Editor—Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly infectious respiratory pathogen disseminated by droplets and aerosols.1 Healthcare providers (HCPs) performing aerosol-generating procedures (AGPs) on coronavirus disease 2019 (COVID-19) patients are at risk of infection. AGPs include, but are not limited to tracheal intubation, extubation, mask ventilation, tracheostomy, oropharyngeal/tracheal aspiration, high-flow air/oxygen delivery, bronchoscopy, esophagogastroduodenoscopy, transoesophageal echocardiography, defibrillation, chest compression, and a range of dental, head and neck, and thoracic surgeries. Variations of Lai's aerosol barrier2 for limiting healthcare provider exposures has been rapidly adopted, but remains incompletely validated.3 , 4 Cubillos and colleagues5 reported qualitative results of vacuum filtration, but clinically actionable time-to-clearance information is lacking. Efficacy of particle elimination by vacuum relates to air flow rates, which can be diminished by in-line viral filters essential to decontamination of outflow. Therefore, empirical testing is needed for each vacuum/filter configuration attached to intubation boxes to determine the particle elimination kinetics. Here, we present experimental data on the time course of active aerosol removal, comparing our hospital in-wall suction system and two low-cost commercially-available vacuums using an intubation box.
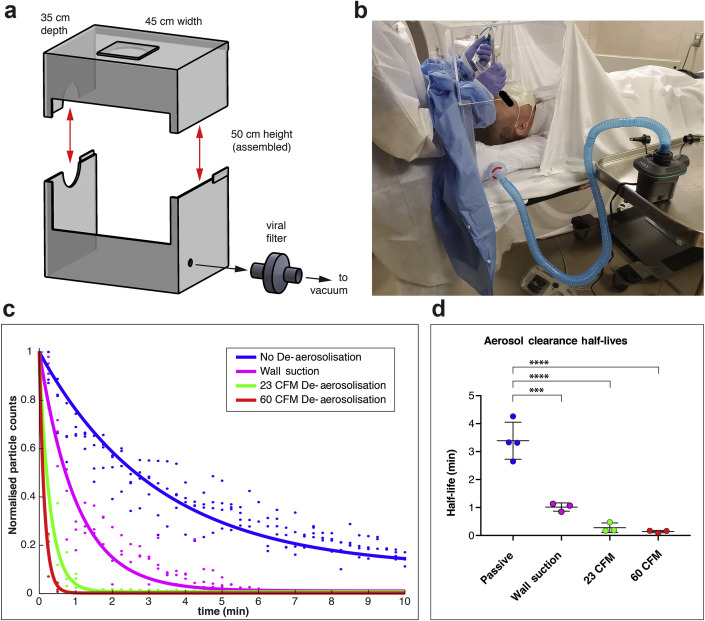
Our two-piece design intubation box6 (Fig. 1 a) includes active aerosol removal by attaching a vacuum with an in-line high-efficiency viral filter (Draeger SafeStar55R, German company). Aerosol removal by such filters could mitigate virus dispersion; this filter has 99.9999% viral filtration efficiency.7 We tested two vacuums, with stated air flow ratings of 60 cubic feet min−1 (CFM; Shop-Vac #9303511) or 23 CFM (Intex (Long Beach, CA, USA) mattress inflator/deflator #66639E), attached via standard airway circuit tubing. Separately, we also attached our hospital wall vacuum through a pressure regulator (Ohio Medical PISA, Gurnee, IL, USA) set to maximum (0.13 kPa) to a 2 L suction canister, then to the filter and box. In our practice, the patient is covered with a sheet or surgical drape (Fig. 1b).
Fig 1.
Intubation box with improved mobility and vacuum filtration. (a) The two-piece intubation box with a vacuum and in-line particulate filter is shown as a schematic with overall dimensions shown, with red arrows showing detachable top. (b) A mock intubation setup is shown with the working window sealed with a gown (disposable) clipped into place, affording proceduralist arm mobility, aerosol enclosure, and vacuum elimination. The gown can be easily detached during airway rescue. (c) Aerosol elimination follows exponential decay kinetics, with hospital wall vacuum and two commercial vacuums improving clearance kinetics. (d) Vacuum aerosol removal significantly decreases particle clearance half-lives from 3.4 min (passive) to 1.0 min (wall suction), and to 0.28 min with the 23 cubic feet min−1 (CFM) vacuum, or 0.14 min with a 60 CFM vacuum. Time series from replicate experiments from 1c were fit to exponential decays after normalisation, and average half-lives (t1/2) were analysed by one-way analysis of variance (anova) (F(3,9)=52, overall P<0.0001). Aerosol clearance was significantly hastened with suction from the wall vacuum, and with the 23 or 60 CFM stand-alone vacuums vs passive clearance. ∗∗∗P=0.0001, ∗∗∗∗P<0.0001, anova with Tukey's multiple comparisons testing, error bars represent standard deviation.
To simulate viral aerosol contamination and clearance, an aerosol particle generator (TSI 8026, Shoreview, MN, USA) was placed inside the covered 35×45×50 cm plexiglass box. An aerosol particle counter (TSI PortaCount 8048) was connected to a 135 cm long sampling tubing inside the box. To measure baseline particle clearance without vacuum applied, we created a stabilised elevated particle count (2.5–6×104 particles cm−3); the particle generator was then turned off and particle count data sampled at 15 s intervals in technical replicates. For active aerosol removal, the vacuum source was turned on at the moment when the particle generator was turned off. Normalised counts were fit as exponential decays (r 2>0.95, Matlab, Natick, MA, USA) and half-lives analysed by one-way analysis of variance (anova) (Prism 7, GraphPad Software, San Diego, CA, USA) with significance set to P<0.05 and Tukey's post hoc pairwise comparisons test.
The 3.4 min half-life baseline aerosol clearance was reduced to 1.0 min with the wall vacuum, 0.28 min with the 23 CFM vacuum, and 0.14 min with the 60 CFM vacuum (Fig. 1c, one-way anova, F(3,9)=52, overall P<0.0001). The two stand-alone vacuum configurations were not statistically distinguishable (P=0.97), though clearance half-lives for each vacuum were shorter than with no vacuum (Fig. 1d, ANOVA post hoc Tukey's test: P=0.001 for passive vs wall suction, P<0.0001 for passive vs 60 CFM, P<0.0001 for passive vs 23 CFM).
We applied a vacuum and viral filter to an enclosed intubation box and determined aerosol clearance times in order to establish parameters for time-to-removal after use. Enclosed boxes with vacuums capable of filtering SARS-CoV-2 dispersed during AGPs are likely safer compared with intubation boxes open to the room. The National Institute of Occupational Safety and Health (NIOSH) ‘hierarchy of controls’ prioritises engineering and administrative controls over personal protective equipment (PPE) for mitigating occupational hazards, and PPE is considered the least effective (albeit indispensable) control.8 Although we promote this engineering control, proper PPE is still recommended despite any additional benefits offered by our system.
The Occupational Safety and Health Administration (OSHA) recommends US operating rooms maintain a minimum of 15 air changes per hour, equivalent to 99% aerosol removal in 18 min.9 Both 23 and 60 CFM vacuum pumps reached 99% clearance of the box in 90 s, and likely reduce collateral contamination of other operating room equipment. The reusable 23 CFM vacuum costs $20, and could save several hundred dollars in operating room time per use.10 Our hospital wall suction significantly reduced clearance times also, but flow rates for wall suction are not routinely controllable nor determinable in clinical practice, precluding broad extrapolation. Aerosol levels outside the box were not assessed, but gases suctioned through a viral filter with 99.9999% efficiency exceed recommended air quality regulations. For longer procedures necessitating aerosol removal, ear plugs should be used and pressures considered.11 Improvements towards lightweight barriers, disposable barriers, or both combining various features can be readily envisioned. Our design may afford improvements in proceduralist mobility restrictions and emergency access to patients, though further testing is warranted to verify patient safety.5 Improvements in control of perioperative inhalational risk may be an unexpected lasting impact of the COVID-19 pandemic, in the same way that universal precautions emerged from the HIV epidemic.
Authors' contributions
Concept and design: TI, SH, GC
Acquisition, analysis, and interpretation of data: TI, SH
Drafting of the manuscript: TI, SH
Critical revision of the manuscript: all authors
Acknowledgements
We are grateful for the assistance of the following members of Memorial Sloan Kettering Cancer Center: Ying Zhen for access to instruments, Alexander Tanchoco for helpful discussions, Craig Goulbourne for construction and design input in assembling intubation boxes, and Gregory W. Fischer for departmental support. No compensation was provided for their assistance of this project.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
National Cancer Institute Cancer Center Support Grant P30CA008748, Department of Anesthesiology and Critical Care Medicine (TI).
References
- 1.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everington K. March 23, 2020. Taiwanese doctor invents device to protect US doctors against coronavirus. Available from: https://www.taiwannews.com.tw/en/news/3902435. [Google Scholar]
- 3.Ortega R., Nozari A., Canelli R. More on barrier enclosure during endotracheal intubation. Reply N Engl J Med. 2020;382:e69. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalli J., Khan M.F., Marsh B., Nolan K., Cahill R.A. Evaluating intubation boxes for airway management. Br J Anaesth. 2020;125:e295–e297. doi: 10.1016/j.bja.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cubillos J., Querney J., Rankin A., Moore J., Armstrong K. A multipurpose portable negative air flow isolation chamber for aerosol-generating procedures during the COVID-19 pandemic. Br J Anaesth Adv. 2020 doi: 10.1016/j.bja.2020.04.059. Access published on April 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MSKCC COVID Safety Innovations Team . 2020. Intubation-extubation boxes v3: thinking inside the box.https://www.mskcc.org/clinical-trials-updates/msk-covid-19-innovation-hub/covid-19-safety-innovations-intubation-extubation-boxes Available from: [Google Scholar]
- 7.Anesthesia Patient Safety Foundation . 2020. Breathing circuit filters by recommended application –for use with anesthesia machine breathing circuits.https://www.apsf.org/wp-content/uploads/patient-safety-resources/covid-19/Breathing-Circuit-Filters.pdf Available from: [Google Scholar]
- 8.National Institute for Occupational Safety and Health National Institute for Occupational Safety and Health . 2015. Hierarchy of controls.https://www.cdc.gov/niosh/topics/hierarchy/default.html Available from: [Google Scholar]
- 9.Centers for Disease Control and Prevention NCfEaZIDN, Division of Healthcare Quality Promotion (DHQP) 2003. Guidelines for environmental infection control in health-care facilities.https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html July 22, 2019. Available from: [Google Scholar]
- 10.Childers C.P., Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153 doi: 10.1001/jamasurg.2017.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chahal A., Van Dewark K., Gooch R., Fukushima E., Hudson Z.M. A rapidly deployable negative pressure enclosure for aerosol-generating medical procedures. medRxiv Adv Access. 2020 doi: 10.1101/2020.04.14.20063958. published on April 21, 2020. [DOI] [Google Scholar]