Abstract
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase implicated in numerous physiological processes and cellular functions through its ability to regulate the function of many proteins, including transcription factors and structural proteins. GSK-3β has been demonstrated to function as a regulator of multiple behavioral processes induced by drugs of abuse, particularly psychostimulant drugs. In this review, we provide an overview of the regulation of GSK-3β activity produced by psychostimulants, and the role of GSK-3β signaling in psychostimulant-induced behaviors including drug reward, associative learning and memory which play a role in the maintenance of drug-seeking. Evidence supports the conclusion that GSK-3β is an important component of the actions of psychostimulant drugs and that GSK-3β is a valid target for developing novel therapeutics. Additional studies are required to examine the role of GSK-3β in distinct cell types within the mesolimbic and memory circuits to further elucidate the mechanisms related to the acquisition, consolidation, and recall of drug-related memories, and potentially countering neuroadaptations that reinforce drug-seeking behaviors that maintain drug dependence.
Keywords: cocaine, amphetamine, addiction, neuroplasticity, mesolimbic pathway, drug reward
1. Introduction
Substance abuse is a global health problem which has devastating impact on individuals, families and communities. Substance use disorders are characterized by compulsive drug seeking and drug use regardless of detrimental consequences. Repeated exposure to drugs of abuse causes neuroadaptations which result in dysfunction of multiple brain systems. These systems include circuits important for motivation, cognitive control, and memory. Dysfunction of these circuits are thought to contribute to high rates of relapse even after long periods of abstinence, making substance use disorder a chronic relapsing disease that is particularly difficult to treat. The biological basis for addiction is not completely understood, but immense progress has been made in elucidating molecules and circuits impacted by drugs of abuse that contribute both to their acute pleasurable and reinforcing effects and to the development of addictive disease. One molecule that has been shown to have importance in these processes is GSK-3β. This review will discuss the evidence that GSK-3β signaling plays an important role in the acute effects of stimulants as well as in the maintenance of drug-seeking behaviors. First, brief summaries of the pharmacology of stimulants, neural circuitry of drug reward and memory, and neurotransmitter systems impacted by drugs of abuse as related to GSK-3β will be presented.
1.1. Psychostimulants and reward circuitry
Cocaine and the amphetamines, including d-amphetamine, methamphetamine, and methylene-dioxy-methamphetamine (MDMA; ‘ecstasy’), belong to the class of drugs of abuse termed psychostimulants. They are sometimes referred to as psychomotorstimulants because they produce CNS excitation and stimulate locomotor activity. Psychostimulants share the mechanism of enhanced monoamine transmission, although this is achieved through distinct molecular mechanisms. Cocaine binds to and inhibits the function of monoamine transporters (i.e., dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET)). As a consequence of inhibition of reuptake by the transporters, synaptic levels of dopamine, serotonin, and norepinephrine are increased. On the other hand, amphetamines promote monoamine efflux producing the similar end result of enhanced synaptic levels of these neurotransmitters. In fact, a common defining feature of all drugs of abuse is their ability to increase dopamine transmission in the mesocorticolimbic circuit and this neurochemical event underlies the acute reinforcing effects of drugs of abuse (DiChara and Imperato, 1988; Carboni et al., 1989).
The mesocorticolimbic pathway is comprised of dopaminergic cell bodies in the ventral tegmental area (VTA) that project to limbic and cortical regions including the nucleus accumbens (ventral striatum), dorsal striatum (caudate putamen), amygdala, hippocampus, and prefrontal cortex (Fields et al., 2007). The nucleus accumbens is divided into two major subfields primarily by afferent and efferent projection patterns and function, and these are called the core and the shell. The shell division is primarily associated with drug reward and the accumbens core and dorsal striatum, contribute to cue-conditioning (Ito and Hayen, 2011). Figure 1 illustrates connections between the nucleus accumbens and other brain areas important in the development and maintenance of drug-seeking behaviors
Figure 1: Schematic depiction of the mesocorticolimbic circuit in the rodent brain important for the development and maintenance of behaviors underlying drug-seeking and influenced by GSK-3β activity.
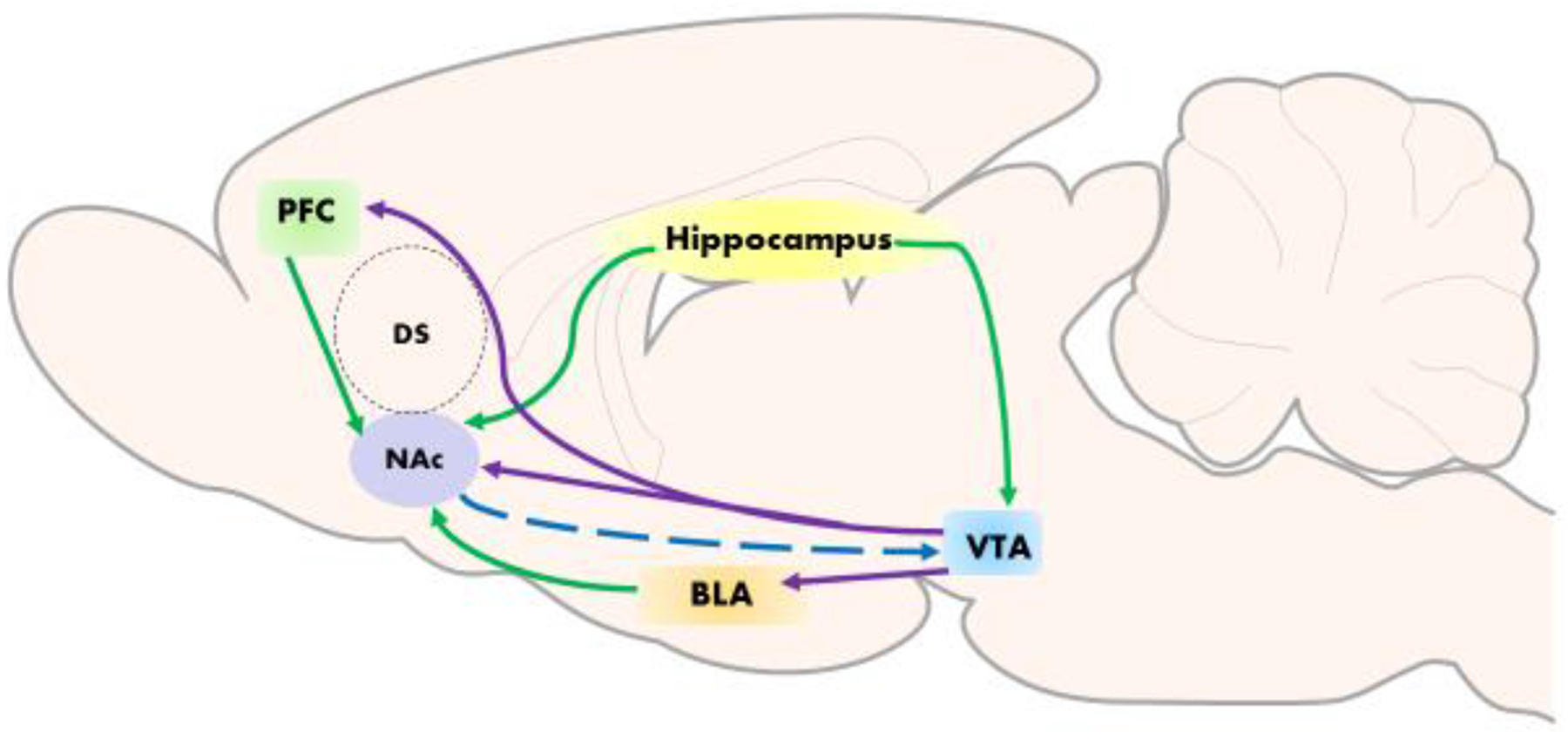
The ventral tegmental area (VTA) sends dopaminergic projections (purple) to the nucleus accumbens (NAc), prefrontal cortex (PFC), and basolateral amygdala (BLA). Glutamatergic projections from the PFC, amygdala, and hippocampus (green) innervate the NAc to modulate GABAergic transmission to the VTA (blue), as well as directly regulate VTA neuronal activity. Additional structures have been omitted for clarity.
The GABAergic medium spiny neurons in the nucleus accumbens receive dopaminergic innervations from the VTA and glutamatergic inputs from cortical, limbic and thalamic brain regions (Britt et al., 2012; Figure 1). The medium spiny neurons in turn project back to the VTA and substantia nigra in the midbrain, and to the ventral pallidum. This circuit has been shown to play a role in goal-directed behaviors, associative learning, and memory formation (consolidation/reconsolidation) (Sesack and Grace, 2010; Phillips et al., 2008). The dopamine surge in the nucleus accumbens is the initial event in psychostimulant-induced reinforcement, and it results in alterations in excitatory glutamate transmission. With repeated exposure to drugs of abuse including psychostimulants, neuroadaptations including synaptic plasticity drive long-term cellular and behavioral effects, and glutamate plays a key role in these events (Wolf et al., 2004). While acute administration of cocaine or amphetamine causes modest elevations of extracellular glutamate in the nucleus accumbens (Reid et al, 1997; McKee and Meshul, 2005), withdrawal from chronic cocaine results in reduced basal levels of extracellular glutamate (Schmidt and Pierce, 2010) and lasting adaptations in corticostriatal excitatory synapses (Conrad et al., 2008; Martin et al., 2006; Pascoli et al., 2014). Drug- or cue-induced craving and relapse are associated with surges in synaptic glutamate coming from cortical, limbic and thalamic regions. Specifically, glutamatergic projections from the medial prefrontal cortex to the nucleus accumbens core mediate reinstated drug-seeking behavior (i.e., relapse behaviors) (Kalivas, 2009; Luscher and Malenka, 2011; Britt et al., 2012). Adaptations in glutamatergic synapses are produced with repeated exposure to psychostimulants and these adaptations include reduced levels of excitatory amino acid transporter 2 (EAAT2) and mGLuR2/3, increased activator of G-protein signaling 3 (AGS-3), and reduced long-term depression (LTD)/long-term potentiation (LTP). Upon exposure to drug-associated cues, there is a rapid surge in glutamate, along with increases in matrix metallopeptidase 9 (MMP-9), AMPA/NMDA ratio, and dendritic spine size (Spencer et al., 2016). Thus, there is significant evidence that enduring changes in excitability and synaptic plasticity of accumbal medium spiny neurons are induced by repeated exposure to cocaine and amphetamine and that these adaptations underlie psychostimulant-specific behaviors (Pierce and Wolf, 2013).
1.2. GSK-3β regulation in brain
GSK-3 is highly expressed in the mammalian brain including in the frontal cortex, nucleus accumbens, dorsal striatum (caudate putamen), hippocampus and amygdala (Leroy and Brion, 1999). There are two isoforms of GSK-3, GSK-3α and GSK-3β, with the beta isoform being the most widely studied in regard to the actions of psychostimulants. GSK-3β is constitutively active via tyrosine phosphorylation (Tyr216-GSK-3β) in resting neurons, promoting substrate accessibility (Hur and Zhou, 2010). GSK-3β activity is influential in that it phosphorylates an extensive variety of substrates including transcription factors, structural proteins, and signaling molecules shown to be critical for many cellular processes including regulation of gene expression and synaptic plasticity (Grimes and Jope, 2001; Peineau et al., 2008). As such, GSK-3β influences cellular architecture and motility, cell survival and apoptosis, neuronal differentiation and DNA transcription (Jope and Johnson 2004; Woodgett, 2001). GSK-3β signaling is implicated in the pathology of several neuropsychiatric and neurodegenerative diseases (reviewed in Jope and Roh, 2006; Duda et al., 2018; Kitagishi et al., 2012).
The activity of GSK-3 is regulated by a number of signaling pathways. GSK-3 activity is reduced by phosphorylation of the N-terminal serine, Ser9-GSK3β and Ser21-GSK3α. Several kinases can phosphorylate GSK-3 and hence inhibit its constitutive activity. These include Akt, a serine threonine kinase downstream of phospho-inositide 3-kinase (PI3K) signaling which is activated by tyrosine kinases, G-protein coupled receptors, and integrin signaling (Hong and Lee, 1997). Akt is activated by phosphorylation of two regulator sites, Thr308 and Ser473 (Alessi et al., 1996) by the kinases PDK1 and PDK2 (Scheid and Woodgett, 2001). Activated Akt directly phosphorylates and negatively regulates the activity of GSK-3β (Cross et al., 1995). A second modulator of GSK-3β activity is CaMKII which also phosphorylates and thus inhibits GSK-3β activity in neurons (Song et al., 2010). Protein kinase C (PKC; Espada et al., 2009; Goode et al., 1992) and protein kinase A (PKA; Li et al., 2000; Shelly et al., 2011; Fang et al., 2000) provide inhibitory regulatory control of GSK-3β signaling as well. In contrast to these kinases, stimulation of GSK-3β activity in neurons can be caused by protein phosphatase 1 and 2 (PP1 and PP2) via dephosphorylation of Ser9-GSK-3β (Bennecib et al., 2000; Morfini et al., 2004). CaMKII, PKC, PKA and other kinases have been implicated in numerous effects of stimulants, with their influence dependent upon the specific drug and brain region examined (reviewed in Lee and Messing, 2008).
1.3. Receptor signaling and GSK-3β regulation as related to psychostimulants
Given the vast array of kinases and phosphatases that can regulate GSK-3β activity, it is clear that many cell surface receptors can signal through GSK-3β including those involved in the actions of psychostimulants. Figure 2 highlights some of these signaling pathways. It is now well-established that dopamine receptors, particularly D2 receptors, can signal via a G-protein independent pathway involving Akt (Beaulieu et al., 2004). Following agonist activation of D2 receptors, β-arrestin2 forms a complex with Akt and PP2A which results in a reduction of Akt phosphorylation and activity. A consequence of reduced Akt activity is the activation of GSK-3β which is normally under inhibitory control of Akt (Beaulieu et al., 2007 & 2009; Mannoury la Cour, 2011; O’Brien et al., 2011). The changes in GSK-3 activity are important for the physiological effects of dopamine and dopaminergic drugs (Li and Gao, 2011). For example, increased dopaminergic neurotransmission resultant from genetic knockout of the dopamine transporter (DAT; DAT −/− mice) produces a hyperactive phenotype which is accompanied by reduced Akt phosphorylation (i.e., Akt inactivation) and reduced phosphorylation of the downstream substrates, GSK-3α and GSK-3β, consequently increasing GSK-3 activity in the striatum (Beaulieu et al., 2004). In addition to D2 receptors, dopamine D1 receptors may also signal via Akt and GSK-3β. D1 receptor knockout mice have lower levels of p-Ser473-Akt in the striatum than wild-type controls (Beaulieu et al., 2007). D1 receptor agonists increase p-Thr308-Akt in striatal neuronal cultures (Brami-Cherrier et al., 2002). A more recent study demonstrates that the dopamine D1 receptor is constitutively associated with GSK-3β. Inhibition of GSK-3β impairs this interaction and D1 receptor membrane expression and activation in cortical neurons (Wang et al., 2017). When the D1 receptor is activated, interaction with GSK-3β participates in β-arrestin-mediated recycling of D1 receptor, increasing D1 receptor signaling (Wang et al., 2017). GSK-3 activity is needed for the full expression of the behavioral effects of D1 receptor agonists, as evidenced by the ability of the GSK3 inhibitor, SB216763, to attenuate hyperlocomotion produced by systemic administration of SKF82958 (Miller et al., 2010). Thus, GSK-3 plays an important role in the cellular responses initiated by dopamine receptor activation.
Figure 2. GSK-3β activity is regulated by several neurotransmitters whose synaptic levels are affected by psychostimulants.
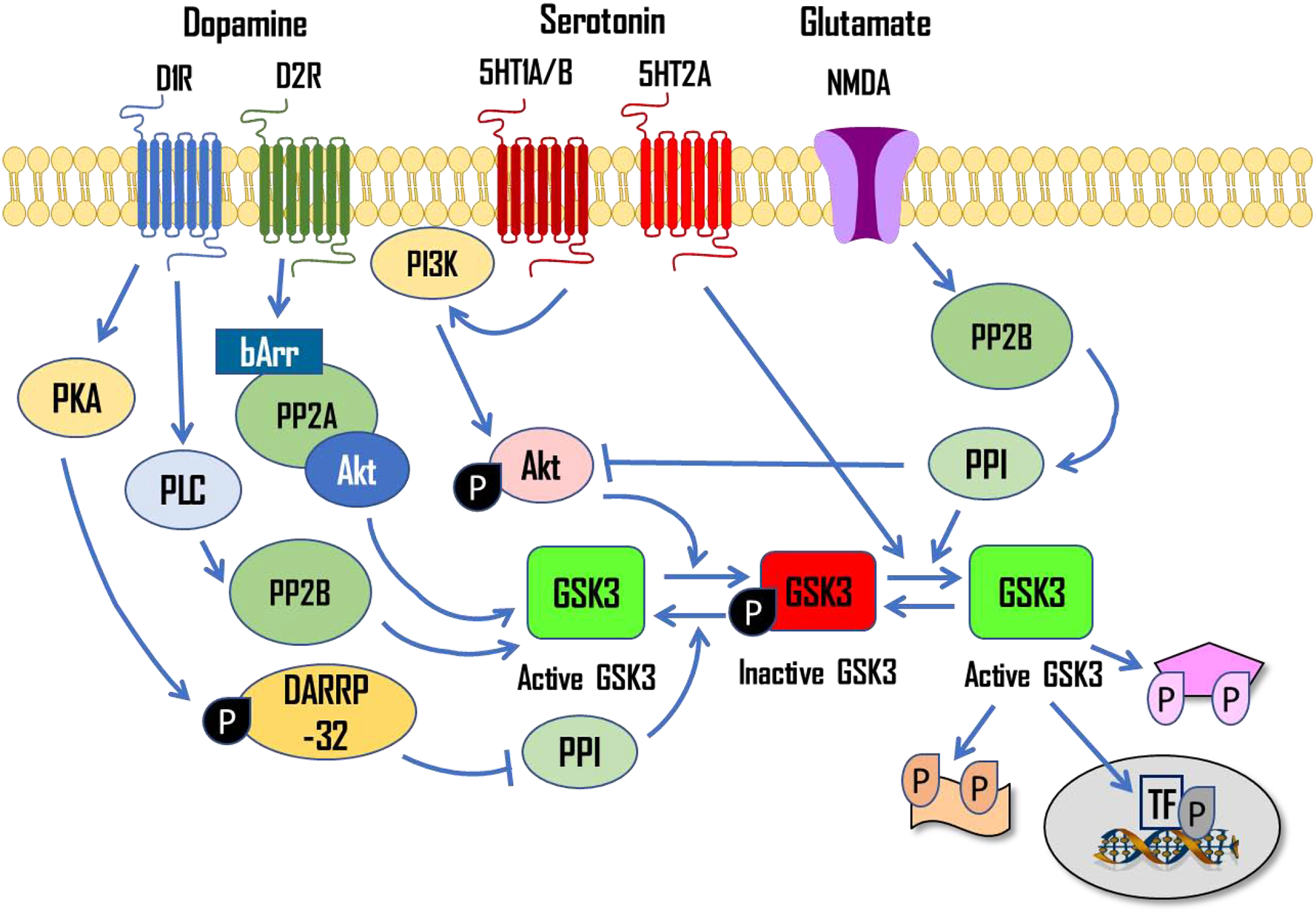
Through their effects on dopamine, serotonin and glutamate transmission, psychostimulants can regulate the activity of GSK-3β. Examples of upstream signaling pathways resulting in GSK-3β regulation are shown. GSK-3β has numerous potential substrates including transcription factors, structural proteins, receptors, signaling molecules and metabolic enzymes. As such, GSK-3β well-positioned to influence neuroplasticity, gene transcription, and learning and memory processes. βArr, beta-arrestin; PKA, protein kinase A; PLC, phospholipase C; PP2, protein phosphatase 2; PP1, protein phosphatase 1; PI3K, phosphatidylinositol 3-kinase; TF, transcription factor; p, phosphorylated.
GSK-3β activity also is under regulatory control of serotonin (5-HT) receptors. In general, 5-HT1 and 5-HT2 receptors have opposite effects on GSK-3β activity. The 5-HT1 receptor agonist 8-OH-DPAT or the 5-HT2 receptor antagonist LY553837 both increase GSK-3β phosphorylation in the brain (Li et al., 2004). The selective serotonin reuptake inhibitor (SSRI) antidepressant, fluoxetine, upregulates phosphorylation of GSK-3β by a 5-HT1A-dependent mechanism (Hui et al., 2014; Polter et al., 2012) and the regulation of GSK-3 contributes to the antidepressant effects of fluoxetine (Polter et al., 2012). Antipsychotics, particularly the atypical antipsychotics such as clozapine and risperidone, have an inhibitory effect on GSK-3β activity which is due to their dual action as 5-HT2A and D2 receptor antagonists (Li et al., 2007; Alimohamad et al., 2005). Thus, 5-HT receptor modulation of GSK-3β activity has been implicated in the mechanism of action of antidepressant (SSRIs) and antipsychotic medications (Beaulieu et al., 2009). Since psychostimulants increase synaptic levels of serotonin (Teneud et al., 1996), regulation of GSK-3 activity through 5-HT receptors may have a role in cellular and behavioral effects of cocaine and amphetamine. In turn, other studies indicate that GSK-3β is able to regulate the function of 5-HT1B and other GPCRs through receptor phosphorylation which impacts cellular localization and association with other intracellular proteins (Chen et al., 2009; Zhou et al., 2012).
Although glutamate receptors are not direct molecular targets of cocaine or amphetamine, psychostimulants can indirectly increase extracellular glutamate in the nucleus accumbens and caudate putamen (Reid et al. 1997; Smith et al. 1995), and glutamate plays an important role in substance use disorders (reviewed in Marquez et al., 2017; Scofield et al., 2016). The activity of GSK-3β can be regulated by glutamatergic NMDA receptors. NMDA application to cultured hippocampal neurons produces a rapid dephosphoylation of Ser9-GSK3β, demonstrating that NMDA receptor signaling activates GSK-3β (Luo et al., 2003). This effect is mediated by the NR2B subunit which increases GSK-3β activity via protein phosphatase-1 (Szatmari et al., 2005). In agreement, NMDA receptor antagonists such as phencyclidine and memantine increase the inhibitory serine-phosphorylation of both GSK-3 isoforms in mouse brain (De Sarno et al., 2006; Svenningsson et al., 2003). The interaction of NMDA receptors and GSK-3β is bidirectional; not only can NMDA receptors affect GSK-3β activity, GSK-3β can regulate NMDA receptor location and function. GSK-3β regulates surface localization of NMDA receptors, including the NR1 and NR2B subunits (Chen et al., 2007; Deng et al., 2014), and the GSK-3 inhibitor, lithium, diminishes NMDA receptor signaling (Nonaka et al., 1998; Hashimoto et al., 2002). Given this relationship, it is not surprising that GSK-3β signaling and LTD/LTP are interconnected. During induction of LTD, calcium enters the cell via the NMDA receptor which triggers the calcium/calmodulin-sensitive enzyme calcineurin (PP2B) and subsequently dephosphorylates inhibitor-1, leading to activation of PP1. PP1 dephosphorylates Ser9-GSK3β and Akt, resulting in enhanced GSK-3β activity (Mulkey et al., 1994; Szatmari et al., 2005; Peineau et al., 2007). Inhibition of GSK-3β prevents the induction of NMDA receptor-LTD but not LTP (Peineau et al., 2007). In contrast to LTD, GSK-3β activity is inhibited during induction of LTP (Hooper et al., 2007; Peineau et al., 2007), and overexpression of GSK-3β inhibits the LTP induction (Zhu et al., 2007).
2. Cocaine and Amphetamine Regulate GSK3β Activity In Vivo
Numerous studies have investigated the effects of acute and repeated psychostimulant administration on GSK-3β activity in mesolimbic circuitry and key afferent pathways. Regulation of GSK-3β is time-dependent following stimulant administration and also depends on the brain region investigated and drug administration protocol. Changes in the phosphorylation state of Akt and GSK-3 in brain tissue have been identified 15 to 120 minutes after a systemic injection of amphetamine or cocaine. Some studies report a rapid increase in phosphorylation of striatal Akt (Shi and McGinty, 2007) or GSK-3β (Svenningsson et al., 2003; Nwaneshiudu and Unterwald, 2010) shortly after stimulant administration (e.g., 15 min). At later times (30–120 min post-injection), GSK-3β is activated by dephosphorylation. For example, cocaine significantly decreases the phosphorylation of Thr308-Akt and Ser9-GSK-3β in the dorsal striatum (caudate putamen) and nucleus accumbens core 30 minutes after administration (Miller et al., 2009 & 2014). Acute amphetamine administration also reduces phosphorylation of Thr308-Akt and GSK-3β in the striatum 90–120 minutes after administration (Beaulieu et al., 2004; Shi and McGinty 2007). Further time-course analysis indicates that D1 receptors regulate Akt at early times (i.e., 15 min) after amphetamine administration, while D2 receptors are responsible for changes in phosphorylation of Akt at later times (i.e., 60 min) (Shi and McGinty, 2011). Blockade of dopamine D2, but not D1 or NMDA receptors, prevents the reduction of pThr308-Akt found 30 minutes after acute cocaine administration, whereas antagonists at dopamine D1, dopamine D2 or glutamatergic NMDA receptors each block cocaine-induced activation of GSK-3β in the caudate putamen (Miller et al., 2014). Thus, cocaine can inhibit the phosphorylation of Akt and GSK-3 via activation of disparate pathways.
Chronic psychostimulant exposure also impacts GSK-3β activity. Decreased phosphorylation of GSK-3β has been revealed in the nucleus accumbens core (Xu et al., 2009 and 2011; Xing et al., 2015) and in the VTA (Wang et al., 2012) after chronic cocaine or methamphetamine administration. While acute cocaine does not alter GSK-3 phosphorylation in the frontal cortex (Miller et al., 2014), repeated cocaine results in increased GSK-3 phosphorylation in this region (Park et al., 2010). Rats injected with cocaine in a binge-pattern (three daily injections 1 hour apart for 1, 3, or 14 days) show lower Akt phosphorylation in the nucleus accumbens and higher in the amygdala after 1 day of binge cocaine administration without significantly altering GSK-3β phosphorylation. Further, phosphorylation of Akt and GSK-3β are significantly lower after 14 days of binge cocaine in the amygdala but not the striatum or hippocampus (Perrine et al., 2008). In summary, although some differences have been reported at early time points, most studies indicate that psychostimulant administration increases GSK-3β activity in the striatum (dorsal and ventral) secondary to increases in neurotransmitter receptor signaling, particularly through dopamine and glutamate receptors.
3. Significance of GSK-3 signaling in psychostimulant-induced behavioral activity and locomotor sensitization
Psychostimulant drugs increase locomotor activity after acute administration, and repeated, intermittent exposure to psychostimulants is known to induce behavioral sensitization (Robinson and Berridge, 1993). Sensitization is defined as an increased effect of a drug due to prior exposure to the drug. In the case of psychostimulants, it is exemplified by an intensification of drug-induced behaviors including locomotor hyperactivity following repeated stimulant administration (Robinson and Berridge, 1993). Evidence suggests that GSK-3 signaling is necessary for stimulant-induced activity and locomotor sensitization. For example, pharmacological inhibition of GSK-3 reduces the hyperlocomotor phenotype exhibited by DAT knockout mice which have elevated striatal dopamine levels (Beaulieu et al., 2004). Dopamine-dependent hyperactivity produced by amphetamine is attenuated in GSK-3β heterozygote mice which have 50% lower levels of GSK-3β compared to wild-type mice (Beaulieu et al., 2004). Several studies demonstrate that systemic administration of GSK-3 inhibitors attenuates hyperactivity induced by amphetamine and cocaine (Miller et al., 2009; Enman and Unterwald, 2012; Zhao et al., 2016). Data from mice with cell-specific deletion of GSK-3β suggest that GSK-3β in D2 receptor-expressing cells may have a predominant role in modulating stimulant-induced locomotor activity, as mice with GSK-3β deletion in D2 receptor-expressing cells have reduced amphetamine- and cocaine-induced locomotion whereas mice with GSK-3 deletion in D1 receptor-expressing cells do not (Urs et al., 2012). However, GSK-3 inhibition dose-dependently reduces ambulatory and stereotypic activity produced by the D1 receptor agonist SKF-82958 (Miller et al., 2010) suggesting a role for GSK-3 in D1 receptor-induced hyperactivity as well. Therefore, hyperlocomotion produced by dopaminergic drugs is mediated at least in part by activation of GSK-3 signaling. These studies indicate that increased dopaminergic neurotransmission activates Akt-GSK3β signaling, and this pathway participates in the resultant hyper-locomotor state.
Repeated administration of psychostimulants can result in locomotor sensitization. Adaptions in dopaminergic and glutamatergic neurotransmission appear to play a role in sensitization. Administration of dopamine D1 or D2 receptor antagonists prior to daily cocaine prevents the development of behavioral sensitization (McCreary and Marsden, 1993; Karler et al., 1994), and transgenic mice lacking the dopamine D1 receptor do not develop locomotor sensitization to cocaine compared to wild-type controls (Karlsson et al., 2008). Further, pretreatment with an NMDA receptor antagonist MK-801 prevents the development of cocaine sensitization (Karler et al., 1989) and cocaine-sensitization produces augmented extracellular glutamate in the core of the nucleus accumbens (Pierce et al., 1996). Therefore, dopamine D1 and D2 receptors and glutamatergic NMDA receptors each appear to play a regulatory role in sensitization produced by repeated administration of psychostimulants. GSK-3 activity in the striatum, and the accumbens core in particular, is critical in the acquisition of behavioral sensitization. Daily pretreatment with systemic GSK-3 inhibitors or their direct infusion into the nucleus accumbens core prior to cocaine, amphetamine or methamphetamine administration prevents the development of locomotor sensitization (Miller et al., 2009; Enman and Unterwald 2012; Xu et al., 2009 and 2011). Additionally, cocaine- and amphetamine-induced locomotor sensitization is reduced in D2 receptor knockout mice and these mice have reduced locomotor response to a D1 agonist (Solis et al., 2019). Together, these findings suggest that D2 receptor activation of Akt-GSK-3 pathway is essential for the development of sensitization. It is still unknown however, how the GSK3 signaling cascade influences stimulant-induced plasticity and sensitization.
Findings by Zhao et al. (2016) demonstrate that acute cocaine or a D1 receptor agonist reduces the firing of medium spiny neurons in the nucleus accumbens of anesthetized rats. This effect of cocaine is blocked by a D1 receptor antagonist, whereas a D2-like receptor agonist or antagonist has no effect on these measures. Pretreatment with a GSK-3 inhibitor attenuates the inhibitory effect of both the D1 receptor agonist and cocaine. GSK-3 inhibition also blocks paired-pulse facilitation by cocaine, suggesting that D1 receptor-mediated GSK-3β activation following cocaine inhibits the presynaptic release of glutamate in the accumbens (Zhao et al., 2016). Therefore, acute cocaine exposure, via blocking dopamine reuptake, may promote feedback inhibition of glutamate release through presynaptic dopamine receptors and this feedback may be regulated by GSK-3 activity.
Cocaine has been shown to increase dopamine and inhibit glutamate transmission from VTA terminals (Adrover et al., 2014). However, reports indicate that extracellular glutamate levels in the nucleus accumbens increase immediately following acute cocaine or amphetamine administration, and this effect is blocked by pretreatment with dopamine D1 or D2 receptor antagonists (Reid et al., 1997, Smith et al., 1995). Repeated cocaine exposure reduces membrane excitability but increases the frequency of miniature excitatory postsynaptic currents (mEPSC) of accumbal D1 receptor-expressing medium spiny neurons and reduces mEPSC in the population of D2 receptor-expressing medium spiny neurons (Kim et al., 2011). Additionally, repeated cocaine or amphetamine reduces firing of nucleus accumbens shell neurons and increases firing of neurons within the accumbens core (Kourich and Thomas, 2009). Therefore, GSK-3 may have differential roles in pre- and post-synaptic signaling in the nucleus accumbens as well as differentially influence neuronal activity depending upon brain region.
Acute and repeated exposure to cocaine activates cholinergic interneurons in the nucleus accumbens shell, and these interneurons are important modulators of medium spiny neuron activity in the striatum (Berlanga et al., 2003; Chuhma et al., 2014). Cholinergic interneuron activity plays a role in associative-learning, including formation of cocaine-context associations (Witten et al., 2010; Lee et al., 2016). Genetic knockdown of GSK-3β expression in the nucleus accumbens shell results in a reduction of action potential firing of cholinergic interneurons, thus demonstrating that GSK-3β activity also plays a role in regulating interneuron activity in the nucleus accumbens (Crofton et al., 2017) although it is unknown if this is due to altered GSK-3β signaling within the interneurons themselves or through altered input from medium spiny neurons or other afferents. In addition, cocaine self-administration is augmented when rats are responding for higher unit doses, but not lower doses of cocaine following GSK-3β knockdown in the accumbens shell (Crofton et al., 2017). It should be noted, however, that GSK-3β activation following stimulant administration has largely been detected in nucleus accumbens core versus the shell region (Xu et al., 2011; Miller et al., 2014). Core and shell neurons differ in responding to afferent input and functionally with the core being more involved in responses to conditioned stimuli and the shell involved in processing of unconditioned stimuli (Meredith et al., 2008; Ito and Hayen, 2011). It remains to be seen if GSK-3 signaling is directly linked to the differential effects of cocaine on the nucleus accumbens core versus shell and action potential firing of specific cell populations within these regions.
Together, studies indicate that exposure to psychostimulants activates GSK-3β in a regional, time, and drug experience dependent manner, and disruption of this GSK-3β signaling interferes with drug-induced behavioral activity. Further study is needed, using techniques with cellular specificity, to determine how GSK-3β activation induced by acute and repeated psychostimulant exposure influences cell-specific synaptic plasticity within the nucleus accumbens core and shell regions as well as afferent and efferent pathways and its influence upon motivated behaviors.
4. Significance of GSK-3 signaling in psychostimulant-induced reward processes
Learned associations between a rewarding or aversive experience and a specific context can be measured using the conditioned place preference or aversion assay, a form of Pavlovian conditioning where preference for a context that was previously paired with a drug is measured. The ability of a drug to produce a conditioned place preference indicates that the drug has rewarding properties (Bardo and Bevins, 2000). Psychostimulants, including cocaine and amphetamine, reliably induce a conditioned place preference in rats and mice. Evidence demonstrates a critical role for both glutamatergic receptor and dopaminergic receptor signaling in the nucleus accumbens in psychostimulant-induced conditioned place preference (Kim et al., 1996; Cervo and Samanin, 1995; Anderson, 2005). Since both of these receptors can regulate GSK-3β activity, a role for GSK-3β signaling is implicated in this process. In support of this, systemic administration of a GSK3 inhibitor prior to cocaine or amphetamine conditioning sessions prevents the development of place preference (Miller et al., 2014; Enman and Unterwald, 2012; Wickens et al., 2017). Intra-accumbal administration of a GSK-3 inhibitor also blocks development of amphetamine-induced place preference (Wickens et al., 2017), whereas GSK-3 inhibition in the VTA does not alter acquisition of cocaine-induced place preference (Li et al., 2014). The acquisition of a contextual fear conditioning response is unaltered by GSK-3 inhibition, demonstrating that impaired GSK-3 signaling does not globally inhibit contextual learning processes (Kimura et al., 2008; Miller et al., 2014). The development of cocaine-induced conditioned place preference is also significantly attenuated in male and female transgenic mice with reduced levels of GSK-3β specifically within the nucleus accumbens, whereas the development of morphine-induced place preference remains intact in these mice (Shi et al., 2019a). This finding agrees with studies indicating that opiates and psychostimulants differentially alter excitatory synapses and neuronal activity in the nucleus accumbens (Graziane et al., 2016; German and Fields, 2007; Sjulson et al., 2018; Hearing, 2019) and can signal through different pathways during the acquisition of conditioned place preference and retrieval of drug-associated contextual memories (Miller et al., 2014, Shi et al., 2014; Wang et al., 2019).
In addition to drug-induced conditioned place preference, conditioned place aversion is regulated by motivational systems in the nucleus accumbens (Wise and Koob, 2014; Wenzel et al., 2015). Dopaminergic transmission in the accumbens is required for conditioned place aversion (Shippenberg et al., 1993; Bals-Kubik et al., 1993). Knockdown of GSK-3β in the nucleus accumbens interferes with the development of place aversion associated with a kappa opioid receptor agonist (Shi et al., 2019a), indicating that accumbal GSK-3β signaling also plays an important role in associations between negative motivational state and environment. Regional differences in cellular subpopulations may be involved in processing reward-predicting cues versus aversive cues (de Jong et al., 2019) differentially impacting target regions (Hikida et al., 2010). However, optogenetic stimulation of D1 receptor- and D2 receptor-expressing medium spiny neurons indicates that activation of both receptors induce reward or aversion depending upon the level of stimulation (Soares-Cunha et al., 2019). Taken together, these observations suggest that acquisition of cocaine- and amphetamine–induced conditioned place preference, as well as aversive cue processing, depends upon activation of GSK-3β within the nucleus accumbens and projections from regions that regulate the development of conditioned responses to cues.
Although most studies have focused upon the nucleus accumbens, a few studies have investigated contributions of GSK-3β activity in other brain regions that regulate the mesolimbic pathway or in a neuron specific manner. GSK-3β signaling in the ventral hippocampus has been shown to play a distinct role in the modulation of responses to contextual cues related to cocaine reward as knockdown of GSK-3β expression in the ventral hippocampus prior to drug conditioning reduces place preference for cocaine (Barr et al., 2020). Multiple studies have demonstrated that input from the ventral hippocampus to nucleus accumbens modulates accumbal neurotransmission and that this input regulates the reinforcing properties of psychostimulants (Ciocchi et al., 2015; LeGates et al., 2018; Sjulson et al., 2018; Zhou et al., 2019; Sikora et al., 2016; Pascoli et al., 2014; Trouche et al., 2019). It is likely that interruption of GSK-3β signaling in the hippocampus interferes with contextual processing important for formation of memories for drug-associated environmental cues through alteration of afferent input to the nucleus accumbens. Dopaminergic transmission in the nucleus accumbens also plays a role in novel object recognition and novel object location memory (Nelson et al., 2010). Knockdown of GSK-3β in either the nucleus accumbens or the ventral hippocampus impairs performance on the object location task, but not object recognition (Shi et al., 2019a; Barr et al., 2020). These results demonstrate a role for GSK-3β in short-term spatial location memory, but not non-spatial memory for objects. The nucleus accumbens contributes to the processing of hippocampal-dependent spatial information, and performance on spatial tasks depends on functional interaction between the hippocampus and accumbens. Therefore, GSK-3β is likely important in regulation of the functional input from the hippocampus to the nucleus accumbens involved in processing contextual information defined by spatial cues as well as interoceptive and emotional cues.
5. Role for GSK-3β signaling in psychostimulant-cue associative memories
In addition to the initial reinforcing effects of psychostimulants, drug-associated stimuli through associative learning can acquire motivational significance and produce drug-seeking behaviors (MacNiven et al., 2018; Kilts et al., 2001; Volkow et al., 2006). Memories of drug-associated stimuli or cues are consolidated for later retrieval. Exposure to these cues causes drug memories to be recalled and this can produce intense drug craving. Cue-induced drug craving activates brain regions involved in stimulus-reward association and motivation, including the basolateral nucleus of the amygdala and the hippocampus, regions that send glutamatergic afferents to the nucleus accumbens and process information about novelty, context and emotions (Gardner, 2011; Childress et al., 1999; Wexler et al., 2001; Fotros et al., 2013). The process of reconsolidation after memory retrieval is thought to strengthen the memory. However, memories can be destabilized after retrieval, enabling the updating of memory by various conditions prior to undergoing reconsolidation (reviewed in Tronson and Taylor, 2007). The nucleus accumbens core, basolateral amygdala, and hippocampus are involved in the reconsolidation of the memories underlying cocaine-related associative memory (Theberge et al., 2010; Li et al., 2016; Wells et al., 2011).
A potential role for GSK-3β in memory reactivation and reconsolidation was demonstrated by Kimura et al., (2008). Mice heterozygous for the GSK-3β gene were able to learn a spatial task at a similar rate compared to wild-type mice. However, GSK-3β heterozygous mice displayed reduced memory of the task at longer time intervals. When a GSK-3 inhibitor was administered before conditioning in a contextual fear conditioning task, memory for the task was not affected when assessed a week later. In contrast, when the GSK-3 inhibitor was administered before re-exposure to the context, memory of the task was impaired when tested one week after re-exposure (Kimura et al., 2008). These observations suggest that activation of GSK-3 is not necessary for acquisition of fear conditioning but disruption of signaling initiated by memory retrieval alters the expression of learned behavior. In support of this, hippocampal GSK-3β has been shown to be activated during memory retrieval in a passive avoidance task (Hong et al., 2012) in which mice learn to avoid an environment in which an aversive stimulus (foot-shock) was previously delivered. Delivery of a GSK-3 inhibitor into the dorsal hippocampus prior to testing interferes with memory retrieval, suggesting the activation of GSK-3 in this region is necessary to produce expression of behavior based upon memory of the task. However, there was no effect when these animals were re-tested 24 hours after re-exposure suggesting a specific effect on retrieval (Hong et al., 2012). GSK-3 is activated following a conditioning session (1 hr after conditioning), during consolidation (24 hr after conditioning) and during reconsolidation (after retrieval) with the reconsolidation period showing the greatest degree of activation (Hong et al., 2012). Genetic knockdown of GSK-3β in the dentate gyrus of the dorsal hippocampus in transgenic mice produces deficits in a spatial memory task (i.e., Morris water maze) as well as contextual fear conditioning (Chew et al., 2015; Liu et al., 2017). Therefore, some degree of GSK-3 activity is likely involved in early stages of memory formation and complete deletion of GSK-3β in certain cell populations likely disrupts neurotransmission or cellular signaling in brain regions involved in these processes. The period following memory retrieval seems to a time in which GSK-3β activity is most critical, and this includes retrieval of memories related to psychostimulants.
Reactivation of cocaine- or methamphetamine-associated memories induced by re-exposure to a context previously paired with the drug, engages the Akt-GSK-3β signaling pathway within the nucleus accumbens, hippocampus and basolateral amygdala resulting in GSK-3β activation (Wu et al., 2011; Shi et al., 2014 & 2019b). These are regions involved in cue-induced drug craving (Gardner, 2011). Systemic injection of a GSK-3 inhibitor following reactivation of drug-cue memories inhibits cue-induced GSK-3β activity and impairs subsequent place preference behavior (Wu et al. 2011; Shi et al., 2014). Loss of place preference appears to be permanent as there is no spontaneous recovery of preference even weeks later (Shi et al., 2014, 2019b, unpublished data). Infusion of a GSK-3 inhibitor into the basolateral amygdala immediately after the reactivation of cue memories also disrupts cocaine memory reconsolidation and prevents cue-induced increases in GSK-3β activity in that region (Wu et al., 2011).
Upon further examination of cellular processes involved in reconsolidation, it was found that administration of glutamatergic NMDA receptor antagonists targeting the NR2A- and NR2B-subtypes following recall of cocaine-associated memories, impairs expression of cocaine place preference one and seven days later, and when administered prior to retrieval, prevents the activation of GSK-3β in the amygdala, nucleus accumbens, and hippocampus produced by cocaine memory reactivation (Shi et al., 2019b). These findings indicate that activation of NMDA receptors is the major upstream element regulating GSK-3β activity by psychostimulant-associated cues. This is in concordance with previous studies demonstrating a role for NMDA receptors in contextual conditioning that is rewarding or aversive (Sikora et al., 2016; Bespalov, 1996). In further investigation of the signaling pathway involved in these processes, protein phosphatase-1 inhibition with okadaic acid blocks activation of GSK3β induced by exposure to cocaine-contextual cues and abolishes a previously established cocaine place preference (Shi et al., 2019b). NMDA receptors containing the NR2B subunit have been shown to increase GSK-3β activity via protein phosphatase-1 (Szatmari et al., 2005; Peineau et al., 2007). Together these studies demonstrate that re-exposure to cocaine-associated environments activates NMDA receptors in the hippocampus, amygdala, and nucleus accumbens, that subsequently dephosphorylate GSK3β through protein phosphatase-1, and this pathway is necessary for reconsolidation of drug-associated memories. The proposed signaling cascade involved in the maintenance of cocaine-associated memories is illustrated in Figure 3. Thus, targeting this pathway could abolish cocaine contextual memories thereby reducing cue-induced craving and relapse.
Figure 3: Potential signaling pathway underlying reconsolidation of stimulant-associated contextual memories.
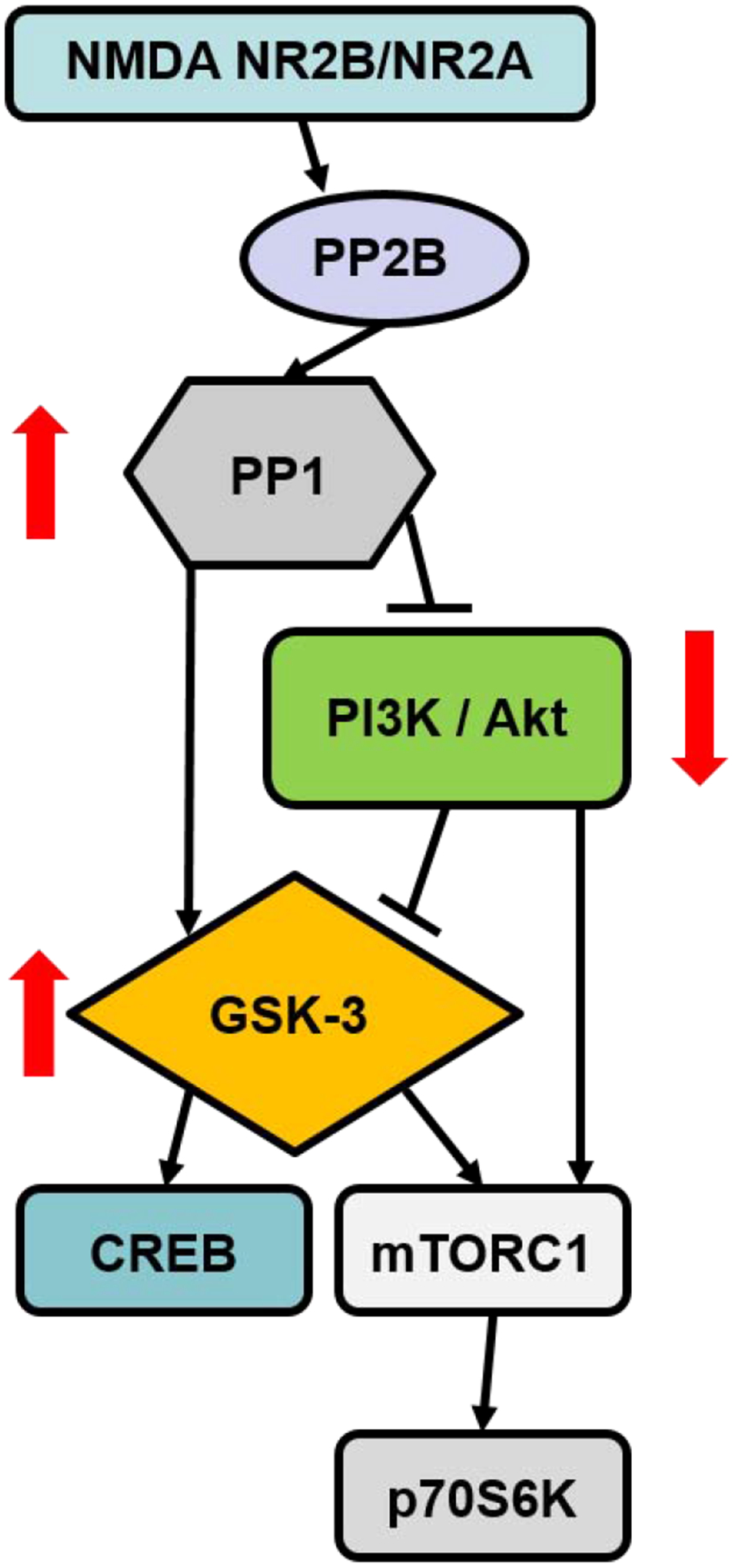
Activation of NMDA receptors in the nucleus accumbens and hippocampus following the reactivation of cocaine-contextual memories stimulates a protein phosphatase cascade, through PP2B and PP1, resulting in dephosphorylation of Akt and GSK-3β. GSK-3β can regulate mTORC1 and CREB. Arrows indicate changes in enzyme activity following reactivation of cocaine-associated memories. GSK, glycogen synthase kinase; mTORC1, mammalian target of rapamycin complex 1; PI3K, phosphatidylinositol 3-kinase; PP1, protein phosphatase 1; PP2B, protein phosphatase 2B. CREB, cAMP response element-binding protein. Adapted from Shi et al., 2014 & 2019b.
6. Conclusions
In summary, a growing body of literature indicates the important role of GSK-3β in the actions of psychostimulants. Specifically, GSK-3β is regulated following exposure to cocaine or amphetamine in a time- and brain region-dependent manner. Dopamine receptors are likely the initiators of GSK-3 activation following acute psychostimulant administration, although glutamate, serotonin, or other receptors may be involved as well. Pharmacological and genetic knockdown studies demonstrate that GSK-3β activation is necessary for the rewarding effects of cocaine and amphetamine. Another important line of research has demonstrated that maintenance of psychostimulant contextual memories relies on GSK-3β signaling secondary to NMDA receptor activation. Inhibition of GSK-3β activity following recall of drug-associated memories interferes with memory reconsolidation, and thereby can erase a previously established place preference. This suggests that GSK-3 inhibitors might be therapeutically useful in the prevention of relapse to psychostimulant use by dampening drug-associated memories and craving in the presence of cues previously associated with drug use. This avenue of research is sure to continue to yield further insights into the importance of this kinase. Targeting the molecular adaptations underlying the persistence of drug-related associative memories suggests a novel approach to diminishing drug-seeking and other behaviors that maintain psychostimulant dependence.
Highlights.
This review discusses the evidence that GSK-3β signaling plays an important role in the acute effects of stimulants as well as in the maintenance of drug-seeking behaviors. Evidence is presented that summarizes how psychostimulant drugs of abuse regulate the function of GSK3 in specific areas of the brain. A role for GSK3 in producing drug reward and reinforcement resulting in drug-seeking behaviors is described. Finally, data to support the involvement of GSK3 signaling in associative learning processes and the maintenance of psychostimulant memories is reviewed.
Acknowledgements:
The authors would like to acknowledge Dr. Xiangdang Shi for her important contributes to this work.
Funding Sources:
This work was supported in part by the National Institutes of Health [R01 DA043988 (EMU) and P30 DA013429 (EMU)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors declare that they have no known competing financial interests or personal relationships that could have appe ared to influence the work reported in this paper.
References
- Adrover MF, Shin JH, & Alvarez VA (2014). Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci, 34(9), 3183–3192. doi: 10.1523/jneurosci.4958-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, & Hemmings BA (1996). Mechanism of activation of protein kinase B by insulin and IGF-1. Embo j, 15(23), 6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Alimohamad H, Rajakumar N, Seah YH, & Rushlow W (2005). Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry, 57(5), 533–542. doi: 10.1016/j.biopsych.2004.11.036 [DOI] [PubMed] [Google Scholar]
- Anderson SM, & Pierce RC (2005). Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther, 106(3), 389–403. doi: 10.1016/j.pharmthera.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, & Shippenberg TS (1993). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther, 264(1), 489–495. [PubMed] [Google Scholar]
- Bardo MT, & Bevins RA (2000). Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl), 153(1), 31–43. doi: 10.1007/s002130000569 [DOI] [PubMed] [Google Scholar]
- Barr JL, Shi X, Zaykaner M, & Unterwald EM (2020). Glycogen Synthase Kinase 3beta in the Ventral Hippocampus is Important for Cocaine Reward and Object Location Memory. Neuroscience, 425, 101–111. doi: 10.1016/j.neuroscience.2019.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, & Caron MG (2007). The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci, 28(4), 166–172. doi: 10.1016/j.tips.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, & Caron MG (2009). Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol, 49, 327–347. doi: 10.1146/annurev.pharmtox.011008.145634 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, & Caron MG (2004). Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A, 101(14), 5099–5104. doi: 10.1073/pnas.0307921101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennecib M, Gong CX, Grundke-Iqbal I, & Iqbal K (2000). Role of protein phosphatase-2A and −1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett, 485(1), 87–93. doi: 10.1016/s0014-5793(00)02203-1 [DOI] [PubMed] [Google Scholar]
- Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, & Alcantara AA (2003). Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience, 120(4), 1149–1156. doi: 10.1016/s0306-4522(03)00378-6 [DOI] [PubMed] [Google Scholar]
- Bespalov A (1996). The expression of both amphetamine-conditioned place preference and pentylenetetrazol-conditioned place aversion is attenuated by the NMDA receptor antagonist (+/−)-CPP. Drug Alcohol Depend, 41(1), 85–88. doi: 10.1016/0376-8716(96)01227-6 [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, & Caboche J (2002). Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci, 22(20), 8911–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, & Bonci A (2012). Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron, 76(4), 790–803. doi: 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, & Di Chiara G (1989). Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience, 28(3), 653–661. doi: 10.1016/0306-4522(89)90012-2 [DOI] [PubMed] [Google Scholar]
- Cervo L, & Samanin R (1995). Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res, 673(2), 242–250. doi: 10.1016/0006-8993(94)01420-m [DOI] [PubMed] [Google Scholar]
- Chen L, Salinas GD, & Li X (2009). Regulation of serotonin 1B receptor by glycogen synthase kinase-3. Mol Pharmacol, 76(6), 1150–1161. doi: 10.1124/mol.109.056994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Gu Z, Liu W, & Yan Z (2007). Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol Pharmacol, 72(1), 40–51. doi: 10.1124/mol.107.034942 [DOI] [PubMed] [Google Scholar]
- Chew B, Ryu JR, Ng T, Ma D, Dasgupta A, Neo SH, … Goh EL (2015). Lentiviral silencing of GSK-3β in adult dentate gyrus impairs contextual fear memory and synaptic plasticity. Front Behav Neurosci, 9, 158. doi: 10.3389/fnbeh.2015.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, & O’Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry, 156(1), 11–18. doi: 10.1176/ajp.156.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Moore H, & Rayport S (2014). Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron, 81(4), 901–912. doi: 10.1016/j.neuron.2013.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, & Klausberger T (2015). Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science, 348(6234), 560–563. doi: 10.1126/science.aaa3245 [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, … Wolf ME (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature, 454(7200), 118–121. doi: 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton EJ, Nenov MN, Zhang Y, Scala F, Page SA, McCue DL, … Green TA (2017). Glycogen synthase kinase 3 beta alters anxiety-, depression-, and addiction-related behaviors and neuronal activity in the nucleus accumbens shell. Neuropharmacology, 117, 49–60. doi: 10.1016/j.neuropharm.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, & Hemmings BA (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature, 378(6559), 785–789. doi: 10.1038/378785a0 [DOI] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, … Lammel S (2019). A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron, 101(1), 133–151.e137. doi: 10.1016/j.neuron.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Bijur GN, Zmijewska AA, Li X, & Jope RS (2006). In vivo regulation of GSK3 phosphorylation by cholinergic and NMDA receptors. Neurobiol Aging, 27(3), 413–422. doi: 10.1016/j.neurobiolaging.2005.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Xiong Z, Chen P, Wei J, Chen S, & Yan Z (2014). beta-amyloid impairs the regulation of N-methyl-D-aspartate receptors by glycogen synthase kinase 3. Neurobiol Aging, 35(3), 449–459. doi: 10.1016/j.neurobiolaging.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, & Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A, 85(14), 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda P, Wisniewski J, Wojtowicz T, Wojcicka O, Jaskiewicz M, Drulis-Fajdasz D, … Gizak A (2018). Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin Ther Targets, 22(10), 833–848. doi: 10.1080/14728222.2018.1526925 [DOI] [PubMed] [Google Scholar]
- Enman NM, & Unterwald EM (2012). Inhibition of GSK3 attenuates amphetamine-induced hyperactivity and sensitization in the mouse. Behav Brain Res, 231(1), 217–225. doi: 10.1016/j.bbr.2012.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada S, Rojo AI, Salinas M, & Cuadrado A (2009). The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: connecting neurotransmission with neuroprotection. J Neurochem, 110(3), 1107–1119. doi: 10.1111/j.1471-4159.2009.06208.x [DOI] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC Jr., Woodgett JR, & Mills GB (2000). Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A, 97(22), 11960–11965. doi: 10.1073/pnas.220413597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, & Nicola SM (2007). Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci, 30, 289–316. doi: 10.1146/annurev.neuro.30.051606.094341 [DOI] [PubMed] [Google Scholar]
- Fotros A, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Gravel P, … Leyton M (2013). Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [(1)(8)F]fallypride study in cocaine dependent participants. Neuropsychopharmacology, 38(9), 1780–1788. doi: 10.1038/npp.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL (2011). Addiction and brain reward and antireward pathways. Adv Psychosom Med, 30, 22–60. doi: 10.1159/000324065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German PW, & Fields HL (2007). Rat nucleus accumbens neurons persistently encode locations associated with morphine reward. J Neurophysiol, 97(3), 2094–2106. doi: 10.1152/jn.00304.2006 [DOI] [PubMed] [Google Scholar]
- Goode N, Hughes K, Woodgett JR, & Parker PJ (1992). Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem, 267(24), 16878–16882. [PubMed] [Google Scholar]
- Graziane NM, Sun S, Wright WJ, Jang D, Liu Z, Huang YH, … Dong Y (2016). Opposing mechanisms mediate morphine- and cocaine-induced generation of silent synapses. Nat Neurosci, 19(7), 915–925. doi: 10.1038/nn.4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, & Jope RS (2001). The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol, 65(4), 391–426. doi: 10.1016/s0301-0082(01)00011-9 [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hough C, Nakazawa T, Yamamoto T, & Chuang DM (2002). Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem, 80(4), 589–597. doi: 10.1046/j.0022-3042.2001.00728.x [DOI] [PubMed] [Google Scholar]
- Hearing M (2019). Prefrontal-accumbens opioid plasticity: Implications for relapse and dependence. Pharmacol Res, 139, 158–165. doi: 10.1016/j.phrs.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, & Nakanishi S (2010). Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron, 66(6), 896–907. doi: 10.1016/j.neuron.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Hong JG, Kim DH, Lee CH, Park SJ, Kim JM, Cai M, … Ryu JH (2012). GSK-3β activity in the hippocampus is required for memory retrieval. Neurobiol Learn Mem, 98(2), 122–129. doi: 10.1016/j.nlm.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Hong M, & Lee VM (1997). Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem, 272(31), 19547–19553. doi: 10.1074/jbc.272.31.19547 [DOI] [PubMed] [Google Scholar]
- Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, … Lovestone S (2007). Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci, 25(1), 81–86. doi: 10.1111/j.1460-9568.2006.05245.x [DOI] [PubMed] [Google Scholar]
- Hui J, Zhang J, Kim H, Tong C, Ying Q, Li Z, … Xi G (2014). Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3beta/beta-catenin signaling. Int J Neuropsychopharmacol, 18(5). doi: 10.1093/ijnp/pyu099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, & Zhou FQ (2010). GSK3 signalling in neural development. Nat Rev Neurosci, 11(8), 539–551. doi: 10.1038/nrn2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, & Hayen A (2011). Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci, 31(16), 6001–6007. doi: 10.1523/jneurosci.6588-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, & Johnson GV (2004). The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci, 29(2), 95–102. doi: 10.1016/j.tibs.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Jope RS, & Roh MS (2006). Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets, 7(11), 1421–1434. doi: 10.2174/1389450110607011421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci, 10(8), 561–572. doi: 10.1038/nrn2515 [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Chaudhry IA, & Turkanis SA (1989). Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci, 45(7), 599–606. doi: 10.1016/0024-3205(89)90045-3 [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Thai LH, & Bedingfield JB (1994). A dopaminergic-glutamatergic basis for the action of amphetamine and cocaine. Brain Res, 658(1–2), 8–14. doi: 10.1016/s0006-8993(09)90003-8 [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, & Holmes A (2008). Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl), 200(1), 117–127. doi: 10.1007/s00213-008-1165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, … Drexler KP (2001). Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry, 58(4), 334–341. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park WK, Jang CG, & Oh S (1996). Inhibition by MK-801 of cocaine-induced sensitization, conditioned place preference, and dopamine-receptor supersensitivity in mice. Brain Res Bull, 40(3), 201–207. doi: 10.1016/0361-9230(96)00006-8 [DOI] [PubMed] [Google Scholar]
- Kim J, Park BH, Lee JH, Park SK, & Kim JH (2011). Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry, 69(11), 1026–1034. doi: 10.1016/j.biopsych.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Kimura T, Yamashita S, Nakao S, Park JM, Murayama M, Mizoroki T, … Takashima A (2008). GSK-3beta is required for memory reconsolidation in adult brain. PLoS One, 3(10), e3540. doi: 10.1371/journal.pone.0003540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagishi Y, Kobayashi M, Kikuta K, & Matsuda S (2012). Roles of PI3K/AKT/GSK3/mTOR Pathway in Cell Signaling of Mental Illnesses. Depress Res Treat, 2012, 752563. doi: 10.1155/2012/752563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, & Thomas MJ (2009). Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci, 29(39), 12275–12283. doi: 10.1523/jneurosci.3028-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Finkelstein J, Choi JY, & Witten IB (2016). Linking Cholinergic Interneurons, Synaptic Plasticity, and Behavior during the Extinction of a Cocaine-Context Association. Neuron, 90(5), 1071–1085. doi: 10.1016/j.neuron.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Messing RO. (2008). Protein kinases and addiction. Ann N Y Acad Sci. 1141:22–57. doi: 10.1196/annals.1441.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, & Thompson SM (2018). Reward behaviour is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature, 564(7735), 258–262. doi: 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy K, & Brion JP (1999). Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J Chem Neuroanat, 16(4), 279–293. doi: 10.1016/s0891-0618(99)00012-5 [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, & Heidenreich KA (2000). Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol, 20(24), 9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Wei YM, Shi HS, Luo YX, Ding ZB, Xue YX, … Yu CX (2014). Glycogen synthase kinase-3beta in the ventral tegmental area mediates diurnal variations in cocaine-induced conditioned place preference in rats. Addict Biol, 19(6), 996–1005. doi: 10.1111/adb.12068 [DOI] [PubMed] [Google Scholar]
- Li X, Rosborough KM, Friedman AB, Zhu W, & Roth KA (2007). Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol, 10(1), 7–19. doi: 10.1017/s1461145706006547 [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, & Jope RS (2004). In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology, 29(8), 1426–1431. doi: 10.1038/sj.npp.1300439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ge S, Li N, Chen L, Zhang S, Wang J, … Wang X (2016). NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience, 315, 45–69. doi: 10.1016/j.neuroscience.2015.11.063 [DOI] [PubMed] [Google Scholar]
- Li YC, & Gao WJ (2011). GSK-3beta activity and hyperdopamine-dependent behaviors. Neurosci Biobehav Rev, 35(3), 645–654. doi: 10.1016/j.neubiorev.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Xie AJ, Zhou Q, Li M, Zhang S, Li S, Wang W, Wang Q, Want JZ (2017) GSK-eb deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory. Sci Rep, 7(1), 5781. doi: 10.1038/s41598-017-06173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo HR, Hattori H, Hossain MA, Hester L, Huang Y, Lee-Kwon W, … Snyder SH (2003). Akt as a mediator of cell death. Proc Natl Acad Sci U S A, 100(20), 11712–11717. doi: 10.1073/pnas.1634990100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, & Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron, 69(4), 650–663. doi: 10.1016/j.neuron.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNiven KH, Jensen ELS, Borg N, Padula CB, Humphreys K, & Knutson B (2018). Association of Neural Responses to Drug Cues With Subsequent Relapse to Stimulant Use. JAMA Netw Open, 1(8), e186466. doi: 10.1001/jamanetworkopen.2018.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoury la Cour C, Salles MJ, Pasteau V, & Millan MJ (2011). Signaling pathways leading to phosphorylation of Akt and GSK-3beta by activation of cloned human and rat cerebral D(2)and D(3) receptors. Mol Pharmacol, 79(1), 91–105. doi: 10.1124/mol.110.065409 [DOI] [PubMed] [Google Scholar]
- Marquez J, Campos-Sandoval JA, Penalver A, Mates JM, Segura JA, Blanco E, … de Fonseca FR (2017). Glutamate and Brain Glutaminases in Drug Addiction. Neurochem Res, 42(3), 846–857. doi: 10.1007/s11064-016-2137-0 [DOI] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, & Bonci A (2006). Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci, 9(7), 868–869. doi: 10.1038/nn1713 [DOI] [PubMed] [Google Scholar]
- McCreary AC, & Marsden CA (1993). Cocaine-induced behaviour: dopamine D1 receptor antagonism by SCH 23390 prevents expression of conditioned sensitisation following repeated administration of cocaine. Neuropharmacology, 32(4), 387–391. doi: 10.1016/0028-3908(93)90161-u [DOI] [PubMed] [Google Scholar]
- McKee BL, & Meshul CK (2005). Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience, 133(2), 605–613. doi: 10.1016/j.neuroscience.2005.02.020 [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, & Kelley AE (2008). The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct, 213(1–2), 17–27. doi: 10.1007/s00429-008-0175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Barr JL, Harper LJ, Poole RL, Gould TJ, & Unterwald EM (2014). The GSK3 signaling pathway is activated by cocaine and is critical for cocaine conditioned reward in mice. PLoS One, 9(2), e88026. doi: 10.1371/journal.pone.0088026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, & Unterwald EM (2009). Cocaine-induced hyperactivity and sensitization are dependent on GSK3. Neuropharmacology, 56(8), 1116–1123. doi: 10.1016/j.neuropharm.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, & Unterwald EM (2010). Inhibition of GSK3 attenuates dopamine D1 receptor agonist-induced hyperactivity in mice. Brain Res Bull, 82(3–4), 184–187. doi: 10.1016/j.brainresbull.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, … Brady ST (2004). A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. Embo j, 23(11), 2235–2245. doi: 10.1038/sj.emboj.7600237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, & Malenka RC (1994). Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature, 369(6480), 486–488. doi: 10.1038/369486a0 [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Thur KE, Marsden CA, & Cassaday HJ (2010). Dissociable roles of dopamine within the core and medial shell of the nucleus accumbens in memory for objects and place. Behav Neurosci, 124(6), 789–799. doi: 10.1037/a0021114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, & Chuang DM (1998). Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A, 95(5), 2642–2647. doi: 10.1073/pnas.95.5.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaneshiudu CA, & Unterwald EM (2010). NK-3 receptor antagonism prevents behavioral sensitization to cocaine: a role of glycogen synthase kinase-3 in the nucleus accumbens. J Neurochem, 115(3), 635–642. doi: 10.1111/j.1471-4159.2010.06973.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT, & Klein PS (2011). Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J Clin Invest, 121(9), 3756–3762. doi: 10.1172/jci45194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Cui FJ, Hwang JY, & Kang UG (2010). Effects of clozapine on behavioral sensitization induced by cocaine. Psychiatry Res, 175(1–2), 165–170. doi: 10.1016/j.psychres.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, & Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature, 509(7501), 459–464. doi: 10.1038/nature13257 [DOI] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, & Collingridge GL (2008). The role of GSK-3 in synaptic plasticity. Br J Pharmacol, 153 Suppl 1, S428–437. doi: 10.1038/bjp.2008.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, … Collingridge GL (2007). LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron, 53(5), 703–717. doi: 10.1016/j.neuron.2007.01.029 [DOI] [PubMed] [Google Scholar]
- Perrine SA, Miller JS, & Unterwald EM (2008). Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem, 107(2), 570–577. doi: 10.1111/j.1471-4159.2008.05632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Vacca G, & Ahn S (2008). A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav, 90(2), 236–249. doi: 10.1016/j.pbb.2007.10.014 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, & Kalivas PW (1996). Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci, 16(4), 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, & Wolf ME (2013). Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harb Perspect Med, 3(2), a012021. doi: 10.1101/cshperspect.a012021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Yang S, Jope RS, & Li X (2012). Functional significance of glycogen synthase kinase-3 regulation by serotonin. Cell Signal, 24(1), 265–271. doi: 10.1016/j.cellsig.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Hsu K Jr., & Berger SP (1997). Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse, 27(2), 95–105. doi: [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Scheid MP, & Woodgett JR (2001). PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol, 2(10), 760–768. doi: 10.1038/35096067 [DOI] [PubMed] [Google Scholar]
- Schmidt HD, & Pierce RC (2010). Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci, 1187, 35–75. doi: 10.1111/j.1749-6632.2009.05144.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, … Kalivas PW (2016). The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev, 68(3), 816–871. doi: 10.1124/pr.116.012484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, & Grace AA (2010). Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology, 35(1), 27–47. doi: 10.1038/npp.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng PL, Gao H, & Poo MM (2011). Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron, 71(3), 433–446. doi: 10.1016/j.neuron.2011.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Barr JL, von Weltin E, Wolsh C, & Unterwald EM (2019a). Differential Roles of Accumbal GSK3beta in Cocaine versus Morphine-Induced Place Preference, U50,488H-Induced Place Aversion, and Object Memory. J Pharmacol Exp Ther, 371(2), 339–347. doi: 10.1124/jpet.119.259283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, & McGinty JF (2007). Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. J Neurochem, 103(2), 706–713. doi: 10.1111/j.1471-4159.2007.04760.x [DOI] [PubMed] [Google Scholar]
- Shi X, & McGinty JF (2011). D1 and D2 dopamine receptors differentially mediate the activation of phosphoproteins in the striatum of amphetamine-sensitized rats. Psychopharmacology (Berl), 214(3), 653–663. doi: 10.1007/s00213-010-2068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, & Unterwald EM (2014). Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology (Berl), 231(16), 3109–3118. doi: 10.1007/s00213-014-3491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, von Weltin E, Barr JL, & Unterwald EM (2019b). Activation of GSK3beta induced by recall of cocaine reward memories is dependent on GluN2A/B NMDA receptor signaling. J Neurochem, 151(1), 91–102. doi: 10.1111/jnc.14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, & Herz A (1993). Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther, 265(1), 53–59. [PubMed] [Google Scholar]
- Sikora M, Tokarski K, Bobula B, Zajdel J, Jastrzebska K, Cieslak PE, … Rodriguez Parkitna J (2016). NMDA Receptors on Dopaminoceptive Neurons Are Essential for Drug-Induced Conditioned Place Preference. eNeuro, 3(3). doi: 10.1523/eneuro.0084-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjulson L, Peyrache A, Cumpelik A, Cassataro D, & Buzsaki G (2018). Cocaine Place Conditioning Strengthens Location-Specific Hippocampal Coupling to the Nucleus Accumbens. Neuron, 98(5), 926–934.e925. doi: 10.1016/j.neuron.2018.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, & Robinson SE (1995). Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res, 683(2), 264–269. doi: 10.1016/0006-8993(95)00383-2 [DOI] [PubMed] [Google Scholar]
- Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, … Rodrigues AJ (2019). Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. doi: 10.1038/s41380-019-0484-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis O, Garcia-Sanz P, Martin AB, Granado N, Sanz-Magro A, Podlesniy P, … Moratalla R (2019). Behavioral sensitization and cellular responses to psychostimulants are reduced in D2R knockout mice. Addict Biol, e12840. doi: 10.1111/adb.12840 [DOI] [PubMed] [Google Scholar]
- Song B, Lai B, Zheng Z, Zhang Y, Luo J, Wang C, … Li M (2010). Inhibitory phosphorylation of GSK-3 by CaMKII couples depolarization to neuronal survival. J Biol Chem, 285(52), 41122–41134. doi: 10.1074/jbc.M110.130351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Scofield M, & Kalivas PW (2016). The good and bad news about glutamate in drug addiction. J Psychopharmacol, 30(11), 1095–1098. doi: 10.1177/0269881116655248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, … Greengard P (2003). Diverse psychotomimetics act through a common signaling pathway. Science, 302(5649), 1412–1415. doi: 10.1126/science.1089681 [DOI] [PubMed] [Google Scholar]
- Szatmari E, Habas A, Yang P, Zheng JJ, Hagg T, & Hetman M (2005). A positive feedback loop between glycogen synthase kinase 3beta and protein phosphatase 1 after stimulation of NR2B NMDA receptors in forebrain neurons. J Biol Chem, 280(45), 37526–37535. doi: 10.1074/jbc.M502699200 [DOI] [PubMed] [Google Scholar]
- Teneud LM, Baptista T, Murzi E, Hoebel BG, & Hernandez L (1996). Systemic and local cocaine increase extracellular serotonin in the nucleus accumbens. Pharmacol Biochem Behav, 53(3), 747–752. doi: 10.1016/0091-3057(95)02087-x [DOI] [PubMed] [Google Scholar]
- Theberge FR, Milton AL, Belin D, Lee JL, & Everitt BJ (2010). The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem, 17(9), 444–453. doi: 10.1101/lm.1757410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, & Taylor JR (2007). Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci, 8(4), 262–275. doi: 10.1038/nrn2090 [DOI] [PubMed] [Google Scholar]
- Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-Dos-Santos V, … Dupret D (2019). A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space. Cell , 176(6), 1393-1406.e1316. doi: 10.1016/j.cell.2018.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Snyder JC, Jacobsen JP, Peterson SM, & Caron MG (2012). Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci U S A, 109(50), 20732–20737. doi: 10.1073/pnas.1215489109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, … Wong C (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci, 26(24), 6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sun LL, Zhu WL, Sun Y, Liu JF, Lu L, & Shi J (2012). Role of calcineurin in the VTA in rats behaviorally sensitized to methamphetamine. Psychopharmacology (Berl), 220(1), 117–128. doi: 10.1007/s00213-011-2461-7 [DOI] [PubMed] [Google Scholar]
- Wang JR, Sun PH, Ren ZX, Meltzer HY, & Zhen XC (2017). GSK-3beta Interacts with Dopamine D1 Receptor to Regulate Receptor Function: Implication for Prefrontal Cortical D1 Receptor Dysfunction in Schizophrenia. CNS Neurosci Ther, 23(2), 174–187. doi: 10.1111/cns.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang H, Cui J, Zhang J, Yin F, Guo H, … Xing B (2019). Opiate-associated contextual memory formation and retrieval are differentially modulated by dopamine D1 and D2 signaling in hippocampal-prefrontal connectivity. Neuropsychopharmacology, 44(2), 334–343. doi: 10.1038/s41386-018-0068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, & Fuchs RA (2011). Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem, 18(11), 693–702. doi: 10.1101/lm.2273111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Rauscher NA, Cheer JF, & Oleson EB (2015). A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem Neurosci, 6(1), 16–26. doi: 10.1021/cn500255p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, & Gore JC (2001). Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry, 158(1), 86–95. doi: 10.1176/appi.ajp.158.1.86 [DOI] [PubMed] [Google Scholar]
- Wickens RH, Quartarone SE, & Beninger RJ (2017). Inhibition of glycogen synthase kinase-3 by SB 216763 affects acquisition at lower doses than expression of amphetamine-conditioned place preference in rats. Behav Pharmacol, 28(4), 262–271. doi: 10.1097/fbp.0000000000000283 [DOI] [PubMed] [Google Scholar]
- Wise RA, & Koob GF (2014). The development and maintenance of drug addiction. Neuropsychopharmacology, 39(2), 254–262. doi: 10.1038/npp.2013.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, … Deisseroth K (2010). Cholinergic interneurons control local circuit activity and cocaine conditioning. Science, 330(6011), 1677–1681. doi: 10.1126/science.1193771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, & Chao SZ (2004). Psychomotor stimulants and neuronal plasticity. Neuropharmacology, 47 Suppl 1, 61–79. doi: 10.1016/j.neuropharm.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Woodgett JR (2001). Judging a protein by more than its name: GSK-3. Sci STKE, 2001(100), re12. doi: 10.1126/stke.2001.100.re12 [DOI] [PubMed] [Google Scholar]
- Xing B, Liang XP, Liu P, Zhao Y, Chu Z, & Dang YH (2015). Valproate Inhibits Methamphetamine Induced Hyperactivity via Glycogen Synthase Kinase 3beta Signaling in the Nucleus Accumbens Core. PLoS One, 10(6), e0128068. doi: 10.1371/journal.pone.0128068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Xue YX, Zhu WL, Li QQ, … Lu L (2011). Glycogen synthase kinase 3beta in the nucleus accumbens core is critical for methamphetamine-induced behavioral sensitization. J Neurochem, 118(1), 126–139. doi: 10.1111/j.1471-4159.2011.07281.x [DOI] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, … Lu L (2009). Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. J Neurochem, 111(6), 1357–1368. doi: 10.1111/j.1471-4159.2009.06414.x [DOI] [PubMed] [Google Scholar]
- Zhao R, Chen J, Ren Z, Shen H, & Zhen X (2016). GSK-3beta inhibitors reverse cocaine-induced synaptic transmission dysfunction in the nucleus accumbens. Synapse, 70(11), 461–470. doi: 10.1002/syn.21922 [DOI] [PubMed] [Google Scholar]
- Zhou W, Chen L, Paul J, Yang S, Li F, Sampson K, … Li X (2012). The effects of glycogen synthase kinase-3beta in serotonin neurons. PLoS One, 7(8), e43262. doi: 10.1371/journal.pone.0043262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhu H, Liu Z, Chen X, Su X, Ma C, … Ma L (2019). A ventral CA1 to nucleus accumbens core engram circuit mediates conditioned place preference for cocaine. Nat Neurosci, 22(12), 1986–1999. doi: 10.1038/s41593-019-0524-y [DOI] [PubMed] [Google Scholar]
- Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, … Wang JZ (2007). Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci, 27(45), 12211–12220. doi: 10.1523/jneurosci.3321-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


