Abstract
Background:
Recent respiratory infection including SARS-CoV-2 is an independent risk factor for acute cerebrovascular disease.
Purpose:
There have been reports linking haemorrhagic strokes to SARS-CoV-2 infection during this pandemic, which lead us to evaluate if SARS-CoV-2 infection could be associated with increased risk of intracerebral haemorrhage (ICH).
Methods:
A retrospective observational study evaluating all stroke cases admitted in our centre in the past one month.
Results:
More than half (56%) had ICH, compared to 22% last year. Two patients with ICH were SARS-CoV-2 positive and they had no or mild respiratory symptoms and had higher occurrence of renal dysfunction.
Conclusion:
There could be possible association between ICH and SARS-CoV-2 infections. However, a prospective study with larger sample size is needed to elucidate the pathogenesis.
Keywords: Hemorrhage, ICH, SARS-CoV-2, stroke
INTRODUCTION
Early December 2019 was associated with health authorities recognizing the emergence of multiple cases of a short, febrile, predominantly respiratory illness in Wuhan, China.[1] This disease was attributed to a beta coronavirus and termed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).[2] The virus bore a close resemblance to SARS-CoV-1, which had led to the SARS epidemic of 2003. SARS-CoV-2 has now swept across the globe and has emerged as a pandemic with over 35,00,000 cases worldwide and above 2,45,000 deaths. India has recorded 41,076 cases with 1,359 mortalities at the time of writing this article, with the number rising every day.
There is evidence to suggest that recent respiratory infection is an independent risk factor for acute cerebrovascular disease.[3,4] Animal model studies have revealed that influenza virus can aggravate ischemic cerebral insult by triggering an inflammatory cytokine cascade. The same also increases the risk of haemorrhagic transformation after thrombolysis.[5] SARS-CoV-2 has been associated with cytokine storms, predisposing to acute ischemic strokes (IS). Severe infections have been associated with elevated D-dimer and low platelet counts,[6] which predispose these patients both to ischemic and haemorrhagic strokes. There have been reports linking haemorrhagic strokes to SARS-CoV-2 infection during this pandemic,[7] which lead us to review our data.
AIM
To evaluate whether SARS-CoV-2 infection could be associated with increased risk of intracerebral haemorrhage (ICH)
METHODS
We conducted an observational retrospective study at the Department of Neurology at AIIMS, New Delhi, India. We reviewed all admitted stroke cases in the past month (lockdown period between 22-3-2020 and 21-4-2020) following SARS-CoV-2 outbreak in India and compared with stroke cases admitted last year during the same period. The demographic profile, patients' address with particular emphasis on whether they hailed from a hotspot of SARS-CoV-2, history, examination, stroke subtype (ischemic/haemorrhagic), routine investigations and SARS-CoV-2 status were documented. Testing for SARS-CoV-2 was done if the patient was clinically suspected or hailing from a known hotspot.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
RESULTS
Of 25 stroke patients admitted, 11 (44%) were IS and 14 (56%) were haemorrhagic strokes. Nineteen (76%) of these stroke patients were tested for SARS-CoV-2 by reverse transcriptase-polymerase chain reaction (RT-PCR) of a nasopharyngeal swab specimen and two were positive.
Case 1
A 56-year-old gentleman with hypertension for past 3 years (well controlled on 5 mg amlodipine), and smoking, presented with sudden onset left hemiparesis and loss of consciousness of 12 hours duration. He had no history of head trauma, vomiting or preceding headache. His blood pressure was 148/80 mm of Hg and non-contrast computed tomography (NCCT) head revealed a right pontine bleed with intraventricular extension [Figure 1]. His blood parameters are described in Table 1. He developed fever and dry cough the following day after admission, for which he was tested for SARS-CoV-2 by RT-PCR and found positive. Chest X-Ray (CXR) was normal, and CT chest revealed multiple fibro-atelectatic parenchymal bands with calcifications involving bilateral upper lobes with emphysematous changes. ECG and ECHO did not show changes of left ventricular hypertrophy. During the course of hospitalization, he needed ventilatory support and is critical.
Figure 1.
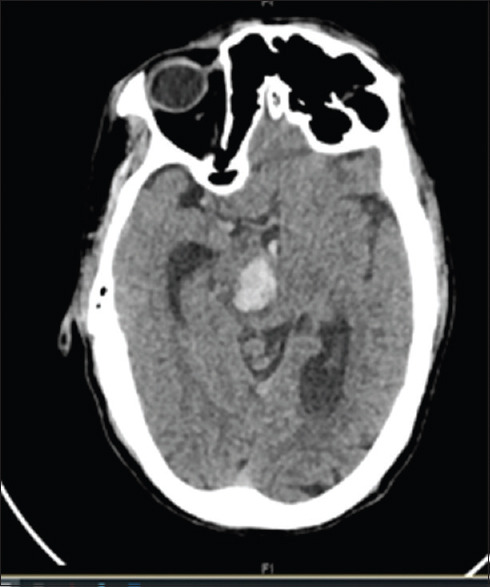
NCCT head showing right pontine bleed with intraventricular extension
Table 1.
Characteristics of SARS-CoV-2 positive ICH patients
| Parameters | Patient 1 | Patient 2 |
|---|---|---|
| Age/Sex (years) | 56/M | 72/M |
| Risk factors | Hypertension, smoking | Diabetes mellitus |
| Hemogram (Hb/TLC/DLC) | 15.1/23, 320/N85L6 | 15/11, 260/N82L14 |
| Platelet count (per cu mm) | 2.2 lakh | 1.65 lakh |
| INR | 1.07 | 1.00 |
| SGOT/PT (IU/ml) | 48/64 | 40/44 |
| Urea/creatinine (mg/dl) | 41/1.2 | 67/1.23 |
| CRP (mg/dl) | 4.11 (<1) | |
| Cholesterol (mg/dl) | 112 | |
| Cause for SARS-CoV-2 testing | Developed symptoms on day 2 of admission | Hotspot area |
Case 2
A 72-year-old gentleman, diabetic, woke-up with complaints of sudden left-sided weakness. There was no history of head trauma, headache, vomiting or loss of consciousness. His blood pressure was 130/84 mmHg and regular pulse rate of 80/minute. NCCT head showed a right frontal lobar bleed with intraventricular extension [Figure 2a]. A possibility of amyloid angiopathy was considered and magnetic resonance imaging (MRI) brain revealed microbleeds in bilateral subcortical white matter zones of cerebral hemispheres, left basal ganglia, thalamus, pons and left cerebellar hemisphere (2B) which are typical sites for hypertensive bleeds [Figure 2b]. SARS-CoV-2 testing was done since the patient was from a hotspot and found positive. He had no cough or fever and CXR was normal. He was managed conservatively with CNS decongestants but expired during the course of hospitalization.
Figure 2.
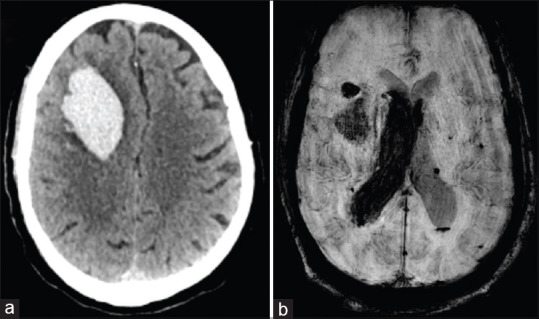
(a) NCCT head revealing large right frontal lobar bleed. (b) SWI sequence of MRI brain showing haemorrhage in right frontal lobe with intraventricular extension and microhaemorrhages in bilateral cerebral hemispheres
DISCUSSION
Stroke is a rare complication described with SARS-CoV-2.[8] Limited available studies have reported its association with IS. A recent report has claimed a seven-fold increase in young strokes amongst individuals without risk factors and mild or no symptoms of COVID-19.[9] Helms et al.[10] reported three cases of IS in an observational study done to document neurologic features in 58 SARS-CoV-2 positive patients who had presented with acute respiratory distress syndrome (ARDS). The diagnosis of IS based on MRI brain was done because of unexplained encephalopathic features, and none of them had focal neurological deficits to arouse suspicion of stroke.
A review by Li et al.[8] on neurological manifestations in 221 patients of SARS-CoV-19 revealed 13 patients (5.9%) with cerebrovascular disease. Only one of them had ICH (0.5%), eleven had IS, and one had cortical vein thrombosis (CVT).
Our study showed an unexpected finding of more cases of ICH (56%), which is in stark contrast to the same time last year which had nine (21.9%) ICH cases amongst 41 stroke admissions. Thirty patients had IS (73.2%) and two had CVT (4.9%). Stroke admission data from the past one year revealed 33% patients had ICH with IS accounting for 61% cases. A possible explanation for this disparity might be that the patients with ICH tend to be sicker and would, therefore, seek medical attention even during this pandemic. However, a difference of this magnitude should not be ruled out on this presumption alone.
Two of our ICH patients were positive for SARS-CoV-2. These were different from usual ICH presentations as there was an absence of prototypical ICH risk factors or hematological abnormalities [Table 2]. Case 1 was younger, and case 2 was a non-hypertensive lobar bleed, and both were asymptomatic for SARS-CoV-2. Their hospital course, however, showed worse outcomes. This has been reported that SARS-CoV-2 patients who have strokes have higher mortality rates.[11] No hematological abnormality such as thrombocytopenia or bleeding diathesis was identified in both cases.
Table 2.
Clinico-hematological parameters of SARS-CoV-2 positive versus negative ICH patients
| ICH patients tested for SARS-CoV-2 | SARS-CoV-2 positive (n=2) | SARS-CoV-2 negative (n=10) |
|---|---|---|
| Mean age (years) | 64 | 53.6 |
| Gender (M:F) | M:F=2:0 | 4:1 |
| Mean platelet count (per cu mm) | 1,92,500 | 1,74,200 |
| Mean serum urea/creatinine (mg/dl) | 54/1.21 | 40.8/1.01 |
| Mean INR | 1.03 | 1.04 |
| Mean serum cholesterol (mg/dl) | 110 | 158.5 |
| Mean TLC (per cu mm) | 17,290 | 11,504 |
They differ from the other reported stroke patients with SARS-CoV-2 infections since stroke was the presenting complaint in both cases and were asymptomatic for the infection. According to the SARS-CoV-2 severity classification by Mao et al.[8], mild cases do not require respiratory intervention; moderate ones require some intervention and severe ones require mechanical ventilation. They also concluded that neurological complications, including stroke, were more likely to occur in the severely afflicted group.[10] Aggarwal et al.[12] also found an approximate 2.5-fold increased odds of severe SARS-CoV-2 infection with cerebrovascular disease. However, our patients would be classified as mild and yet presented with ICH.
The mean serum urea of patients with SARS-CoV-2 was 54 compared to 40.8 in the negative group. This could be significant as an early marker for infection during this pandemic since there are reports of acute kidney injury occurring in 5%-30% cases, occasionally requiring dialysis, with SARS-CoV-2 infection.[12]
This association between SARS-CoV-2 positivity and ICH could be co-incidental considering that stroke is a common disorder with an individual developing a stroke every 20 seconds in India. However, we record these as unusual non-pulmonary presentations of SARS-CoV-2 infection in our institute. These unusual presentations highlight the potentially unexplained pathogenesis and route of infection with SARS-CoV-2 strain of coronavirus. The potential possibilities leading to ICH include an infective arteriopathy, viral infection-induced platelet dysfunction or thrombocytopenia, activation of pro-inflammatory cascade leading to increased cytokine levels culminating in a cytokine storm or severe viral infection leading to consumption coagulopathy and multi-organ dysfunction.
Our patients did not have thrombocytopenia or evidence of liver dysfunction. However, slight impairment of renal function was present and the presence of systemic inflammation could be commented upon by raised CRP levels.
CONCLUSION
The effect of SARS-CoV-2 on neurological function is not well established and not the effects on the outcomes after treatment of the neurological illness. During this pandemic, every atypical presentation of a common illness or clustering of a common finding needs to view with suspicion of having an association with SARS-CoV-2 infection. The exact pathogenesis of ICH due to SARS-CoV-2 is not yet known and its disease phenotype is continuously evolving. We would like to propose an association between ICH and SARS-CoV-2 infection. However, a prospective study with larger sample size and extensive investigations is needed to elucidate the exact pathogenesis of the same.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkind MS. Why now? Moving from stroke risk factors to stroke triggers. Curr Opin Neurol. 2007;20:51–7. doi: 10.1097/WCO.0b013e328012da75. [DOI] [PubMed] [Google Scholar]
- 4.Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: A self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018:51. doi: 10.1183/13993003.01794-2017. pii: 1701794 doi: 101183/1399300301794-2017 Print 2018 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhammad S, Haasbach E, Kotchourko M, Strigli A, Krenz A, Ridder DA, et al. Influenza virus infection aggravates stroke outcome. Stroke. (42) 2011:783–91. doi: 10.1161/STROKEAHA.110.596783. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020 doi: 10.1002/jmv.25748. doi: 101002/jmv 25748 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: Causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. doi: 101001/jamaneurol 20201127 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. pii: jnnp-2020-323586 doi: 101136/jnnp-2020-323586 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. doi: 101056/NEJMc2008597 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal G, Lippi G, Henry BM. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stoke. 2020 doi: 10.1177/1747493020921664. DOI: 101177/1747493020921664 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Wendling P. KidneyKidney complications in COVID-19 send hospitals scrambling. Medscape. 2020 [Google Scholar]


