Abstract
Biological systems overwhelmingly comprise charged entities generating electrical activity that can have significant impact on biological structure and function. This intrinsic bio-electrical activity can also be harnessed for overcoming the tissue matrix and cell membrane barriers, which have been outstanding challenges for targeted drug delivery, by using rationally designed cationic carriers. The weak and reversible long-range electrostatic interactions with fixed negatively charged groups facilitate electro-diffusive transport of cationic therapeutics through full-tissue thickness to effectively reach intra-tissue, cellular, and intracellular target sites. This article presents a perspective on the promise of using rationally designed cationic biomaterials in targeted drug delivery, the underlying charge-based mechanisms, and bio-transport phenomena while addressing outstanding concerns around toxicity and methods to mitigate them. We also discuss electrically charged drugs that are currently being evaluated in clinical trials and identify areas of further development that have the potential to usher in new treatments.
Keywords: drug delivery, electrostatic interactions, cell-penetrating peptides, cationic drug carriers, multilevel targeting, cytotoxicity
Introduction
Our growing understanding of disease pathogenesis at molecular, genetic, cellular, and tissue levels is paving way for the discovery of an increasing number of therapeutic targets. This provides the opportunity and a critical need to develop systems that can enable effective local delivery of biological cargo to target tissue, cellular, and nuclear sites with specificity.1 Functional delivery systems are increasingly becoming part of the drug development process rather than being viewed as product enhancements.
Biological systems overwhelmingly comprise electrically charged entities, including tissues consisting of fixed negatively charged proteoglycans (PGs), cells with negatively charged glycocalyx, phospholipid cell and nuclear bilayer membranes, and several types of charged macromolecules, proteins, amino acids, and electrolytes. The fixed electrically charged regions within a tissue or cell generate short-range local electric potentials across boundaries of adjacent tissues (or within tissues) of varying chemical composition or across the cell membrane.2 These potential differences, existing over nano-length scales, produce strong local electrical fields, and the resulting transport of mobile charges creates electric currents in the microenvironment. For example, bioelectric resting potential across a cell membrane is about 50 mV.3 Cartilage tissue contains a high density of negatively charged aggrecan, comprising of sulfated glycosaminoglycans (GAGs), that generates a similar magnitude of electric potential drop at the synovial fluid-cartilage interface over nanometer length scales, creating strong inward pointing electric fields of 106–107 V/m, equivalent to that required for dielectric breakdown of air.2
These bio-electrical phenomena have very significant impacts on both biological structure and function. For example, negatively charged PGs in tissues provide hydration, swelling pressures, compressive stiffness, and signal transduction. Aggrecans in cartilage hold water and resist compressive loading due to electrostatic repulsion between negative charges as they come closer. Small PGs such as decorin, biglycan, fibromodulin, and lumican are closely associated with collagen fibrils and are believed to regulate collagen fibril diameter.4 At the cell membrane, PGs stabilize ligand-receptor binding, triggering signaling complexes that regulate cell proliferation, migration, matrix adhesion, and endocytosis.4 Disruptions to this micro-electrical environment can, therefore, impair active transport of biomolecules (both direction and speed), protein conformational changes, signaling pathways, and downstream transcriptional changes, leading to complex degenerative diseases.5
This intrinsic bio-electrical activity can also be harnessed for overcoming the tissue matrix and cell membrane barriers, which have been outstanding challenges for targeted drug delivery, by using rationally designed cationic peptide carriers (CPCs), protein-, lipid-, and polymer-based drug carriers.6–9 Their weak and reversible long-range electrostatic interactions (that persist over nanometer length scales)10,11 with fixed negatively charged groups facilitate their electro-diffusive transport through the full-tissue thickness to reach intra-tissue and intracellular target sites. While incorporating positive charges is the underlying design mechanism, carrier specificity can be further tuned based on the net fixed charge density (FCD) of the target tissue, state of disease, where cell targets reside, and the physicochemical properties of the relevant drug.6 Recent advances have brought the potential of bioelectricity in targeted drug delivery to the forefront, and increased research and clinical interest in the field as in the case of cell penetrating peptides (CPPs) that interact with negatively charged GAG moieties on the plasma membrane to enhance their uptake across the membrane barrier.
Here, we review the mechanisms underlying charge-based bio-transport and their applications to achieve multilevel targeting in the tissue, cellular, and nuclear domains (Fig. 1). We explore research advances and discuss outstanding questions, including those around toxicity concerns and methods to mitigate them. We also discuss a few charged materials that are being evaluated in clinical trials and identify areas of further development of charged delivery systems that have the potential to usher in treatments for a set of largely unaddressed diseases.
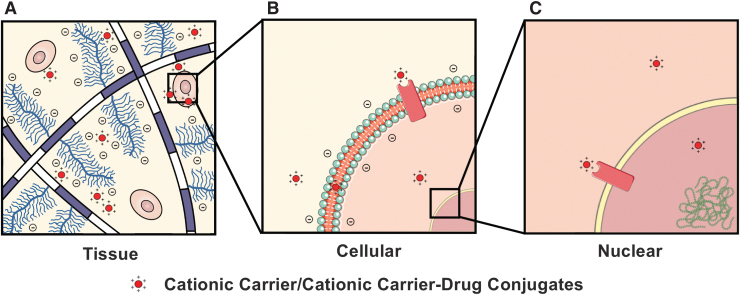
FIG. 1.
Cationic carrier or carrier-drug conjugates can interact with negatively charged fixed intra-tissue and cellular moieties, enabling multilevel drug targeting at tissue, cellular, and intracellular levels. (A) Cationic carriers can rapidly penetrate the tissue in high concentrations owing to electrostatic interactions with the high density of negatively charged matrix GAGs, after which they can successively penetrate (B) the cell and (C) the nucleus to reach their target sites via charge-mediated pathways. GAGs, glycosaminoglycans.
Cationic Biomaterials for Drug Delivery at the Tissue, Cellular, and Intracellular Level
Tissue targeting
There exist a number of dense, negatively charged tissues in the body such as the musculoskeletal tissues, including cartilage, meniscus, and intervertebral disks (IVDs), vitreous humor of the eye and mucosal membrane that remain an outstanding challenge in the field of drug delivery. Their degeneration is associated with several common diseases, such as osteoarthritis, back pain, macular degeneration, inflammatory bowel disease, etc., that affect millions of people worldwide. Several of these tissues are also avascular, rendering systemic methods of drug delivery ineffective in reaching intra-tissue cellular sites. In addition, the complex extracellular matrix of such avascular tissues consists of a dense meshwork of collagen fibrils, PGs with highly negatively charged GAG chains, and cells, which hinders transport of locally injected macromolecules, especially if they are anionic.
Despite the existence of chemical entities and biologics that have been shown to inhibit or reverse disease progression in preclinical studies, many degenerative diseases affecting these tissues remain untreated due to a lack of effective drug delivery methods.12 Targeted therapies that can penetrate through the full thickness of these tissues to reach their matrix, cellular, and intracellular target sites and provide sustained drug release can enable the clinical translation of these drugs and address several untreated diseases of avascular, negatively charged tissues.
Recent research has shown that the high negative FCD of tissues provides an opportunity, rather than a challenge, for targeted drug delivery by modifying therapeutics to add optimally charged cationic domains, thus utilizing the weak, reversible nature of electrostatic interactions to enhance their intra-tissue transport, uptake, and retention.2,6,13,14 The concentration of cationic carriers administered locally increases sharply at the interface of negatively charged tissues by a Donnan partitioning factor, K, due to the internal electrical field created by the negative FCD of the tissue (Fig. 2A).2 The enhanced interface concentration of cationic carriers (KC versus C in the bulk bath) induces steep concentration gradients across the tissue thickness, resulting in faster intra-tissue transport rates and higher uptake compared with neutral molecules.2 In fact, positively charged carriers have shown to be uptaken up to 400 times more than neutral versions of the same drug carriers in cartilage.6,7 These cationic carriers also demonstrate increased intra-tissue residence time due to the presence of a high density of negatively charged groups that enable binding with the cationic carriers.
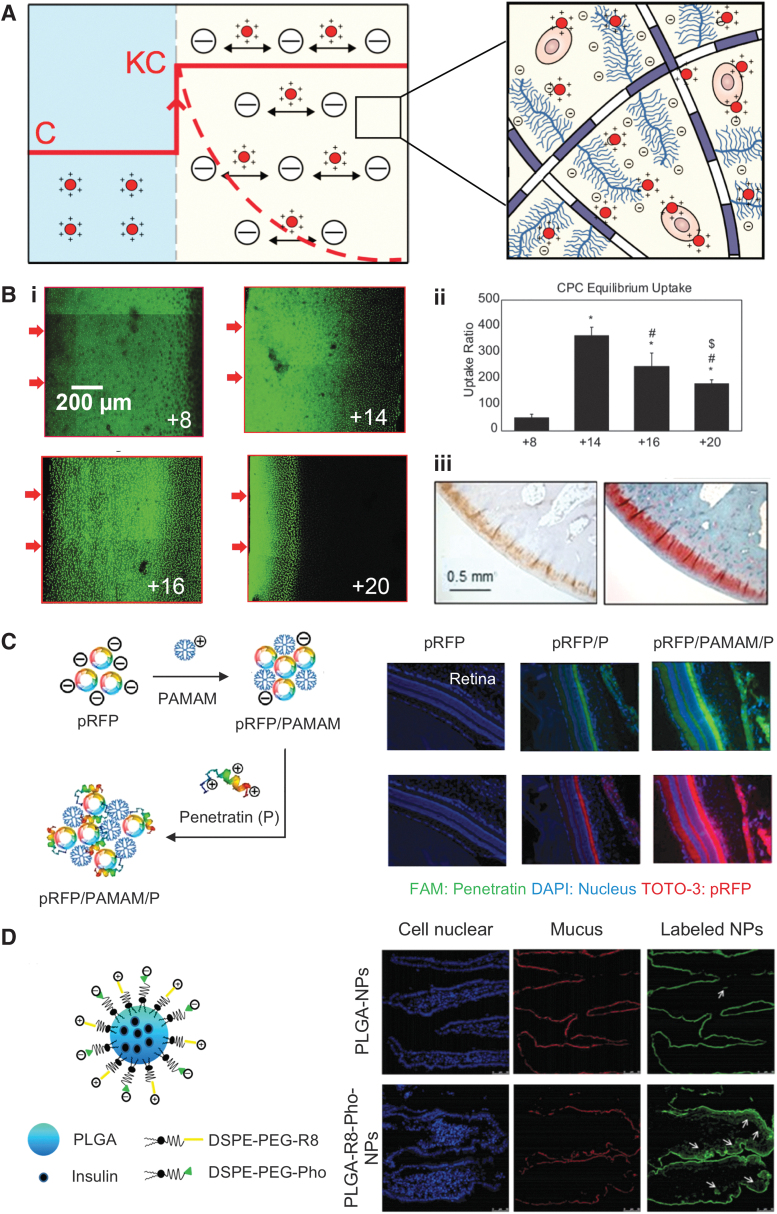
FIG. 2.
(A) Transport mechanism of cationic carriers in negatively charged tissues. Concentration of the cationic carrier is enhanced from C at the administration site to KC at the interface of negatively charged tissue due to Donnan partitioning effect, which increases the intra-tissue concentration gradients, resulting in enhanced electro-diffusive transport and high uptakes. Weak-reversible binding interactions enable carriers to penetrate through the full-tissue thickness to effectively reach their cell targets in sufficient doses. Dashed line represents early initial concentration profile of the cationic carrier across the tissue, whereas solid line across the tissue refers to intra-tissue concentration of cationic carriers after equilibrium has been reached. (B) Targeted drug delivery to cartilage by using optimally charged cationic carriers. (i) Confocal images showing depth of penetration of CPCs in cartilage within 24 h. (ii) Charge-dependent equilibrium uptake of CPCs of various charge (between +8 and +20) in cartilage. Figure adapted with permission from Vedadghavami et al.6 (iii) Immunohistochemical analysis showing presence of Avidin through the full thickness of rabbit articular cartilage even at 3 weeks post-ACLT, which correlates with the spatial GAG density stained by Safranin-O (red). Figure adapted with permission from Bajpayee et al.22 (C) Targeted drug delivery to ocular tissues by using an electrostatically formed complex of pRFP, PAMAM, and penetratin peptide. Fluorescent images of rat retina 4 h after topical administration of the complexes (blue, green, and red represent cell nuclei, penetratin, and pRFP, respectively). Figure adapted with permission from Liu et al.25 (D) Drug delivery across mucosal barrier. Insulin-encapsulated PLGA nanoparticles coupled with cationic R8 and negatively charged Pho, capable of dissociation through digestion with IAP and resulting in a net positive charge. R8 and Pho were coupled to PLGA by using a DSPE-PEG bridge. Fluorescent images of rat intestinal villi post-oral administration (white arrows show absorption of particles in the interior of intestinal villi). Figure adapted with permission from Wu et al.29 ACLT, anterior cruciate ligament transected; CPCs, cationic peptide carriers; DAPI, 4,6-diamidino-2-phenylindole; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino-(poly{ethylene glycol})]; FAM, 5-carboxyfluorescein; IAP, intestinal alkaline phosphatase; NPs, nanoparticles; PAMAM, poly(amidoamine); Pho, phosphoserine; PLGA, poly(lactic-co-glycolic-acid); pRFP, red fluorescent protein plasmid.
Although the positive charge of solutes facilitates tissue penetration in dense, negatively charged, avascular tissues such as cartilage, it has been observed that the effectiveness of tissue penetration is highly dependent on the magnitude of net charge.6,15 Mechanistic transport studies using peptides with different net charges across tissues of varying FCD (cartilage, vitreous of eye, mucin, skin) have shown that an optimal charge can be determined for a peptide carrier to take advantage of Donnan partitioning-induced enhanced transport such that the electrostatic interactions are weak enough so the carriers can rapidly penetrate the tissue superficial zones but strong enough to bind within the deep zones for long-term retention.6,14,16,17
Conversely, carriers with excessive high net positive charge can get trapped at the tissue surface because of the strong binding that will hamper their ability to reversibly bind and reach target sites.6,16 Recently, the effect of net positive charge on the transport of arginine-rich short-length CPCs of varying net charge (between +8 and +20) was investigated in cartilage.6 The depth of penetration of fluorescently labeled CPCs in cartilage was determined by using confocal microscopy. It was noted that CPC +8 rapidly penetrated through full thickness of cartilage due to weak and reversible charge interactions with negatively charged cartilage aggrecans. However, CPC +20 was halted within the cartilage superficial zone because of strong binding resulting from its high net positive charge, impeding its further intra-tissue diffusion (Fig. 2B-i).6 In addition, equilibrium uptake of CPCs in cartilage was measured 24 h after submerging cartilage explants in baths of fluorescently labeled CPCs. The highest uptake was demonstrated by CPC +14 while still maintaining full-tissue thickness penetration (Fig. 2B-ii).6
Transport properties of cationic peptides of varying net charge (between +7 and +23) were also studied in the vitreous humor of the eye, which comprises a meshwork of negatively charged hyaluronic acid. It was discovered that peptides of up to +15 charge effectively diffused through the vitreous humor while increasing the net charge beyond this threshold to +19 and +23, resulting in their complete immobilization in the vitreous humor.16 The effect of length of polyarginine in enhancing penetration of nanostructured lipid carriers across the skin was also investigated by using polyarginines of varying length between 8 and 15 residues. Nanoparticles (NPs) modified with 11-arginine residues (R11) showed the highest intra-skin penetration compared with ones modified by the other peptides.17 Therefore, modulating the net positive charge of a carrier depending on target tissue FCD is an important design criterion for effective tissue targeting, penetration, and uptake.
Cationic carriers are increasingly being utilized for targeted drug delivery to negatively charged tissues. Avidin, a positively charged glycoprotein, due to its optimal size and net charge, demonstrated 200 × higher intra-cartilage uptake and rapid penetration through the full thickness of cartilage after its intra-articular administration into the knee joints of rats and rabbits.18–21 Avidin, when conjugated with an anti-inflammatory drug, dexamethasone (Dex), was found to be present in the full thickness of knee articular cartilage of anterior cruciate ligament transected (ACLT) rabbits even after 2 weeks following its intra-articular injection (Fig. 2B-iii). A single low dose of this cationic conjugate effectively suppressed ACLT-induced joint inflammation and catabolic activity through sustained Dex release from its intra-cartilage drug depot over 3 weeks; its efficacy was significantly greater than the unmodified Dex.22 Recently, multiarm Avidin nano-constructs with enhanced drug loading content have also been developed.23–25
Similarly, poly(amidoamine) (PAMAM) dendrimers were used to locally deliver insulin like growth factor 1 (IGF-1) to rat cartilage.9 Sixth-generation PAMAM dendrimers were partially PEGylated to minimize NP's cytotoxic response. The amines were covalently conjugated to IGF-1, and the remaining amine groups on PAMAM provided the positive charge to facilitate electrostatic interactions with the cartilage matrix. PAMAM conjugation significantly enhanced joint residence time of IGF-1; a single intra-articular injection of PAMAM–IGF-1 provided 30.4 days of drug at therapeutic concentrations, whereas the free IGF-1 injection only provided 2.9 days of effective therapeutic response. Cartilage targeting using cationic carriers via the systemic route has also been explored. Recently, cationic cysteine-dense peptides (CDPs) with at least three reducible disulfide bonds were shown to accumulate in cartilage and IVDs at a higher concentration compared with blood, muscle, and liver after its systemic administration in rats whereas uncharged neutral CDPs did not, further reinforcing the importance of charge interactions in targeting negatively charged tissues.26 The cysteine-knotted folds preserved the peptide from proteolytic degradation. The CDP was then conjugated to triamcinolone acetonide by using a hydrolysable dimethyl adipic acid for selective targeting to joint tissues on intravenous injection. The conjugate accumulated at 11 × and 33 × higher concentrations in knee cartilage and IVDs, respectively, compared with the free drug and significantly reduced the ankle diameter in a rat arthritic joint within 24 hours after the first dosing.
The first identified CPP, trans-acting transcriptional activator (TAT), isolated from HIV, has been applied to a number of drug delivery systems since its discovery. This cationic CPP has been used to enhance penetration of PEG-poly(lactic-co-glycolic-acid) (PLGA) NPs through the ocular barrier after topical administration.27 The NP was further modified with an integrin αvβ3 specific binding peptide to selectively target choroidal neovascularization (CNV). The dual modified NPs demonstrated superior corneal penetration (5.5 × higher than unmodified NPs) and specific targeting toward CNV. A gene delivery system using a combination of 3rd-generation PAMAM and penetratin peptide was developed for reaching posterior eye tissues.28 Complexes for gene delivery were formed by using the anionic red fluorescent protein plasmid (pRFP) gene model with PAMAM and penetratin (P) (pRFP/PAMAM/P) using electrostatic interactions and were administered topically in rat eyes. Free pRFP failed to reach the retina 4 hours post topical administration, whereas pRFP/P complex was able to penetrate into the retina. Further, the ultimate pRFP/PAMAM/P complex demonstrated higher accumulation in the retina compared with pRFP/P (Fig. 2C).
In addition, PLGA NPs capable of reversing their net charge from net neutral to positive have been developed to overcome both mucosal and epithelium barriers to enable oral delivery of insulin.29 Insulin encapsulated PLGA NPs were functionalized with octa-arginine (R8) and dissociable negatively charged phosphoserine (Pho) to form PLGA-R8-Pho NPs. The initial neutral charge of the systems allows rapid penetration through negatively charged mucus barrier. Thereafter, in presence of the epithelium, intestinal alkaline phosphatase cleaves the negatively charged Pho groups, giving the NP an overall positive charged that enhances epithelium uptake of the NPs. This charged-reversal strategy significantly enhanced penetration of PLGA-R8-Pho through the mucosal and epithelium barriers into the bloodstream compared with PLGA NPs (Fig. 2D).
Cell targeting
The negatively charged, GAG-containing glycocalyx and the anionic phospholipid bilayer of cell membranes serve as the first barriers to cellular entry by especially repelling negatively charged, hydrophobic, or large-molecular-weight solutes. This leads to poor intracellular targeting and uptake of therapeutics.30 Cationic CPPs utilize electrostatic interactions to overcome the cell membrane barrier and facilitate delivery of various cargoes to the cytoplasm and nuclear sites via direct penetration, endocytosis, or via transitory modification of structure to form inverted micelles (Fig. 3).31 Cationic solutes can interact electrostatically with the negatively charged plasma membrane, temporarily disrupting it and forming transient pores to facilitate direct penetration and cellular uptake.32,33 Peptides with amphipathic properties (containing both hydrophobic and charged regions) can help temporarily disrupt the bilayer to further promote direct penetration into the cell.34 CPPs, particularly regions with polyarginine residues, exhibit strong binding affinity to negatively charged PGs, especially with heparin sulfates in the glycocalyx, leading to aggregation of these drug carriers in high concentrations, facilitating rapid cellular uptake via receptor-mediated or nonspecific endocytosis.35–37
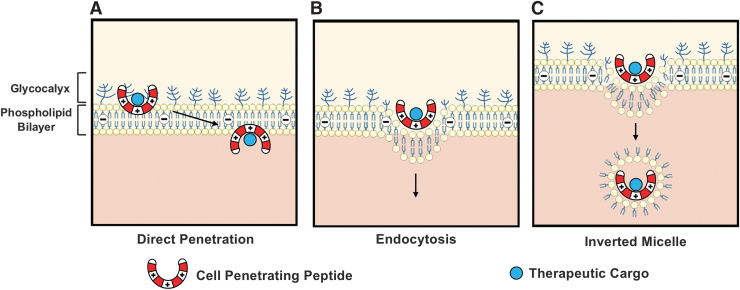
FIG. 3.
Mechanisms facilitating cell membrane translocation of cell-penetrating peptides: (A) direct penetration, (B) receptor-mediated or nonspecific endocytosis, and (C) formation of transitory structures such as inverted micelles.
For example, internalization of arginine-rich CPPs such as TAT happens through macropinocytosis, a nonspecific form of fluid-phase endocytosis where large wavy cellular extensions trap extracellular solutes and bring them into the interior of the cell.38 Finally, cationic peptides (or other types of carriers) can also achieve membrane translocation through the formation of transitory structures within the membrane, specifically inverted micelles. Biologically, micelles are single-layer phospholipid membranes, typically oriented with the hydrophilic phospholipid heads on the outside, associating with the polar solvent, whereas the hydrophobic tails are shielded within the structure. Cationic CPPs associate with negatively charged phospholipids through electrostatic interactions and form an intracellular vesicle with the negatively charged hydrophilic phospholipid heads surrounding the positively charged CPP, and the nonpolar hydrophobic lipid tails pointing outward (inverted micelles), allowing the CPP to remain in a hydrophilic environment as they enter the cell.39,40
Nucleus targeting
The cell nucleus represents a critical biological target in the delivery of a number of therapeutics. Many anti-cancer drugs exert cytotoxic effects by inhibiting the unwrapping and transcription of DNA, thus preventing replication and leading to cell death.41–43 In addition, gene therapy and genome editing have the potential to address countless genetically linked diseases and conditions.44 Therefore, efficient delivery of therapeutics to the nucleus remains a major area of interest. However, unmodified drugs exhibit relatively low transport efficiency into the nucleus, limiting their therapeutic effect.45 As such, several peptide-based drug delivery systems have been developed to specifically target the cell nucleus and subnuclear regions.46–48 Cationic CPPs display a similar ability to translocate across the nucleus envelope, comprising a two phospholipid bilayer membrane, and several sequences have been identified that display affinity for nuclear and subnuclear targets.49
For example, portions of the cationic antimicrobial peptide 18 were shown to exhibit high cellular internalization and accumulation within nuclei. Further, when conjugated to the anti-cancer agent doxorubicin, which interferes with DNA transcription, the CAP18 conjugate showed greater cytotoxicity than the control, indicating successful delivery to the nucleus.50 In a similar study, a highly basic nuclear factor kappa-light-chain-enhancer of activated B cells peptide, CB5005, displayed effective cellular internalization and accumulation within the nuclei of human glioma cells and tumor spheroids. In addition, in vivo, CB5005 was conjugated to doxorubicin, resulting in reduced tumor volume and initiating apoptosis in a glioma-bearing mouse model.51 Cationic co-polypeptides combining various ratios of histidine-rich peptide, a known CPP, and nuclear localization sequences have been developed for gene therapy, demonstrating significant cellular uptake, nuclear localization, and successful delivery of small interfering RNA to the nucleus.52
Undesired Biological Outcomes of Cationic Carriers
Despite several advantages that cationic biomaterials offer in multilevel drug targeting to penetrate the tissue and successively reach its tissue, cellular, and nuclear target sites, their long-term biological activity largely remains unknown. Concerns around their side-effects associated with the use of high doses persist and need to be addressed.
At the tissue level, high cationic charge of biomaterials can cause charge shielding, reducing the electrostatic repulsion effect between negatively charged PGs and thereby reducing intra-tissue swelling pressures (Fig. 4A).53 This can lead to decreased water content and some loss of PGs, which can, subsequently, affect organization of collagen fibrils, intra- and intermolecular crosslinks, interaction of PGs with extracellular matrix proteins such as collagen, as well as with cells, leading to altered downstream signaling and changes at sub-cellular levels. These effects are, however, highly dose dependent. For example, a cationic glycoprotein, Avidin, an effective drug carrier for intra-cartilage targeting,7,23,24 was shown to cause GAG loss only at the very high concentration of 100 μM (which is two to three orders of magnitude higher than that used for drug delivery).18,22 Even at this high dose, it did not cause any chondrotoxicity or changes in biosynthesis rates of proteins and PGs.18 Similarly, recent work on cartilage penetrating cationic peptides showed small but measurable changes in tissue Donnan osmotic swelling pressures but only at very high peptide concentrations.6 The change in swelling pressure was insignificant and did not cause any GAG loss.6,18 In addition, no effects on cell viability or biosynthesis were observed.
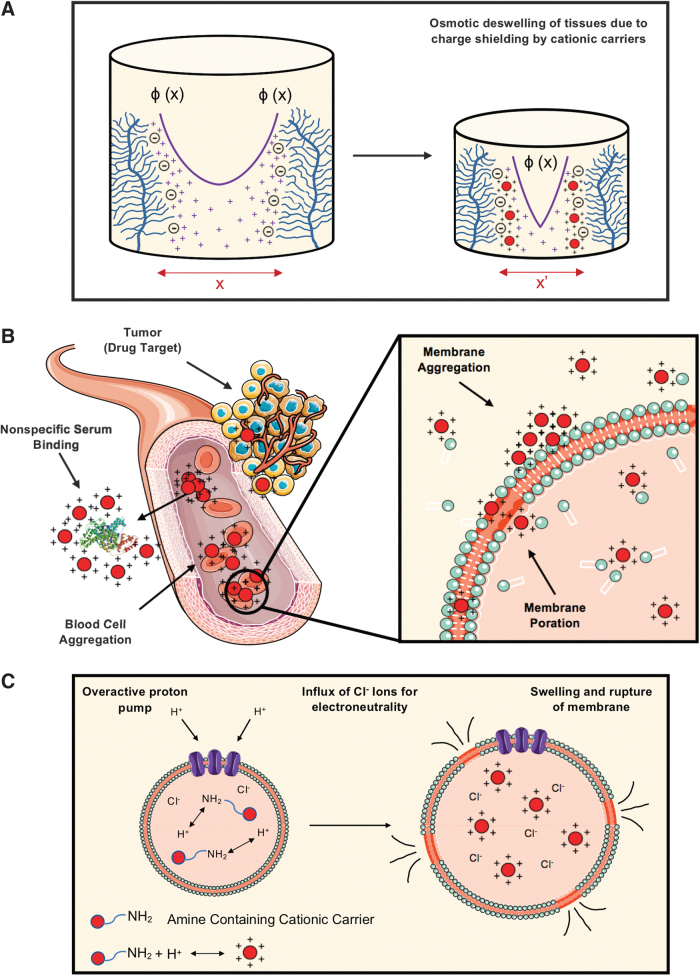
FIG. 4.
Undesired bioactivity of cationic carriers. (A) High concentrations of cationic carriers can shield the intra-tissue electrostatic repulsion between fixed negatively charged groups. Highly positively charged carriers can displace the sodium ions (Na+) to create a higher density of positive charges near the fixed negative groups, thereby resulting in a steeper exponential drop of electric potential, ɸ (x) and reducing the spacing between the negatively charged groups (x′ < x) causing osmotic deswelling of tissues. (B) Undesired nonspecific binding of cationic drug carriers with serum proteins and blood cells, resulting in formation of aggregates and hindering their delivery to desired target sites. These aggregates can also disrupt the phospholipid cell membrane and facilitate poration. (C) Excessive pumping of protons to help ionization of amines after intracellular uptake of highly charged cationic carriers results in an excessive inward flow of chloride ions (Cl−) and water molecules to maintain charge electroneutrality in the endosome (proton-sponge theory), resulting in osmotic swelling and rupture of the endosome.
Similarly, cationic CPPs such as TAT and penetratin have shown only minor effects on cell proliferation and membrane integrity at concentrations up to 50 μM.54 Transcriptomic studies have also indicated insignificant genetic alterations with TAT in a variety of cell lines.55,56 High doses of octa-arginine, however, were found to upregulate several immune-related genes in a macrophage cell line.57 Increasing amphiphilicity of peptides, on the other hand, can cause membrane disruption and changes in cellular metabolism at much lower doses due to the increased ability of highly amphipathic peptides to penetrate the cell membrane.58 This principle has also been confirmed by recent proteomics studies.59
When administered in the circulatory system or close to the target organ, heavily charged cationic biomaterials can interact with negatively charged serum proteins such as albumin60 and red blood cells, precipitate in large clusters, and adhere to the cell surface. This can destabilize the cell membrane and induce immediate toxicity, including eliciting an immune response.61 Once out of circulation, if used in high doses, cationic drug carriers can aggregate in cell glycocalyx regions with high concentrations of PGs, causing poration of cellular membranes (Fig. 4B).62 In addition, opsonization of cationic liposomes by serum proteins increases their uptake by macrophages of the reticuloendothelial system (mainly in liver and spleen), resulting in rapid clearance of the cationic liposomes from blood circulation post-systemic administration.63,64 PEGylation has been used for enhancing circulation time of cationic liposomes by providing a hydrophilic shield surrounding the liposome, which prevents opsonization and clearance of the cationic liposomes.65,66
After cellular uptake and on reaching the lysosomal compartment, the amine group on the material surface could also perturb proton pump activity.67 The proton sponge theory suggests that the high H+ buffering capacity of titratable amines could interfere in endosomal acidification, which can lead to exaggerated proton pump activity, osmotic swelling, and, ultimately, the rupture of the endosome (Fig. 4C), causing a reduced rate of mitosis and vacuolization of the cytoplasm.68 A recent study showed that high doses of positively charged liposomes and nanocarriers synthesized from polymer polyethyleneimine and chitosan competitively interacted with Na+/K+-ATPase cationic binding sites, resulting in overload of Na+/K+ in cells and causing cytoplasm swelling. This resulted in cell necrosis and leakage of mitochondrial DNA and other damage-associated molecular patterns, triggering severe inflammatory response.69
Modification Methods to Overcome Toxicity Concerns and Improve Target Specificity
In addition to controlling the dose, the net charge and its spatial distribution on a biomaterial must also be optimized to ensure that targeting benefits outweigh toxicity concerns. In the past few years, new modification techniques for commonly used cationic biomaterials and delivery systems have been devised to overcome this problem. For example, PAMAM dendrimers have shown generation, surface charge, and concentration-dependent toxicity.9,70 To overcome this, PEGylation has been used as a strategy to partially shield cationic charge to minimize cytotoxic effects while not hindering its tissue or cell targeting effectiveness.9,23,24,71 Other methods utilize the incorporation of acid-labile linkers within large-molecular-weight polymers to allow for rapid degradation into low-molecular-weight structures in acidic endosomes, which has shown to significantly reduce toxicity.72 Disulfide bonds can also be incorporated in polymer or peptide designs to enable easy cellular reduction and enhance cellular transfection while minimizing cytotoxicity.73 To avoid cationic carriers from associating with negatively charged serum proteins, spatially distributing the positive charge along the peptide sequence has been investigated. This approach involves using neutral amino acids as spacers without affecting the net charge, or importing a heterocyclic ring such as imidazolium, guanidinium, or pyridinium as a substitution for the linear amine head group in vector design to help spread the positive charge of the headgroup.61,74
Charge-based binding can be further stabilized by utilizing synergistic effects of short-range hydrogen bonds and hydrophobic interactions that are strongest over angstrom length scales, as demonstrated recently by arginine-rich cartilage-penetrating peptide carriers that showed enhanced retention in arthritic cartilage with significantly reduced negative FCD.6,75 The specificity of cationic carriers can be further improved by incorporating specific cell receptors or matrix binding motifs.76–78 Their cellular uptake and delivery of drug can be controlled by using activatable linkers that are responsive to their microenvironment. This approach has mainly been used in tumor-affected tissues where the microenvironment has upregulated proteases, acidic pH, lower membrane potential, and hypoxia, including in a breast cancer model where the cleavage of the linker allowed the activation of the polycationic CPP.79–81 For example, a 54 amino acid, lysine-rich CPP known as KRP was screened out of phage display for its high affinity to CD105, or endoglin, an accessory receptor for transforming growth factor-β that is upregulated in rapidly proliferating endothelial cells. KRP's hydrophilicity, basicity, large molar mass, and affinity for cancerous endothelial cells allow for selective targeting of the tumor and its acidic microenvironment, and improved cellular uptake due to the enhanced permeation and retention effect. KRP-doxorubicin conjugates were shown to exhibit significantly stronger tumor selectively, cellular internalization, and anti-tumor properties in human osteosarcoma cells compared with a noncancerous control cell line.82
In a second study, a novel, polyarginine CPP named RIPL was rationally designed as a homing peptide for hepsin, an extracellular protease overexpressed in numerous cancer cells. By exploiting the upregulation of proteases, acidic pH, and lower membrane potential of the tumor microenvironment, cationic RIPL-liposomes were able to effectively target and enhance intracellular drug delivery to hepsin-expressing cell lines.83 In addition, RIPL-liposome-docetaxel conjugates displayed significantly greater intracellular drug delivery in hepsin-expressing human ovarian and prostate cancer cells, while inhibiting tumor growth in an in vivo model of ovarian cancer.84 These studies indicate that CPP sequences can be fine-tuned to target specific tumor cell lines by harnessing the properties of the tumor microenvironment, displaying their potential for delivering chemotherapeutics with higher specificity.
Clinical Translation
The promising preclinical data highlighting the successful application of cationic carriers in targeted therapies have resulted in their clinical translation for treatment of various diseases (Table 1). Cationic liposomes have gained attention as a promising candidate for targeted drug delivery for cancer treatment due to their preferential binding with angiogenic blood vessels compared with normal blood vessels.85 The lack of glycocalyx barrier on the surface of tumor endothelia exposes the negatively charged phospholipid bilayer of the epithelium, facilitating preferential binding and internalization of the cationic liposomes through electrostatic interactions.86 In addition, the acidic microenvironment of the tumors87 can further ionize cationic liposomes, making them more positively charged and resulting in enhanced selective uptake.
Table 1.
Cationic Carriers in Clinical Trials
| Compound | Cationic carrier | Therapeutic cargo | Company | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| EndoTAG™-1 | Cationic liposome | Paclitaxel | SynCore Biotechnology | NCT00377936 |
| NCT00448305 | ||||
| NCT00542048 | ||||
| NCT01537536 | ||||
| NCT03002103 | ||||
| NCT03126435 | ||||
| SGT-53 | Cationic liposome | p53 | SynerGene Therapeutics | NCT00470613 |
| NCT02340117 | ||||
| NCT02340156 | ||||
| NCT02354547 | ||||
| KAI-9803 | TAT | δ-protein kinase C inhibitor peptide | KAI Pharmaceuticals | NCT00093197 |
| NCT00785954 | ||||
| XG-102 | TAT | c-Jun N-terminal kinase inhibitor peptide | Xigen | NCT01570205 |
| NCT02235272 | ||||
| NCT02508337 | ||||
| AM-111 | TAT | c-Jun N-terminal kinase inhibitor peptide | Auris Medical | NCT00802425 |
| NCT02561091 | ||||
| NCT02809118 | ||||
| RT001 | TAT derivative | Botulinum toxin type A | Revance Therapeutics | NCT00968942 |
| NCT00888914 | ||||
| NCT01064518 | ||||
| NCT01124552 | ||||
| PsorBan | R7 | Cyclosporine A | Cell Gate | N/A |
| AZX100 | PTD-4 | AZX100 | Capstone Therapeutics | NCT00811577 |
| NCT00825916 | ||||
| NCT00892723 |
PTD, protein transduction domain; TAT, trans-acting transcriptional activator.
EndoTAG™-1 (SynCore Biotechnology) is a cationic liposomal formulation encapsulating the chemotherapeutic paclitaxel, which has advanced to clinical studies for targeted cancer treatment.88 By exploiting the negatively charged tumor microenvironment, these positively charged drug carriers bind preferentially to actively dividing endothelial cells and deliver chemotherapeutics that prevent angiogenesis and inhibit tumor growth. In a phase I trial, EndoTAG-1 was generally well tolerated.89 Combined administration of EndoTAG-1 and a standard chemotherapy drug increased the survival rate of patients with advanced pancreatic cancer86 and advanced triple-negative breast cancer (TNBC)90 in separate randomized controlled phase II clinical trials. Efficacy of EndoTAG-1 combined with other chemotherapy agents in patients with visceral metastatic TNBC is currently under investigation in a phase III clinical trial.91
A similar cationic liposomal drug carrier for cancer therapeutics, SGT-53 (SynerGene Therapeutics), encapsulates wild-type p53 and features surface transferrin receptor antibodies as a targeting moiety. Loss of the tumor suppressor gene p53 is implicated in a vast majority of human cancers; therefore, this gene therapy technique attempts to restore function of p53, which improves tumor sensitivity to radiation and chemotherapeutics.92 Transferrin receptor levels are correlated to cell proliferation rate and are greater in actively dividing cancer cells. Cationic SGT-53 actively targets tumors undergoing angiogenesis by harnessing the overexpression of transferrin receptors, basic tumor microenvironment, and decreased membrane potential to achieve intracellular delivery of p53. In a phase Ib trial, combined SGT-53 and docetaxel was well tolerated after systematic administration, with only 2 out of 14 patients experiencing the dose-limiting toxicity of neutropenia.93 Three patients experienced partial response with significant tumor shrinkage, whereas another two patients showed no further disease progression.94
Several cationic peptide-based drug delivery systems, mainly consisting of the TAT peptide, have also advanced to clinical studies. KAI-9803 (KAI Pharmaceuticals), a δ-protein kinase C inhibitor peptide conjugated to TAT peptide, was used to restore blood flow after myocardial infarction.95,96 In vivo studies showed that TAT peptide enabled enhanced, rapid penetration of KAI-9803 in tissues such as heart, kidney, and liver on intravenous injection in rats compared with the cargo peptide and also showed superior internalization in cardiomyocyte cells in vitro.97 In a phase I/II trial, KAI-9803 demonstrated a safe and tolerable profile in a dose-escalated study when injected intra-coronarily during angioplasty.96 Although the study suggested improvement trends compared with the concurrent placebo, the study was not powered to conclude efficacy of the treatment using biomarkers.98 In a follow-up phase II clinical trial, KAI-9803 failed to reduce infarct size in patients with ST elevation myocardial infarction through intravenous injection.99 It remains unclear whether change in route of administration, choice of patients, sample size, or ineffectiveness of the treatment contributed to the contradictory results.
XG-102 (Xigen), a TAT coupled to a c-Jun N-terminal kinase inhibitory peptide, was designed with the goal of reducing ocular inflammation. XG-102 has shown to accumulate in various parts of ocular tissues, including iris epithelium, retinal pigment epithelium, and neural retina, upon intravitreal injection.100 Safety and tolerability of XG-102 was confirmed by using intravenous injection in a dose-escalating phase I clinical trial in healthy individuals101 and upon subconjunctival injection in patients with intraocular inflammation in a phase Ib study.102 In a phase II randomized, double-blind, parallel-group, controlled, multicenter trial, a single-dose subconjunctival injection of XG-102 was shown to be noninferior to repeated Dex eye drop administration for the treatment of postoperative ocular inflammation within 28 days post-surgery.103 The same peptide electrostatically formulated in a hyaluronic acid gel has also been investigated for prevention of acute inner ear hearing loss under AM-111 (Auris Medical), which has received Fast Track Designation by the Food and Drug Administration.104 The TAT peptide enables accumulation of AM-111 in cochlear hair cells and neurons, whereas the therapeutic cargo prevents the cell apoptosis initiated by extreme noise exposure.105 Intratympanic administration of AM-111 demonstrated safety and promising improvement in hearing of patients with severe-to-profound acute sensorineural hearing loss in a double-blind, randomized, placebo-controlled phase II study.106 Further, in a phase III study, significant treatment effect of AM-111 was confirmed in patients with profound idiopathic sudden sensorineural hearing loss.107
RT001 (Revance Therapeutics, Inc.), a topical gel formulation for the treatment of facial wrinkles, was able to reach phase III clinical trials.108 RT001 is composed of 150 kDa botulinum toxin type A (BoNTA) and a cationic peptide that is a reverse sequence of basic residues of TAT.109 The peptide electrostatically binds to BoNTA such that its protein transduction domains (PTDs) are directed outward to attach to cell membranes.110 Phase II clinical trials were promising; however, in phase III clinical trials, RT001 did not achieve its co-primary endpoints, and as a result, Revance Therapeutics has developed an injectable formulation for future clinical trials.110
Cationic peptides other than TAT have also advanced to clinical studies. PsorBan (Cell Gate, Inc.) consisting of cyclosporine A conjugated to a polyarginine carrier was developed for treatment of psoriasis through topical administration.111 Phases I and IIa clinical trials of PsorBan demonstrated effective transdermal penetration of the conjugate and potential benefit in treatment of mild-to-moderate psoriasis.111 However, future clinical trials were terminated, as the release rate of the cyclosporine drug was not fast enough to compete with clearance despite efficient uptake.111 Capstone Therapeutics developed AZX100 for treatment of dermal scars resulting from surgical operations.112,113 AZX100 comprises phosphorylated peptide analog of heat shock protein 20 coupled to PTD-4 peptide (YARAAARQARA), an alanine-substituted TAT sequence.78,114 However, AZX100 was later not pursued owing to financial reasons.115 The safety and tolerability of the cationic carriers as demonstrated in these clinical trials emphasizes that using cationic carriers for drug delivery applications is practical if the dosing needed to induce an effective biological response remains below the cytotoxic concentration of the carrier.
Conclusion
The increasing number of cationic carrier-drug conjugates that are reaching the clinical trial stage is a testament to the promise they hold in translating targeted therapies naturally aided by the overwhelming number of negatively charged entities in our bodies. These negative charges play critical structural and functional roles but can also be harnessed for targeted drug delivery. The associated cytotoxicity concerns can be minimized through rational design of carriers. Methods include incorporating just enough charge on the carrier for effective tissue, cellular, or intracellular drug delivery; incorporating structure modifications that include PEGylation, spatial charge distribution, and the use of reducible or acid labile linkers for easy breakdown; and controlling dose. By utilizing the body's internal electric fields and incorporating rational design, bio-electroceuticals with reversible, tunable, and dynamic properties are becoming an exciting new direction of research and clinical work, with the promise of translating targeted therapies for treatment of several unaddressed diseases.
Authors' Contributions
All authors contributed toward the research, drafting, and revising of the article, and figure creation. The final version of the article was reviewed and approved by all authors. This article has been submitted solely to this journal and is not published, in press, or submitted elsewhere. The responsible authors for the integrity of this article are: C.C.Y., A.V., and A.G.B.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the United States Department of Defense through the Congressionally Directed Medical Research Programs under contract W81XWH-17-1-0085, National Institutes of Health (NIH) R03 EB025903-1, and NIH Trailblazer R21 EB028385-01.
References
- 1.Škalko-Basnet N. Biologics: The role of delivery systems in improved therapy. Biol Targets Ther 2014;8:107–114. DOI: 10.2147/BTT.S38387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: Can electrostatic interactions help? Nat Rev Rheumatol 2017;13:183–193. DOI: 10.1038/nrrheum.2016.210 [DOI] [PubMed] [Google Scholar]
- 3.Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol 1987;385:207–242. DOI: 10.1113/jphysiol.1987.sp016492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon JH, Halper J. Tendon proteoglycans: Biochemistry and function. J Musculoskelet Neuronal Interact 2005;5:22–34 [PubMed] [Google Scholar]
- 5.Fadeel B, Xue D. The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Crit Rev Biochem Mol Biol 2009;44:264–277. DOI: 10.1080/10409230903193307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vedadghavami A, Wagner EK, Mehta S, et al. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater 2019;93:258–269. DOI: 10.1016/j.actbio.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajpayee AG, Wong CR, Bawendi MG, et al. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials 2014;35:538–549. DOI: 10.1016/J.BIOMATERIALS.2013.09.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo C, Miao L, Zhao Y, et al. A novel cationic lipid with intrinsic antitumor activity to facilitate gene therapy of TRAIL DNA. Biomaterials 2016;102:239–248. DOI: 10.1016/j.biomaterials.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger BC, Wang S, Padera RF, et al. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med 2018;10:eaat8800. DOI: 10.1126/scitranslmed.aat8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motiejunas D, Wade RC. Structural, energetic, and dynamic aspects of ligand-receptor interactions. In: Taylor JB, Triggle DJ, Mason JS, eds. Comprehensive Medicinal Chemistry II, Vol. 4. Amsterdam, Netherlands: Elsevier, 2007: 193–212
- 11.Pinak M. Enzymatic recognition of radiation-produced oxidative DNA lesion. Molecular dynamics approach. In: Starikov EB, Lewis JP, Tanaka S, eds. Modern Methods for Theoretical Physical Chemistry of Biopolymers. Amsterdam: Elsevier, 2006: 191–210. [Google Scholar]
- 12.Mehta S, Akhtar S, Porter RM, et al. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res Ther 2019;21:238. DOI: 10.1186/s13075-019-2003-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson LN, Cashman SM, Read SP, et al. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res 2010;50:686–697. DOI: 10.1016/j.visres.2009.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li LD, Crouzier T, Sarkar A, et al. Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophys J 2013;105:1357–1365. DOI: 10.1016/j.bpj.2013.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan Y, Rees HA, Rossitto CP, et al. Green fluorescent proteins engineered for cartilage-targeted drug delivery: Insights for transport into highly charged avascular tissues. Biomaterials 2018;183:218–233. DOI: 10.1016/j.biomaterials.2018.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Käsdorf BT, Arends F, Lieleg O. Diffusion regulation in the vitreous humor. Biophys J 2015;109:2171–2181. DOI: 10.1016/J.BPJ.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah PP, Desai PR, Channer D, et al. Enhanced skin permeation using polyarginine modified nanostructured lipid carriers. J Control Release 2012;161:735–745. DOI: 10.1016/j.jconrel.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajpayee AG, Scheu M, Grodzinsky AJ, et al. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J Orthop Res 2014;32:1044–1051. DOI: 10.1002/jor.22630 [DOI] [PubMed] [Google Scholar]
- 19.Bajpayee AG, Scheu M, Grodzinsky AJ, et al. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J Orthop Res 2015;33:660–667. DOI: 10.1002/jor.22841 [DOI] [PubMed] [Google Scholar]
- 20. Bajpayee AG, Grodzinsky A, Wong CR, et al., inventors; Massachusetts Institute of Technology, assignee. Surface binding of nanoparticle based drug delivery to tissue. United States patent US 9,289,506. 2016 Mar 22
- 21. Bajpayee AG, Grodzinsky A, Wong CR, et al., inventors; Massachusetts Institute of Technology, assignee. Surface binding of nanoparticle based drug delivery to tissue. United States patent US 10,226,427. 2019 Mar 12
- 22.Bajpayee AG, De la Vega RE, Scheu M, et al. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. Eur Cell Mater 2017;34:341–364. DOI: 10.22203/eCM.v034a21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajpayee AG, Quadir MA, Hammond PT, et al. Charge based intra-cartilage delivery of single dose dexamethasone using Avidin nano-carriers suppresses cytokine-induced catabolism long term. Osteoarthr Cartil 2016;24:71–81. DOI: 10.1016/j.joca.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He T, Zhang C, Vedadghavami A, et al. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J Control Release 2020;318:109–123. DOI: 10.1016/j.jconrel.2019.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, He T, Vedadghavami A, et al. Avidin-biotin technology to synthesize multi-arm nano-construct for drug delivery. MethodsX 2020;7:100882. DOI: 10.1016/j.mex.2020.100882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook Sangar ML, Girard EJ, Hopping G, et al. A potent peptide-steroid conjugate accumulates in cartilage and reverses arthritis without evidence of systemic corticosteroid exposure. Sci Transl Med 2020;12:eaay1041. DOI: 10.1126/scitranslmed.aay1041 [DOI] [PubMed] [Google Scholar]
- 27.Chu Y, Chen N, Yu H, et al. Topical ocular delivery to laser-induced choroidal neovascularization by dual internalizing RGD and TAT peptide-modified nanoparticles. Int J Nanomedicine 2017;12:1353–1368. DOI: 10.2147/IJN.S126865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Jiang K, Tai L, et al. Facile Noninvasive retinal gene delivery enabled by Penetratin. ACS Appl Mater Interfaces 2016;8:19256–19267. DOI: 10.1021/acsami.6b04551 [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Zheng Y, Liu M, et al. Biomimetic viruslike and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers for oral insulin delivery. ACS Appl Mater Interfaces 2018;10:9916–9928. DOI: 10.1021/acsami.7b16524 [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Qin X, Kong F, et al. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv 2019;26:328–342. DOI: 10.1080/10717544.2019.1582730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derakhshankhah H, Jafari S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed Pharmacother 2018;108:1090–1096. DOI: 10.1016/j.biopha.2018.09.097 [DOI] [PubMed] [Google Scholar]
- 32.Herce HD, Garcia AE. Cell penetrating peptides: How do they do it? J Biol Phys 2007;33:345–356. DOI: 10.1007/s10867-008-9074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y, Liu X, Sinha SK, et al. Translocation thermodynamics of linear and cyclic nonaarginine into model dppc bilayer via coarse-grained molecular dynamics simulation: Implications of pore formation and nonadditivity. J Phys Chem B 2014;118:2670–2682. DOI: 10.1021/jp412600e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler A. Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv Drug Deliv Rev 2008;60:580–597. DOI: 10.1016/j.addr.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 35.Gräslund A, Madani F, Lindberg S, et al. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011;2011:414729. DOI: 10.1155/2011/414729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusnati M, Tulipano G, Spillmann D, et al. Multiple interactions of HIV-I Tat protein with size-defined heparin oligosaccharides. J Biol Chem 1999;274:28198–28205. DOI: 10.1074/jbc.274.40.28198 [DOI] [PubMed] [Google Scholar]
- 37.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007;446:1030–1037. DOI: 10.1038/nature05817 [DOI] [PubMed] [Google Scholar]
- 38.Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat Biotechnol 2001;19:713–714. DOI: 10.1038/90741 [DOI] [PubMed] [Google Scholar]
- 39.Plénat T, Deshayes S, Boichot S, et al. Interaction of primary amphipathic cell-penetrating peptides with phospholipid-supported monolayers. Langmuir 2004;20:9255–9261. DOI: 10.1021/la048622b [DOI] [PubMed] [Google Scholar]
- 40.Deshayes S, Heitz A, Morris MC, et al. Insight into the Mechanism of Internalization of the Cell-Penetrating Carrier Peptide Pep-1 through Conformational Analysis. Biochemistry 2004;43:1449–1457. DOI: 10.1021/bi035682s [DOI] [PubMed] [Google Scholar]
- 41.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 2013;65:157–170. DOI: 10.1111/j.2042-7158.2012.01567.x [DOI] [PubMed] [Google Scholar]
- 42.Cao Z, Li D, Wang J, et al. Direct nucleus-targeted drug delivery using cascade pHe/photo dual-sensitive polymeric nanocarrier for cancer therapy. Small 2019;15:1902022. DOI: 10.1002/smll.201902022 [DOI] [PubMed] [Google Scholar]
- 43.Ingato D, Edson JA, Zakharian M, et al. Cancer cell-derived, drug-loaded nanovesicles induced by sulfhydryl-blocking for effective and safe cancer therapy. ACS Nano 2018;12:9568–9577. DOI: 10.1021/acsnano.8b05377 [DOI] [PubMed] [Google Scholar]
- 44.Haapaniemi E, Botla S, Persson J, et al. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med 2018;24:927–930. DOI: 10.1038/s41591-018-0049-z [DOI] [PubMed] [Google Scholar]
- 45.Seynhaeve ALB, Dicheva BM, Hoving S, et al. Intact Doxil is taken up intracellularly and released doxorubicin sequesters in the lysosome: Evaluated by in vitro/in vivo live cell imaging. J Control Release 2013;172:330–340. DOI: 10.1016/j.jconrel.2013.08.034 [DOI] [PubMed] [Google Scholar]
- 46.Feni L, Neundorf I. The current role of cell-penetrating peptides in cancer therapy. Adv Exp Med Biol 2017;1030:279–295. DOI: 10.1007/978-3-319-66095-0_13 [DOI] [PubMed] [Google Scholar]
- 47.Ramaker K, Henkel M, Krause T, et al. Cell penetrating peptides: A comparative transport analysis for 474 sequence motifs. Drug Deliv 2018;25:928–937. DOI: 10.1080/10717544.2018.1458921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel SG, Sayers EJ, He L, et al. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci Rep 2019;9:1–9. DOI: 10.1038/s41598-019-42456-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaro JL, Vekich JE, Tran T, et al. Nuclear localization of cell-penetrating peptides is dependent on endocytosis rather than cytosolic delivery in CHO cells. Mol Pharm 2009;6:337–344. DOI: 10.1021/mp800239p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gronewold A, Horn M, Neundorf I. Design and biological characterization of novel cell-penetrating peptides preferentially targeting cell nuclei and subnuclear regions. Beilstein J Org Chem 2018;14:1378–1388. DOI: 10.3762/bjoc.14.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, Zhang Y, Tai L, et al. Functionalized cell nucleus-penetrating peptide combined with doxorubicin for synergistic treatment of glioma. Acta Biomater 2016;42:90–101. DOI: 10.1016/j.actbio.2016.06.031 [DOI] [PubMed] [Google Scholar]
- 52.Rahbek UL, Howard KA, Oupicky D, et al. Intracellular siRNA and precursor miRNA trafficking using bioresponsive copolypeptides. J Gene Med 2008;10:81–93. DOI: 10.1002/jgm.1120 [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg SR, Grodzinsky AJ. The kinetics of chemically induced nonequilibrium swelling of articular cartilage and corneal stroma. J Biomech Eng 1987;109:79–89. DOI: 10.1115/1.3138647 [DOI] [PubMed] [Google Scholar]
- 54.El-Andaloussi S, Järver P, Johansson HJ, et al. Cargo-dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: A comparative study. Biochem J 2007;407:285–292. DOI: 10.1042/BJ20070507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eguchi A, Meade BR, Chang YC, et al. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol 2009;27:567–571. DOI: 10.1038/nbt.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldeck W, Pipkorn R, Korn B, et al. Transporter molecules influence the gene expression in HeLa cells. Int J Med Sci 2009;6:18–27. DOI: 10.7150/ijms.6.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo J hua S, Jan M shiou, Lin YL, et al. Interactions between octaarginine and U-937 human macrophages: Global gene expression profiling, superoxide anion content, and cytokine production. J Control Release 2009;139:197–204. DOI: 10.1016/j.jconrel.2009.07.006 [DOI] [PubMed] [Google Scholar]
- 58.Saar K, Lindgren M, Hansen M, et al. Cell-penetrating peptides: A comparative membrane toxicity study. Anal Biochem 2005;345:55–65. DOI: 10.1016/j.ab.2005.07.033 [DOI] [PubMed] [Google Scholar]
- 59.Kilk K, Mahlapuu R, Soomets U, et al. Analysis of in vitro toxicity of five cell-penetrating peptides by metabolic profiling. Toxicology 2009;265:87–95. DOI: 10.1016/j.tox.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 60.Brown S, Pistiner J, Adjei IM, et al. Nanoparticle properties for delivery to cartilage: The implications of disease state, synovial fluid, and off-target uptake. Mol Pharm 2019;16:469–479. DOI: 10.1021/acs.molpharmaceut.7b00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv H, Zhang S, Wang B, et al. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release 2006;114:100–109. DOI: 10.1016/j.jconrel.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 62.Verdurmen WPR, Brock R. Biological responses towards cationic peptides and drug carriers. Trends Pharmacol Sci 2011;32:116–124. DOI: 10.1016/j.tips.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 63.Immordino ML, Dosio F, Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006;1:297–315. DOI: 10.2217/17435889.1.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sercombe L, Veerati T, Moheimani F, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol 2015;6:286. DOI: 10.3389/fphar.2015.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016;99:28–51. DOI: 10.1016/j.addr.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamidi M, Azadi A, Rafiei P. Pharmacokinetic consequences of PEGylation. Drug Deliv 2006;13:399–409. DOI: 10.1080/10717540600814402 [DOI] [PubMed] [Google Scholar]
- 67.Xia T, Kovochich M, Liong M, et al. Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2008;2:85–96. DOI: 10.1021/nn700256c [DOI] [PubMed] [Google Scholar]
- 68.Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 2006;6:1794–1807. DOI: 10.1021/nl061025k [DOI] [PubMed] [Google Scholar]
- 69.Wei X, Shao B, He Z, et al. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res 2015;25:237–253. DOI: 10.1038/cr.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winnicka K, Wroblewska M, Sosnowska K, et al. Evaluation of cationic polyamidoamine dendrimers' dermal toxicity in the rat skin model. Drug Des Devel Ther 2015;9:1367–1377. DOI: 10.2147/DDDT.S78336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan H, Chen CY, Chai GH, et al. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm 2013;10:1865–1873. DOI: 10.1021/mp300649z [DOI] [PubMed] [Google Scholar]
- 72.Kim YH, Park JH, Lee M, et al. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release 2005;103:209–219. DOI: 10.1016/j.jconrel.2004.11.008 [DOI] [PubMed] [Google Scholar]
- 73.Read ML, Bremner KH, Oupický D, et al. Vectors based on reducible polycations facilitate intracellular release of nucleic acids. J Gene Med 2003;5:232–245. DOI: 10.1002/jgm.331 [DOI] [PubMed] [Google Scholar]
- 74.Van Der Woude I, Wagenaar A, Meekel AAP, et al. Novel pyridinium surfactants for efficient, nontoxic in vitro gene delivery. Proc Natl Acad Sci U S A 1997;94:1160–1165. DOI: 10.1073/pnas.94.4.1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeffrey GA. An Introduction to Hydrogen Bonding. Oxford, UK: Oxford University Press, 1997
- 76.Svensen N, Walton JGA, Bradley M. Peptides for cell-selective drug delivery. Trends Pharmacol Sci 2012;33:186–192. DOI: 10.1016/j.tips.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 77.Reissmann S. Cell penetration: Scope and limitations by the application of cell-penetrating peptides. J Pept Sci 2014;20:760–784. DOI: 10.1002/psc.2672 [DOI] [PubMed] [Google Scholar]
- 78.Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol Sci 2017;38:406–424. DOI: 10.1016/j.tips.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 79.Aguilera TA, Olson ES, Timmers MM, et al. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr Biol 2009;1:371–381. DOI: 10.1039/b904878b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olson ES, Aguilera TA, Jiang T, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol 2009;1:382–393. DOI: 10.1039/b904890a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 2008;60:1615–1626. DOI: 10.1016/j.addr.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 82.Yu M, Li X, Huang X, et al. New cell-penetrating peptide (KRP) with multiple physicochemical properties endows doxorubicin with tumor targeting and improves its therapeutic index. ACS Appl Mater Interfaces 2019;11:2448–2458. DOI: 10.1021/acsami.8b21027 [DOI] [PubMed] [Google Scholar]
- 83.Kang MH, Park MJ, Yoo HJ, et al. RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes for enhanced intracellular drug delivery to hepsin-expressing cancer cells. Eur J Pharm Biopharm 2014;87:489–499. DOI: 10.1016/j.ejpb.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 84.Kang MH, Yoon HY, Choi YW. RIPL peptide as a novel cell-penetrating and homing peptide: Design, characterization, and application to liposomal nanocarriers for hepsin-specific intracellular drug delivery. Nanostructures Cancer Ther 2017;129–157. DOI: 10.1016/B978-0-323-46144-3.00005-2 [DOI] [Google Scholar]
- 85.Thurston G, Mclean JW, Rizen M, et al. Cationic liposomes target angiogenic endothelial cells cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest 1998;101:1401–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Löhr JM, Haas SL, Bechstein WO, et al. Cationic liposomal paclitaxel plus gemcitabine or gemcitabine alone in patients with advanced pancreatic cancer: A randomized controlled phase II trial. Ann Oncol 2012;23:1214–1222. DOI: 10.1093/annonc/mdr379 [DOI] [PubMed] [Google Scholar]
- 87.Feng L, Dong Z, Tao D, et al. The acidic tumor microenvironment: A target for smart cancer nano-theranostics. Natl Sci Rev 2018;5:269–286. DOI: 10.1093/nsr/nwx062 [DOI] [Google Scholar]
- 88.Eichhorn ME, Ischenko I, Luedemann S, et al. Vascular targeting by EndoTAGTM-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int J Cancer 2010;126:1235–1245. DOI: 10.1002/ijc.24846 [DOI] [PubMed] [Google Scholar]
- 89.Fasol U, Frost A, Büchert M, et al. Vascular and pharmacokinetic effects of EndoTAG-1 in patients with advanced cancer and liver metastasis. Ann Oncol 2012;23:1030–1036. DOI: 10.1093/annonc/mdr300 [DOI] [PubMed] [Google Scholar]
- 90.Awada A, Bondarenko IN, Bonneterre J, et al. A randomized controlled phase ii trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (tnbc). Ann Oncol 2014;25:824–831. DOI: 10.1093/annonc/mdu025 [DOI] [PubMed] [Google Scholar]
- 91.ClinicalTrials.gov. A trial evaluating the efficacy and safety of EndoTAG®-1 in combination with paclitaxel and gemcitabine compared with paclitaxel and gemcitabine as first-line therapy in patients with visceral metastatic triple-negative breast cancer. Available at https://clinicaltrials.gov/ct2/show/study/NCT03002103. Accessed March13, 2020
- 92.Xu L, Pirollo KF, Tang WH, et al. Transferrin-liposome-mediated systemic p53 gene therapy in combination with radiation results in regression of human head and neck cancer xenografts. Hum Gene Ther 1999;10:2941–2952. DOI: 10.1089/10430349950016357 [DOI] [PubMed] [Google Scholar]
- 93.ClinicalTrials.gov. Safety study of infusion of SGT-53 to treat solid tumors. Available at https://clinicaltrials.gov/ct2/show/NCT00470613. Accessed March19, 2020
- 94.Pirollo KF, Nemunaitis J, Leung PK, et al. Safety and efficacy in advanced solid tumors of a targeted nanocomplex carrying the p53 gene used in combination with docetaxel: A phase 1b study. Mol Ther 2016;24:1697–1706. DOI: 10.1038/mt.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.ClinicalTrials.gov. Safety and efficacy study of KAI-9803 to treat subjects with ST elevation myocardial infarction (heart attack). Available at https://clinicaltrials.gov/ct2/show/NCT00785954. Accessed March9, 2020
- 96.ClinicalTrials.gov. Safety of KAI-9803 for injection with angioplasty following heart attack. Available at https://clinicaltrials.gov/ct2/show/NCT00093197. Accessed March9, 2020
- 97.Miyaji Y, Walter S, Chen L, et al. Distribution of KAI-9803, a novel δ-protein kinase C inhibitor, after intravenous administration to rats. Drug Metab Dispos 2011;39:1946–1953. DOI: 10.1124/dmd.111.040725 [DOI] [PubMed] [Google Scholar]
- 98.Bates E, Bode C, Costa M, et al. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation 2008;117:886–896. DOI: 10.1161/CIRCULATIONAHA.107.759167 [DOI] [PubMed] [Google Scholar]
- 99.Lincoff AM, Roe M, Aylward P, et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: Results of the PROTECTION AMI randomized controlled trial. Eur Heart J 2014;35:2516–2523. DOI: 10.1093/eurheartj/ehu177 [DOI] [PubMed] [Google Scholar]
- 100.Touchard E, Omri S, Naud MC, et al. A peptide inhibitor of c-Jun n-terminal kinase for the treatment of endotoxin-induced uveitis. Investig Ophthalmol Vis Sci 2010;51:4683–4693. DOI: 10.1167/iovs.09-4733 [DOI] [PubMed] [Google Scholar]
- 101.ClinicalTrials.gov. Safety, tolerability and PK of a single iv infusion of 10, 40, and 80 μg/kg XG-102 administered to healthy volunteers. Available at https://clinicaltrials.gov/ct2/show/study/NCT01570205. Accessed March9, 2020
- 102.Beydoun T, Deloche C, Perino J, et al. Subconjunctival injection of XG-102, a JNK inhibitor peptide, in patients with intraocular inflammation: A safety and tolerability study. J Ocul Pharmacol Ther 2015;31:93–99. DOI: 10.1089/jop.2013.0247 [DOI] [PubMed] [Google Scholar]
- 103.Chiquet C, Aptel F, Creuzot-Garcher C, et al. Postoperative ocular inflammation: A single subconjunctival injection of XG-102 compared to dexamethasone drops in a randomized trial. Am J Ophthalmol 2017;174:76–84. DOI: 10.1016/j.ajo.2016.10.012 [DOI] [PubMed] [Google Scholar]
- 104.ClinicalTrials.gov. AM-111 in the treatment of acute inner ear hearing loss. Available at https://clinicaltrials.gov/ct2/show/NCT02561091. Accessed March9, 2020
- 105.Coleman JKM, Littlesunday C, Jackson R, et al. AM-111 protects against permanent hearing loss from impulse noise trauma. Hear Res 2007;226:70–78. DOI: 10.1016/j.heares.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 106.Suckfuell M, Lisowska G, Domka W, et al. Efficacy and safety of AM-111 in the treatment of acute sensorineural hearing loss: A double-blind, randomized, placebo-controlled phase II study. Otol Neurotol 2014;35:1317–1326. DOI: 10.1097/MAO.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 107.Staecker H, Jokovic G, Karpishchenko S, et al. Efficacy and safety of AM-111 in the treatment of acute unilateral sudden deafness—A double-blind, randomized, placebo-controlled phase 3 study. Otol Neurotol 2019;40:584–594. DOI: 10.1097/MAO.0000000000002229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.ClinicalTrials.gov. Safety and efficacy study of RT001 to treat moderate to severe lateral canthal liness. Available at https://clinicaltrials.gov/ct2/show/NCT00884234. Accessed March9, 2020
- 109.Brandt F, O'Connell C, Cazzaniga A, et al. Efficacy and safety evaluation of a novel botulinum toxin topical gel for the treatment of moderate to severe lateral canthal lines. Dermatologic Surg 2010;36:2111–2118. DOI: 10.1111/j.1524-4725.2010.01711.x [DOI] [PubMed] [Google Scholar]
- 110.Fonfria E, Maignel J, Lezmi S, et al. The expanding therapeutic utility of botulinum neurotoxins. Toxins (Basel) 2018;10:208. DOI: 10.3390/toxins10050208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta Rev Cancer 2008;1786:126–138. DOI: 10.1016/j.bbcan.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 112.ClinicalTrials.gov. A phase 2a study to evaluate the safety and efficacy of AZX100 in trocar sites of arthroscopic shoulder surgery patients. Available at https://clinicaltrials.gov/ct2/show/NCT00811577. Accessed March10, 2020
- 113.ClinicalTrials.gov. A study to evaluate the safety and efficacy of additional doses of AZX100 drug product following excision of Keloid scars. Available at https://clinicaltrials.gov/ct2/show/NCT00892723. Accessed March10, 2020
- 114.Ho A, Schwarze SR, Mermelstein SJ, et al. Synthetic protein transduction domains: Enhanced transduction potential in vitro and in vivo. Cancer Res 2001;61:474–477 [PubMed] [Google Scholar]
- 115.Snell NJC. Discontinued drug projects in the respiratory therapeutic area during 2012. Expert Opin Investig Drugs 2014;23:411–415. DOI: 10.1517/13543784.2014.873785 [DOI] [PubMed] [Google Scholar]