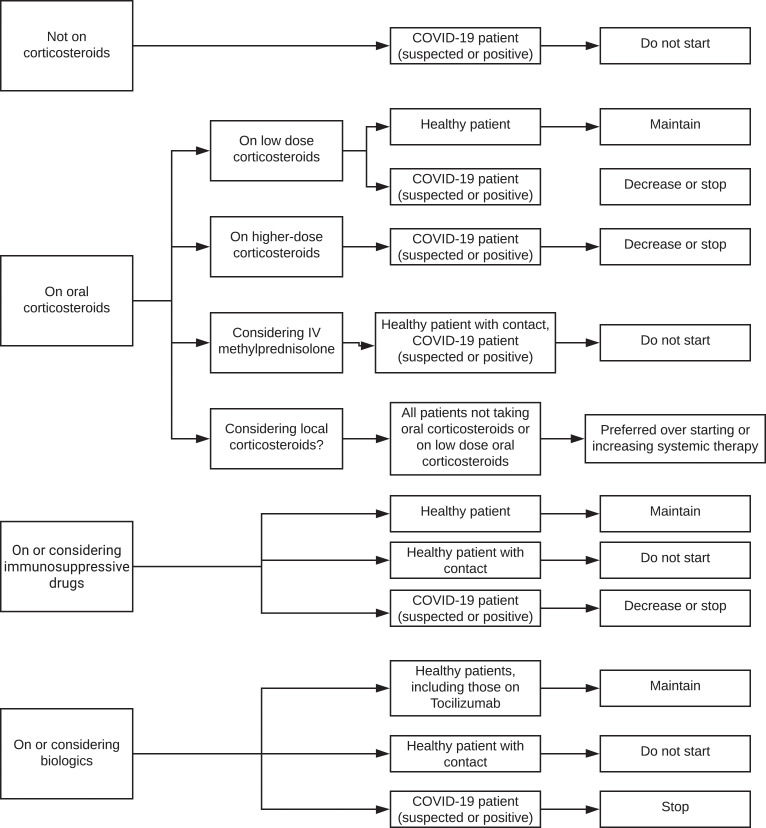
Consensus statements for the management of IMT in very high-risk patients with NIU are presented in table 3, figure 2 and online supplementary tables 3A and 3B. In otherwise healthy patients with NIU, low-dose corticosteroids should be maintained, with no need to taper and stop the medications. Irrespective of a COVID-19 contact history, local corticosteroids are preferred to starting systemic therapy or to increasing the dose of systemic therapy. In healthy patients with a contact history, intravenous methylprednisolone should be avoided. With regard to conventional immunosuppressive drugs and biologics in healthy patients, medications should be maintained, with no need to stop the treatment. In the case of patients with a contact history, conventional immunosuppressive drugs and biologics should not be started.
Table 3.
Consensus guidelines on immunomodulatory therapy in times of COVID-19 in very high-risk patients* (n=139)
| Healthy patients | Healthy patients with contact | Sick patient—COVID-19 suspected | Sick patient—COVID-19 positive | |||
|---|---|---|---|---|---|---|
| Oral corticosteroids | Pt not on oral corticosteroids | To be started | 80 (57.6%) | 40 (28.8%) | 12 (8.6%) | 9 (6.5%) |
| Pt on low-dose oral corticosteroids | To be maintained | 112 (80.6%) | 66 (47.5%) | 31 (22.3%) | 27 (19.4%) | |
| To be decreased | 43 (30.9%) | 84 (60.4%) | 105 (75.5%) | 110 (79.1%) | ||
| To be tapered and stopped | 33 (23.7%) | 73 (52.5%) | 102 (73.4%) | 107 (77%) | ||
| Pt on higher-dose oral corticosteroids | To be maintained | 74 (53.2%) | 35 (25.2%) | 9 (6.5%) | 7 (5%) | |
| To be decreased | 72 (51.8%) | 98 (70.5%) | 124 (89.2%) | 126 (90.6%) | ||
| To be tapered and stopped | 43 (30.9%) | 80 (57.6%) | 108 (77.7%) | 115 (82.7%) | ||
| Intravenous methyl prednisolone | To be started | 57 (41%) | 24 (17.3%) | 9 (6.5%) | 5 (3.6%) | |
| Local corticosteroids | Pt not on oral corticosteroids | To be preferred to systemic therapy | 106 (76.3%) | 121 (87.1%) | 123 (88.5%) | 121 (87.1%) |
| Pt on low-dose oral corticosteroids | To be preferred to increasing the dose of systemic therapy | 113 (81.3%) | 127 (91.4%) | 126 (90.6%) | 124 (89.2%) | |
| Conventional IMT | To be started | 78 (56.1%) | 30 (21.6%) | 5 (3.6%) | 2 (1.4%) | |
| To be maintained | 111 (79.9%) | 60 (43.2%) | 21 (15.1%) | 12 (8.6%) | ||
| To be decreased | 41 (29.5%) | 84 (60.4%) | 118 (84.9%) | 126 (90.6%) | ||
| To be stopped | 25 (18%) | 59 (42.4%) | 116 (83.5%) | 125 (89.9%) | ||
| Biologics | To be started | 64 (46%) | 26 (18.7%) | 11 (7.9%) | 8 (5.8%) | |
| To be continued | 109 (78.4%) | 60 (43.2%) | 17 (12.2%) | 11 (7.9%) | ||
| To be stopped | 30 (21.6%) | 74 (53.2%) | 118 (84.9%) | 124 (89.2%) | ||
| Tocilizumab | To be started | 72 (51.8%) | 46 (33.1%) | 41 (29.5%) | 42 (30.2%) | |
| To be continued | 115 (82.7%) | 90 (64.7%) | 63 (45.3%) | 62 (44.6%) | ||
| To switch to | 47 (33.8%) | 38 (27.3%) | 46 (33.1%) | 52 (37.4%) | ||
Consensus for ‘No’
Consensus for ‘Yes’
*Very high-risk category: patients with uveitis or rheumatologic disease with two or more risk factors.
IMT, immunosuppressive therapy; Pt, patients.
Regarding high-risk and very high-risk patients with NIU who have suspected or confirmed COVID-19, there was consensus that they should not be started on oral corticosteroids, and if on systemic corticosteroids, the dose should be decreased and/or the corticosteroids stopped. Local corticosteroids are preferred to starting systemic therapy or increasing the dose of systemic therapy. Conventional immunosuppressive drugs and biologics should not be started or maintained, but should be decreased and/or stopped. These statements are presented in tables 2 and 3, and figures 2 and 3.
Figure 3.
Flow chart of treatment recommendations for very high-risk patients.
Consensus on general recommendations
For the management of NSAIDs in the treatment of scleritis, there was consensus for starting or maintaining medications, and not decreasing or stopping them, only in those patients who were otherwise healthy and with no history of a COVID-19 contact. By contrast, there was no consensus to either start, maintain, decrease or stop NSAIDs in healthy patients with a contact history or patients with possible or confirmed COVID-19 (online supplementary tables 4A and 4B). There was consensus that hydroxychloroquine should be continued in all patients already receiving it, but there was no consensus around the recommendation to start the drug in any group (healthy, healthy with contact, sick with suspected COVID-19, or sick with confirmed COVID-19) (online supplementary tables 5A and 5B).
Regarding general recommendations for patients with NIU on conventional IMT or biologics, experts agreed on hand personal hygiene, avoiding crowds, use of masks, avoiding unproductive attendances at the hospital, postponing long-term follow-up, self-isolation, and in really sick patients with no symptoms of respiratory infection, urgent doctor appointment.
Consensus based on experience of uveitis specialist and geographical location of practice
In increased risk, high-risk and very high-risk patients, experts across all experience and geographical locations agreed that corticosteroids, intravenous methylprednisolone, conventional immunosuppressive drugs and biologics should not be initiated in sick patients with suspected or confirmed COVID-19 (online supplementary tables 1A, 1B, 2A, 2B, 3A, 3B). The vast majority of general recommendations regarding hygiene and physical distancing were all supported and consistent among all experts, regardless of years of experience or geographical location (online supplementary tables 6A and 6B). In sick patients with COVID-19 who have scleritis, in Asia, Africa, Europe and South America, experts agreed with stopping NSAIDS (79% to 100%), while experts in North America and Oceania were less likely to recommend stopping NSAIDS (57% to 62%) (online supplementary tables 4A and 4B). Across all levels of experience and geography, experts were uniformly in consensus in continuing the use of hydroxychloroquine in patients already receiving it (online supplementary tables 5A and 5B).
DISCUSSION
In the midst of a pandemic, there is an urgent need for accurate information. The rheumatology specialists have responded by incorporating The COVID-19 Global Rheumatology Alliance with over 300 rheumatologists, scientists and patients.12 Similar to their efforts, our study was an international, expert-led consensus initiative with the aim of developing experience-based recommendations for the management of IMT in patients with NIU during the COVID-19 pandemic. Uveitis experts concluded that management decisions were influenced by the patient’s COVID-19 infection status and systemic risk factors, including severity of uveitis and number of IMT agents required for the NIU. The survey questions distinguish between patients with suspected and confirmed COVID-19, but consensus opinion among the experts was similar for both these groups of patients. This is likely because in many regions of the world, even industrialised countries, diagnostic testing is not widely available.13
Corticosteroids represent the first-line therapy to achieve quiescence in NIU, followed by systemic immunosuppressive, commonly used as a second-line therapy in those patients whose disease is not sufficiently controlled with corticosteroids.1 To summarise our results, surveyed uveitis experts recommended to not begin systemic corticosteroid or immunosuppression for NIU treatment in sick patients with suspected or confirmed COVID-19, irrespective of risk group. Among sick patients receiving high-dose corticosteroid, consensus was to taper the dose in all risk groups and to taper even low-dose corticosteroid in high-risk patients or very high-risk patients. Tapering instead of abrupt cessation of the oral corticosteroids was recommended in view of the risk of adrenal insufficiency. In healthy patients, experts agreed to start oral corticosteroids only in increased risk patients and not in high-risk or very high-risk patients. Low-dose oral corticosteroids and conventional IMT should be maintained, while only in increased risk or high-risk patients, higher-dose corticosteroids should not be tapered and stopped and biologics should be continued, including tocilizumab. In healthy patients with a contact history, the overall agreement is lower. Experts agreed not to start biologics in high-risk and very high-risk patients. Low oral dose corticosteroids and conventional IMT should be maintained in increased risk patients.
Although first-line treatment for NIU consists of local or systemic corticosteroids, overall consensus emerged that in the setting of COVID-19 pandemic, the use of systemic corticosteroids should be avoided in sick patients and local therapy (regional corticosteroid injections) should be preferred to systemic treatment in all patients, irrespective of their risk and health, except in healthy patients not already on corticosteroids. Systemic corticosteroids might be harmful, given their mechanism of action that inhibits the immune responses and affects the pathogen clearance. In such a setting, local therapy proves to be extremely useful, because by targeting the site of inflammation with high concentration of the drug, it may be as effective as systemic therapy while reducing systemic exposure.14 This allows at least temporary control of the sight-threatening complications of uveitis, delaying initiation of second-line immunosuppression, until further research on the impact of immunosuppressive agents in patients with COVID-19 becomes available.
Experts agreed that for all sick patients irrespective of the risk, conventional IMT and biologics should not be started, the dose should be at least decreased, or the medication be stopped. This is because of the higher risk for COVID-19 infection in immunosuppressed patients, particularly those on TNF-α inhibitors, as IMT blocks the immune pathways adapted to protect the host, making the patient more susceptible to infections.15
Notably, regarding the treatment of scleritis, consensus was reached to initiate or maintain NSAIDs in otherwise healthy individuals. The US Food and Drug Administration has stated that there was no evidence connecting the use of NSAIDs in patients with severe COVID-19.16
Importantly, with the unprecedented and dynamic nature of the COVID-19 pandemic, our understanding of the SARS-CoV-2 virus and the epidemiology of COVID-19 are rapidly evolving.17 Consensus options may change as we learn more about the virus and the effects of immunosuppression on the outcomes of patients with NIU and they should be revisited regularly. To further decrease the risk of infection when patients with uveitis present for follow-up, physical distancing, hand hygiene and use of masks should be routinely implemented. When possible, electronic or telehealth communication with patients is encouraged to monitor for signs of infection and to minimise non-urgent visits to the clinic.
Numerous medications are under investigation for the treatment of COVID-19. The role of interleukin (IL)-6 inhibitors in the treatment of COVID-19 has yet to be determined with clinical trials underway.18 Accordingly, expert consensus was reached only in the scenario of continuing tocilizumab in otherwise healthy patients with NIU. Although tocilizumab improves visual acuity and reduces intraocular inflammation in NIU,19 the use of tocilizumab for NIU in a patient with COVID-19 is unknown. Evidence of hydroxychloroquine for treatment of COVID-19 remains controversial with the need for randomised controlled trials to assess effectiveness.20 The uncertain utility of hydroxychloroquine is reflected in the survey, as uveitis specialists only reached consensus for maintaining hydroxychloroquine in those patients who are already on the medication.
One of the most important concerns related to IMT is the increased risk of viral infections, as these drugs interfere with patients’ immune response. However, while it has been demonstrated that patients on IMT are at higher risk of developing severe complications of influenza, which has not been shown for coronavirus induced disease.21–25 Studies of past SARS-CoV and Middle East respiratory syndrome coronavirus outbreaks, and the most recent studies of COVID-19, have not associated immunosuppressed status with increased risk of adverse outcomes, such as death or admission to intensive care.22– 25 A recent case series by Monti et al evaluated the clinical courses of COVID-19 in eight Italian patients (four with confirmed and four with suspected COVID-19) who were being treated with IMT for rheumatoid arthritis or spondyloarthritis.8 At the time of symptom onset, these patients stopped their immunosuppressive drugs. The authors reported that these patients did not have an increased risk of life-threatening complications compared with the general population.8 Of note, the Global Rheumatology Alliance has initiated a registry to evaluate the outcomes of patients receiving IMT for rheumatologic diseases who develop COVID-19.12 The evaluation of patients with NIU within this registry will provide further insights on the use of IMT during the COVID-19 pandemic.
Without overlooking the effect of IMT on the immune system in relation to the spreading of COVID-19 infection, new insights on the pathogenesis of the coronavirus disease process have emerged. In particular, the dysregulation and exacerbation of innate immune responses following the infection seem to play a key role in the course of tissue damage, representing a relevant concern in patients with a weaker immune status caused by immunosuppressive treatments.24 25 The cytokine storm induced by the infection seems to have a crucial role in disease progress. Increased levels of pro-inflammatory molecules, including IL-6, TNF-alfa, IL-2, IL-7, IL-10, granulocyte-colony stimulating factor, interferon-γ-inducible protein, monocyte chemoattractant protein and macrophage inflammatory protein 1 alpha, were found in the plasma of patients with COVID-19 and were linked to the severity of disease course.26 27 The considerable production of cytokines derived from pathogenic T cells and inflammatory monocytes, that are rapidly activated by the infection, causes the pro-inflammatory storm. This results in alveolar–capillary exchange dysfunction and impaired oxygen diffusion, eventually leading to pulmonary failure.22 In addition, the dysregulation between Th1 and Th2 lymphocyte subtypes negatively affects B lymphocytes and antibody production.
The potential role of some anti-rheumatic drugs in the management of patients with COVID-19 had been hypothesised, potentially acting as direct antivirals or targeting host immune response. Hydroxychloroquine may alter the lysosomal proteases that mediate the viral entry into the cell and have demonstrated efficacy in improving the infection. Baricitinib has both antiviral and anti-inflammatory properties. Checkpoint inhibitors such as anti-CD200 and anti-PD1 could have a role in the treatment of COVID-19.28
IL-6 seems to have a crucial role in the pro-inflammatory storm and subsequent disease progress, because high levels of IL-6 have been demonstrated to be predictive of severe pneumonia.29 30 Thus, interference with the IL-6 pathway might be a potential therapeutic strategy, and tocilizumab, a recombinant humanised anti-human IL-6 receptor monoclonal antibody that inhibits IL-6 signal transduction, has been proven effective in limited clinical trials in patients with severe COVID-19 disease.11
Our study is limited by the lack of evidence-based literature regarding immunosuppression during the COVID-19 pandemic. However, due to the urgent need for guidance on immunosuppression during the COVID-19 pandemic, expert opinions of uveitis specialists represent an important source for consensus statements. Our survey reports the expert opinions of uveitis specialists from six continents. At the time of the survey, some countries had not experienced major outbreaks and thus specialist survey responses may change over time as the number of COVID-19 cases increase in those regions. Also, NIU is a category of many complex diseases, each with its own distinct clinical course, visual outcomes and requirement for various treatments. In particular, some NIU (eg, Behçet or Vogt–Koyanagi–Harada disease) may not tolerate rapid reduction in IMT without visual loss, which could end up being permanent. Therefore, these guidelines may not apply to all diseases equally within the category of NIU, and uveitis specialists need to assess each individual patient in their particular situation.
CONCLUSION
This is the first survey of uveitis experts worldwide to provide the evolving consensus-based guidance for managing patients requiring IMT for NIU in the COVID-19 pandemic era. Management decisions were influenced primarily by the patient’s potential COVID-19 infection status and baseline systemic risk factors for COVID-19-related complications, which must be balanced with the severity of the NIU and the IMT that is judged necessary to avoid sight-threatening flares of inflammation. Management of IMT during the COVID-19 pandemic involves many challenges; thus, any decision-making on IMT will benefit from multidisciplinary teams of experts involved in the care of the patient with suspected or confirmed COVID-19 infection.
Footnotes
Correction notice: This paper has been corrected since it was published online. Author Christoph Tappeiner’s first name and surname were transposed.
Twitter: EdmundTsuiMD.
Contributors: RA conceptualised this study and was supported by all the authors and COVID-19 IMT study group. The writing committee was comprised of IT, CL, ET and MB, and all these authors worked with RA on writing the first draft of the manuscript, analysis of the data and generation of the tables and figures. The group listed in the appendix participated in administering the survey.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for any of the authors.
Competing interests: None declared.
Data sharing statement: Data are available upon reasonable request.
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1. Dick AD, Rosenbaum JT, Al-Dhibi HA , et al. . Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: Fundamentals Of Care for UveitiS (FOCUS) initiative. Ophthalmology 2018;125:757–73. 10.1016/j.ophtha.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 2. Jabs DA. Immunosuppression for the uveitides. Ophthalmology 2018;125:193–202. 10.1016/j.ophtha.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Organization WH, Others WHO director-general’s opening remarks at the media briefing on COVID. Geneva, Switzerland, 2020. [Google Scholar]
- 4. Holroyd CR, Seth R, Bukhari M , et al. . The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology 2019;58:372 10.1093/rheumatology/key298 [DOI] [PubMed] [Google Scholar]
- 5. Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum 2010;39:327–46. 10.1016/j.semarthrit.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 6. Arentz M, Yim E, Klaff L , et al. . Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winthrop KL, Curtis JR, Lindsey S , et al. . Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol 2017;69:1960–8. 10.1002/art.40189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monti S, Balduzzi S, Delvino P , et al. . Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020;79:667–8. 10.1136/annrheumdis-2020-217424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Health Service England Clinical guide for the management of rheumatology patients during the coronavirus pandemic. NHS England and NHS Improvement 2020. Available: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/clinical-guide-rheumatology-patients-v1-19-march-2020.pdf (accessed 11 Apr 2020).
- 10. Ascierto PA, Fox BA, Urba WJ , et al. . SITC statement on anti-IL-6/IL-6R for COVID-19 - Society for Immunotherapy of Cancer (SITC). The Society for Immunotherapy of Cancer (SITC) CONNECT. 2020. Available https://www.sitcancer.org/research/covid-19-resources/il-6-editorial (accessed 11 Apr 2020).
- 11. Xu X, Han M, Li T , et al. . Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv 2020;202003:v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson PC, Yazdany J. The COVID-19 global rheumatology alliance: collecting data in a pandemic. Nat Rev Rheumatol 2020. 10.1038/s41584-020-0418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC Coronavirus disease 2019 (COVID-19). Centers for Disease Control and Prevention. 2020. Available https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html (accessed 11 Apr 2020).
- 14. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473–5. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon WG, Symmons DPM, Lunt M , et al. . Serious infection following anti-tumor necrosis factor α therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum 2007;56:2896–904. 10.1002/art.22808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Center for Drug Evaluation, Research FDA advises patients on use of NSAIDs for COVID-19 US Food and Drug Administration, 2020. Available https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19 (accessed 11 Apr 2020). [Google Scholar]
- 17. Fauci AS, Lane HC, Redfield RR. COVID-19: navigating the uncharted. N Engl J Med 2020;382:1268–9. 10.1056/NEJMe2002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta P, McAuley DF, Brown M , et al. . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sepah YJ, Sadiq MA, Chu DS , et al. . Primary (Month-6) outcomes of the STOP-Uveitis study: evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol 2017;183:71–80. 10.1016/j.ajo.2017.08.019 [DOI] [PubMed] [Google Scholar]
- 20. Kim AHJ, Sparks JA, Liew JW , et al. . A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med 2020. 10.7326/M20-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Memoli MJ, Athota R, Reed S , et al. . The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 2014;58:214–24. 10.1093/cid/cit725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou F, Yu T, Du R , et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. C-C L, Liu YH, Wang C-Y , et al. . Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl 2020. 10.1002/lt.25756 [DOI] [PubMed] [Google Scholar]
- 25. Ferro F, Elefante E, Baldini C , et al. . COVID-19: the new challenge for rheumatologists. Clin Exp Rheumatol 2020;38:175–80. [PubMed] [Google Scholar]
- 26. Chen N, Zhou M, Dong X , et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang D, Hu B, C H , et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus: infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ceribelli A, Motta F, De Santis M , et al. . Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun 2020;109:102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao Y, Li T, Han M , et al. . Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol 2020. 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang C, Wang Y, Li X , et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjophthalmol-2020-316776s001.pdf (254.5KB, pdf)
bjophthalmol-2020-316776s002.pdf (83.3KB, pdf)
bjophthalmol-2020-316776s003.pdf (174.7KB, pdf)
bjophthalmol-2020-316776s004.pdf (218.5KB, pdf)
bjophthalmol-2020-316776s005.pdf (216.2KB, pdf)
bjophthalmol-2020-316776s006.pdf (103.1KB, pdf)
bjophthalmol-2020-316776s007.pdf (87.9KB, pdf)
bjophthalmol-2020-316776s008.pdf (92.3KB, pdf)