Abstract
OBJECTIVES:
Evaluate effects of a multicomponent intervention (human papillomavirus [HPV] vaccine-specific brochure and recalls) on HPV vaccination and secondarily examine if race/ethnicity moderates effects.
METHODS:
Unvaccinated girls aged 11 to 18 years attending 4 safety-net pediatric clinics and their parent/guardian (n = 814 dyads) were randomized to (1) active comparison (general adolescent vaccine brochure), or (2) intervention consisting of a HPV vaccine-specific brochure, telephone recalls to parents who declined, and recalls to patients overdue for doses 2 and 3. HPV 1-dose and 3-dose coverages were assessed via electronic health records 12 months after randomization. Multivariate logistic regressions estimated adjusted odds and marginal predicted vaccine coverage by study arm and race/ethnicity.
RESULTS:
Intent-to-treat analyses found no main effect of the HPV vaccine-specific brochure on 1-dose coverage (42.0% vs 40.6%); however, secondary analyses found race/ethnicity was a significant moderator such that the intervention was effective only for Hispanic individuals (adjusted odds ratio [AOR] 1.43; 95% confidence interval [CI] 1.02–2.02), and not effective for black individuals (AOR 0.64; 95% CI 0.41–1.13). Recalls to parents who declined the vaccine during the index visit were not effective, but recalls to patients overdue for doses 2 and 3 were effective at increasing 3-dose coverage regardless of race/ethnicity (AOR 1.99; 95% CI 1.16–3.45).
CONCLUSIONS:
Educational materials describing only the HPV vaccine were effective for Hispanic but not black individuals. Future research should test mechanisms that may mediate intervention effects for different racial/ethnic groups, such as different informational needs or vaccine schemas (experiences, beliefs, norms).
What’s Known on This Subject:
Previous studies have evaluated separately the effects of brief education and reminder/recall intervention strategies to increase human papillomavirus vaccine coverage. None have examined if intervention effects varied by race/ethnicity.
What This Study Adds:
When compared with a general adolescent vaccine brochure, human papillomavirus vaccine-specific educational materials increased 1-dose coverage among Hispanic but not black individuals. Recalls for doses 2 and 3 were effective in increasing 3-dose coverage for both racial/ethnic groups.
Human papillomavirus (HPV) vaccination could reduce cervical cancer disparities if populations suffering disproportionately from HPV-related mortality (minority, low-income, underserved) use this prevention strategy. 1 , 2 Safety-net clinics are an important medical home for underserved adolescents and have tremendous potential to address disparities. 3 , 4
HPV vaccine delivery is challenging because of the dosing schedule, target population, and parental ambivalence. 5 – 9 Unlike other adolescent vaccines, guidelines recommend 3 doses over a 6-month period. 10 Although most adolescents have a regular place for health care (96% in the United States; 73% among the uninsured 11 ), recent contact with providers is less frequent (89% teens aged 12–17 compared with 96.7% children aged 0–4 visited a provider in the past year 11 ). Several studies document parental ambivalence and poor motivation about the HPV vaccine, attributing it to several reasons, including low perceived risk, beliefs that the vaccine is not important or efficacious, and concerns about side effects. 12 – 14 Given these challenges, it is critically important to offer both education and vaccine opportunities at all health care visits. 8 , 15 – 17 Reminders and recalls have been shown to increase immunization rates, 18 , 19 but less is known about their effectiveness for improving immunizations among adolescents attending safety-net clinics. 20 , 21 Few have assessed multicomponent interventions providing both brief education and reminders/recalls for HPV vaccination. 22 – 24
Standard practice in most pediatric clinics is to give educational materials, such as Vaccine Information Statements by the Centers for Disease Control and Prevention (CDC) during rather than before a visit. 25 This does not provide adequate time for parents to review materials and formulate questions for their provider. 25 – 27 Such extra time to read and think may be important for safety-net populations because of lower literacy levels 28 and controversial media portrayal of immunizations. 6 , 29 We conducted a 2-arm randomized controlled trial in 4 safety-net clinics to evaluate a multicomponent intervention. We hypothesized that
HPV-specific compared with general materials (Active Comparison group) mailed 1 to 2 weeks before a visit would increase 1-dose coverage;
Among parents declining the vaccine during the visit, delivery of a telephone recall 2 weeks after the visit would increase 1-dose coverage; and
Delivery of telephone recalls if overdue for doses 2 or 3 would increase 3-dose coverage.
Given differences in HPV vaccine attitudes and uptake among different races/ethnicities 30 – 32 and potential for vaccine messages to have harmful effects, 33 we also explored whether intervention effects were moderated by race/ethnicity.
Methods
Setting
This study was conducted in Parkland Health and Hospital System, the safety net for Dallas County, the ninth largest and one of the most ethnically diverse US counties. From Parkland’s system of 10 neighborhood-based pediatric clinics, we identified 4 with the largest volume of patients aged 11 to 18 (43% of adolescent population). All clinics use EpicCare electronic health record (EHR; Verona, WI), participate in the Vaccines for Children Program, and have a standing order policy offering all Advisory Committee on Immunization Practices recommended vaccines at preventive, acute, and nurse-only visits.
Study Design and Patient Population
From February to December 2011, all unvaccinated female patients aged 11 to 18 with an upcoming appointment (hereafter index visit) at 1 of the 4 clinics were selected and randomized. The target population was parents/guardians (Fig 1) because consent is required for immunization delivery. Patient age (at index visit) was restricted to 11 to 18 based on guidelines 10 , 34 and Vaccines for Children eligibility. The EHR was used to verify and monitor HPV vaccine status, and obtain patient information including name, address, telephone number, birth date, race, ethnicity, preferred language for communicating with provider (English/Spanish), and upcoming appointment details (visit type, time). This study was registered at ClinicalTrials.gov (NCT01729429).
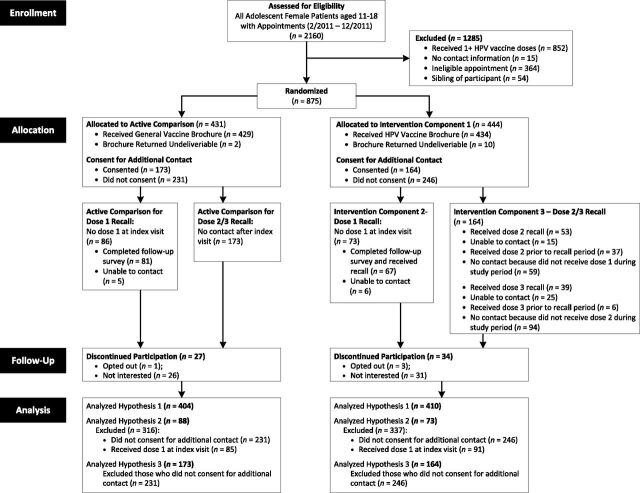
FIGURE 1.
Enrollment and randomization of eligible adolescent female patients and their parent/guardian (dyad) and construction of analytic samples for study hypotheses. Dyads were randomized when upcoming appointments were identified via EHR. Recalls were delivered via telephone.
Patient-parent dyads were excluded if (1) patient had ≥1 HPV vaccine doses, (2) no contact information, (3) appointment was not with a primary care provider (eg, social worker) or did not allow for mailing of materials 1 to 2 weeks before the visit, (4) sibling enrolled in study, (5) parents did not speak English or Spanish, or (6) patient had an HPV vaccine contraindication (eg, pregnant). Exclusions were assessed by using EHR review and during participant contact. University of Texas Southwestern Medical Center’s Institutional Review Board approved this study as follows: waiver of informed consent for Intervention Component 1 (educational materials) and informed consent for Components 2 and 3 (recalls).
Randomization
From weekly EHR reports identifying patients with upcoming appointments, we selected all age-eligible, unvaccinated adolescent females and used SQL Rand function (Microsoft, Redmond, WA) to randomly assign half to Intervention and half to Active Comparison. Approximately 1 to 2 weeks before the visit, staff mailed invitation letters and educational materials to parents in the EHR-documented preferred language. The letter requested participation in a “project to improve patient satisfaction with medical care and vaccine delivery,” and included a telephone number to opt out.
A few days later, a blinded bilingual research assistant (RA) called parents/guardians who did not opt out. The RA explained the study, verified eligibility, and invited the parent to meet 20 minutes before the visit. RAs attempted contact up to 6 times.
Families who agreed met a bilingual RA to obtain consent for additional contact, including previsit and follow-up surveys, and intervention components 2 and 3. Previsit surveys were administered to parents and adolescents. The parent survey included items assessing usefulness of educational materials; the patient’s health care use; the parent’s HPV vaccine knowledge, perceived risk, perceived benefits and barriers, decision stage according to the Precaution Adoption Process Model 35 – 37 ; and parent demographics. The adolescent survey asked about Internet use, information seeking, and communication with provider. Families agreeing to additional contact received a $20 gift card for participation.
Intervention Component 1: Educational Materials
To develop theory-based, HPV-specific materials, we conducted focus groups and interviews with parents of Parkland patients. We asked what information beyond that provided in the CDC’s Vaccine Information Statement would help parents in the HPV vaccine decision process. Based on qualitative findings, we created a brochure focusing on 3 theoretical constructs: perceived risk, vaccine efficacy, and perceived barriers, particularly safety concerns. The brochure was translated and underwent cognitive testing with English and Spanish speakers. Both versions were reviewed by a community advisory board of local social services agency leaders, providers, and parents. Adjustments were made to ensure cultural sensitivity and fifth-grade reading level.
Intervention patients were mailed this brochure with their invitation letter. Those in Active Comparison received a CDC brochure about all Advisory Committee on Immunization Practices recommended vaccines.
Intervention Component 2: Dose 1 Recall
For vaccine-eligible children, EHR programming requires providers to document in a discrete field parents’ vaccine decision (given, refused, out of stock) at every encounter. Staff used weekly EHR reports to identify parents who declined at the index visit. Two weeks after the visit, a nurse called parents who consented for additional contact. She administered a short follow-up survey assessing HPV vaccine decisional stage, perceived risk, information seeking, self-efficacy for initiation, and provider recommendation. If randomized to Intervention, she used a script reminding the parent that Parkland providers strongly recommended the vaccine and offered to schedule a nurse-only immunization appointment. Active Comparison parents were called and only completed the survey.
Intervention Component 3: Dose 2/3 Recalls
Dose 2/3 recalls used similar methods except that Active Comparison dyads were not contacted. Staff used weekly EHR reports to monitor HPV dose 2/3 administration among Intervention patients who received dose 1 at index visit. The nurse called parents 4 weeks overdue for either dose 2 or 3 to administer a survey assessing HPV vaccine decisional stage, perceived risk, information seeking, and self-efficacy for completion. She stressed importance of receiving all 3 doses and offered to schedule a nurse-only appointment. Up to 6 attempts were made to deliver recalls for each dose.
Outcomes and Covariates
Depending on the hypothesis, our primary outcome was HPV vaccine 1-dose or 3-dose coverage (yes/no) as documented in the EHR 12 months after randomization. We obtained EHR reports through December 2012 to ascertain outcomes and data on demographics, health care utilization before the index visit, insurance status, and clinic location at the index visit.
Sample Size
Review of similar interventions found effect sizes up to 29% (median 16%). 18 Our review of Parkland clinics found baseline 1- and 3-dose coverage rates of 30.0% and 6.5%. 3 To detect our a priori 10% difference between groups to justify clinical impact, with 80% power and α of 0.05, we needed 350 participants per group.
Statistical Analysis
To examine if patient demographic and contact characteristics differed between Intervention and Active Comparison groups, we conducted 2-sided χ2 tests. Analyses for hypothesis 1 followed an intent-to-treat principle. Because the institutional review board required consent for recall intervention components, analyses for hypotheses 2 and 3 were conducted on the subset of parents who consented (Fig 1).
To test all hypotheses, we ran a series of univariate logistic regressions, then a multivariate model controlling for significant covariates, and finally an interaction model to explore whether race/ethnicity moderated intervention effects. The first univariate model compared Intervention with Active Comparison. Other univariate models examined influence of patient race/ethnicity, age group, clinic location, and parental consent for additional contact on vaccine outcomes. Covariates significant at P < .05 were entered into the multivariate model. Interaction models were run even if main intervention effects and race/ethnicity were not significant. We multiplied intervention assignment and race/ethnicity to determine if moderation was present on a synergistic scale and retained the main effects of intervention assignment, race/ethnicity, and covariates. Hispanic individuals randomized to Active Comparison were selected as the referent, as they were the largest racial-ethnic group. For significant interactions, we plotted estimated marginal effects (expected coverage if all aforementioned covariates were balanced across racial/ethnic group) and 95% confidence interval (CI) separately by race/ethnicity. Analyses were performed by using SAS 9.3 (SAS Institute, Inc, Cary, NC).
Results
Half of the participants (n = 814) were 11 to 12 years old at index visit (Table 1). Most were Hispanic (68%) or non-Hispanic black (28%) and most parents preferred communicating in Spanish (62%). Nearly all were publicly insured (74%) or uninsured (24%). HPV vaccine refusal rate before index visit was low (10.6%). Approximately 41% of parents consented for additional contact. There were no differences between Intervention and Active Comparison groups (Table 1). Compared with nonconsenters, parents who agreed to additional contact had younger adolescents, were Hispanic, preferred to communicate in Spanish, and attended clinic 3 (data not shown). Analytic samples for each hypothesis are described in Fig 1.
TABLE 1.
Demographic and Contact Characteristics of Adolescent Female Patients Enrolled in the Randomized Trial in Safety-Net Clinics, Overall and by Study Arm (n = 814)
| Characteristics | Overall, n (%) | Intervention, n (%) | Active Comparison, n (%) |
|---|---|---|---|
| n | 814 | 410 | 404 |
| Race/Ethnicity | |||
| Hispanic | 555 (68.2) | 288 (70.2) | 267 (66.1) |
| Non-Hispanic black | 228 (28.0) | 108 (26.3) | 120 (29.7) |
| Non-Hispanic white/other a | 31 (3.8) | 14 (3.4) | 17 (4.2) |
| Age group | |||
| 11–12 y | 408 (50.1) | 214 (52.2) | 194 (48.0) |
| 13–18 y | 406 (49.9) | 196 (47.8) | 210 (52.0) |
| Language preference, parent | |||
| Spanish | 503 (61.8) | 261 (63.7) | 242 (59.9) |
| English | 311 (38.2) | 149 (36.3) | 162 (40.1) |
| Insurance, index visit | |||
| Public | 603 (74.1) | 305 (74.4) | 298 (73.8) |
| Private | 16 (2.0) | 9 (2.2) | 7 (1.7) |
| No insurance | 195 (24.0) | 96 (23.4) | 99 (24.5) |
| Clinic, index visit | |||
| Clinic 1 | 282 (34.6) | 139 (33.9) | 143 (35.4) |
| Clinic 2 | 183 (22.5) | 94 (22.9) | 89 (22.0) |
| Clinic 3 | 238 (29.2) | 124 (30.2) | 114 (28.2) |
| Clinic 4 | 111 (13.6) | 53 (12.9) | 58 (14.4) |
| HPV vaccine refusal before index visit | |||
| Yes | 86 (10.6) | 45 (11.0) | 41 (10.1) |
| No | 728 (89.4) | 365 (89.0) | 363 (89.9) |
| Parent consented for additional contact | |||
| No consent | 477 (58.6) | 246 (60.0) | 231 (57.2) |
| Yes, consented | 337 (41.4) | 164 (40.0) | 173 (42.8) |
We combined non-Hispanic white, Asian, Native American, and multiracial individuals and patients classified as “other” into 1 category because of small numbers.
Effect of HPV-Specific Materials on 1-Dose Coverage (Hypothesis 1)
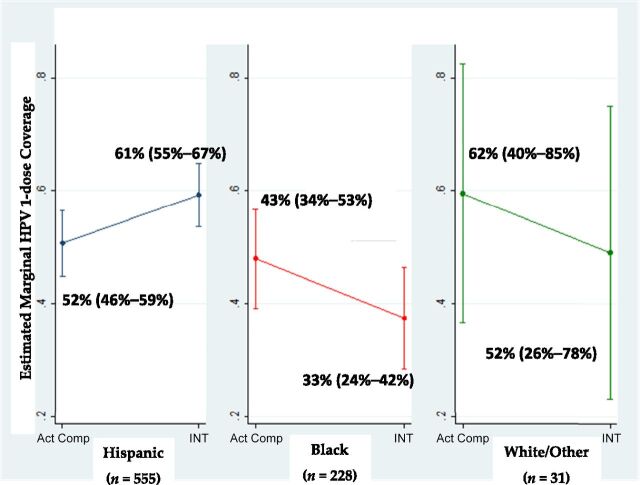
We found no main effect of mailed HPV-specific compared with general materials on 1-dose coverage (42.0% vs 40.6%; adjusted odds ratio [AOR] 1.11, 95% CI 0.84–1.47; Table 2); however, the interaction model (Table 2) revealed that race/ethnicity moderated the intervention effect. Hispanic individuals exposed to HPV-specific materials were more likely to get the first dose than Hispanic individuals mailed general materials (AOR 1.43, 95% CI 1.02–2.02). Although not significant, the magnitude and direction of estimate for black individuals (AOR 0.64, 95% CI 0.37–1.10) suggested a possible harmful effect of HPV-specific materials. Also, the odds ratio (OR) magnitude and direction suggested that black (versus Hispanic) individuals, regardless of intervention assignment, were less likely to start the HPV vaccine series (AOR 0.68, 95% CI 0.41–1.13). Figure 2 depicts the model marginal estimates by study arm and racial-ethnic group. The estimated intervention effect for Hispanic individuals is a 9 percentage point increase in 1-dose coverage rates but a 10 percentage point decrease for black individuals. The small number of white individuals and other races (n = 31) argues for caution when interpreting those estimates.
TABLE 2.
Predictors of 1 Dose HPV Vaccine Coverage at 12 Months After Randomization: Univariate, Multivariate, and Interaction Logistic Regression Models (n = 814)
| Univariate Models | Multivariate Model | Interaction Model | |
|---|---|---|---|
| OR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| −2 Log L a | — | 1088.059 | 1081.359 |
| Study group | |||
| AC | Reference | Reference | Reference |
| I | 1.12 (0.85–1.48) | 1.11 (0.84–1.47) | 1.43 (1.02–2.02) c |
| Race/Ethnicity | |||
| Hispanic | Reference | Reference | Reference |
| Non-Hispanic black | 0.61 (0.45–0.84) b | 0.46 (0.31–0.69) b | 0.68 (0.41–1.13) |
| Non-Hispanic white | 0.99 (0.48–2.04) | 1.06 (0.50–2.23) | 1.53 (0.56–4.23) |
| Study group × race/ethnicity | |||
| Hispanic: I versus AC | — | — | 1.43 (1.02–2.02) c |
| Non-Hispanic black: I versus AC | — | — | 0.64 (0.37–1.10) |
| Non-Hispanic white I versus AC | — | — | 0.65 (0.15–2.76) |
| Age group at randomization | |||
| 11–12 y | Reference | Reference | Reference |
| 13–18 y | 0.63 (0.48–0.84) b | 0.67 (0.50–0.90) b | 0.67 (0.50–0.90) b |
| Clinic, first appointment | |||
| Clinic 1 | Reference | Reference | Reference |
| Clinic 2 | 1.06 (0.73–1.54) | 0.72 (0.47–1.12) | 0.71 (0.46–1.10) |
| Clinic 3 | 0.93 (0.66–1.32) | 0.54 (0.35–0.83) c | 0.54 (0.35–0.84) c |
| Clinic 4 | 0.93 (0.60–1.45) | 0.61 (0.36–1.01) | 0.61 (0.37–1.02) |
| Parent consented for additional contact | |||
| No consent | Reference | Reference | Reference |
| Yes, consented | 1.73 (1.30–2.29) b | 1.67 (1.25–2.23) b | 1.67 (1.25–2.24) b |
AC, active comparison; I, intervention.
Likelihood ratio test comparing multivariate and interaction models (1088.059–1081.359 = 6.7 with 10–8 = 2 DF) is significant at P = .035.
P < .01.
P < .05.
FIGURE 2.
Model-adjusted marginal predicted HPV vaccine 1-dose coverage estimates and 95% CI 12 months after randomization among adolescent female patients attending 4 safety-net clinics. Estimates controlled for all variables in Table 2’s interaction model. Act Comp, active comparison Group; INT, intervention group.
Table 2 also shows that 1-dose coverage rates were lower for older (versus younger) adolescents and patients of clinic 3 (versus clinic 1). Parents who consented for additional contact were more likely to receive the first HPV dose than nonconsenters (AOR 1.67, 95% CI 1.25 – 2.24). At 12 months after randomization, 36.0% and 44.5% of the Intervention and Active Comparison groups did not receive any HPV vaccine doses even though all had at least 1 clinic opportunity.
Effect of Recall on 1-Dose Coverage (Hypothesis 2)
For parents who consented to additional contact and declined the vaccine at the index visit (n = 159), we found no main effect of telephone recall on 1-dose coverage after 12 months. HPV 1-dose rates were similar across Intervention and Active Comparison groups (19.2% [14/73] and 12.8% [11/86], respectively). In univariate analyses, race/ethnicity, patient age, or clinic was not associated with 1-dose coverage. Few patients started the series after declining (n = 25), precluding multivariate analyses.
Effect of Recalls on 3-Dose Coverage (Hypothesis 3)
Among dyads consenting for additional contact, there was a significant main effect such that Intervention patients were more likely to receive all 3 doses 12 months after randomization compared with Active Comparison patients (28.7% vs 15.6%, respectively).
In univariate and multivariate analyses, age and clinic site but not race/ethnicity were associated with 3-dose coverage (Table 3). Older patients aged 13 to 18 were less likely to complete than patients aged 11 to 12 (AOR 0.56, 95% CI 0.31–1.00). Clinic 4 had significantly lower completion rates than clinic 1 (AOR 0.21, 95% CI 0.06–0.80). There was no significant interaction between intervention assignment and race/ethnicity (P = .47).
TABLE 3.
Predictors of 3-Dose HPV Vaccine Coverage at 12 Months After Randomization Among Parent-Adolescent Patient Dyads Who Consented for Additional Contact: Univariate, Multivariate, and Interaction Logistic Regression Models (n = 337)
| Univariate Models | Multivariate Model | |
|---|---|---|
| OR (95% CI) | AOR (95% CI) | |
| Study group | ||
| Active comparison | Reference | Reference |
| Intervention | 2.17 (1.28–3.70) a | 1.99 (1.16–3.45) b |
| Race/Ethnicity | ||
| Hispanic | Reference | Reference |
| Non-Hispanic black | 1.07 (0.59, 1.94) | 0.84 (0.39–1.77) |
| Non-Hispanic white | 2.78 (0.60–12.83) | 3.41 (0.62–18.88) |
| Age group at randomization | ||
| 11–12 y | Reference | Reference |
| 13–18 y | 0.46 (0.26–0.80) a | 0.56 (0.31–1.00) b |
| Clinic, first appointment | ||
| Clinic 1 | Reference | Reference |
| Clinic 2 | 0.91 (0.45–1.86) | 0.87 (0.39–1.97) |
| Clinic 3 | 0.86 (0.47–1.58) | 0.76 (0.36–1.61) |
| Clinic 4 | 0.20 (0.06–0.69) b | 0.21 (0.06–0.80) b |
P < .01.
P < .05.
Discussion
This randomized trial was novel in evaluating whether effects of a multicomponent intervention on initial and subsequent HPV vaccine doses were moderated by race-ethnicity. We found that HPV-specific materials mailed before a clinic visit increased 1-dose coverage for Hispanic but not black individuals. Recalls to parents declining the vaccine during the visit were not effective at encouraging initiation; however, recalls to those overdue for doses 2 and 3 were effective at increasing 3-dose coverage for both blacks and Hispanic individuals. Small numbers of white individuals in our study precluded examination of whether intervention effects differed for them. Although results are encouraging, at the end of the 12-month study period, many did not receive any HPV vaccine doses despite opportunity and clinic policies that strongly recommend the vaccine and support offering it at every visit. 3
Ours was the first randomized trial to compare the effect of HPV-specific materials to an active comparator emphasizing all recommended vaccines on vaccine behavior. 38 Past studies were limited because they assessed intervention impact on intention, 39 – 43 or used a pre/post design with no control group. 39 , 40 , 44 Pragmatic trial designers investigating whether interventions work in usual care settings recommend (1) using EHR because outcomes are objectively measured, and (2) comparing interventions to the best alternative management strategy currently available. 45 In this context, the best comparator is educational materials describing all recommended vaccines because providers are encouraged to embed HPV vaccine recommendation in a broader discussion about the benefits of all vaccines. 17 , 46
Our findings suggest that effect of HPV-specific materials differed by race/ethnicity: positive effect on Hispanic individuals and no effect on black individuals (point estimate was negative but CI was wide and spanned 1.0). Several studies documented differing levels of HPV vaccine acceptability, concerns, and behavior for Hispanic and black individuals. 32 , 47 , 48 These differences between racial/ethnic groups may stem from differences in HPV vaccine knowledge or general vaccine schemas: worldviews based on experiences, values, beliefs, and norms. 49 Compared with black individuals, Hispanic individuals (particularly recent immigrants) may be less aware of the HPV vaccine, have witnessed more vaccine-preventable illnesses, report greater trust in providers and health care systems, and report fewer concerns about vaccine safety and financial motivations of government officials. 50 – 52 Although our materials explained how the HPV vaccine is routinely recommended like other vaccines, it is possible that exclusive focus on the HPV vaccine may have triggered parents’ skepticism and negative schemas about new medical technologies. Our findings parallel work by Nyhan and colleagues 33 who found effects of provaccine messages varied by parental attitudes, such that parents with less favorable attitudes had lower intentions. Others have found that parents of unvaccinated adolescents are a heterogeneous group composed of those who would easily accept the HPV vaccine if given basic information and the opportunity as well as those who are hesitant and need more information addressing safety concerns, the recommended age, and mistrust of pharmaceutical companies. 32 , 53 Black parents, in particular, may need information addressing concerns. 32 , 54 Researchers could use our intervention effect estimates to power future trials that confirm this finding and examine why intervention effects differ by race/ethnicity (eg, less favorable attitudes). 55 , 56
The positive effect of our dose 2 and 3 telephone recalls on improving completion corresponds with other studies of urban, underserved adolescent populations. 21 , 23 , 57 , 58 Only 5% to 25% of US pediatric practices use reminder/recall interventions. 59 , 60 Key implementation barriers include lack of human and financial resources, low confidence that immunization histories are up-to-date, and need for accurate contact information. Future research should monitor if EHR adoption facilitates reminder/recall implementation.
This study has some strengths and limitations. Our evaluation design for the HPV-specific materials followed many recommended elements of a pragmatic trial: randomization of all eligible patients, an active comparison group representing the best alternative management strategy, using the EHR to unobtrusively and objectively measure outcomes, and an intent-to-treat analysis plan. 45 Because we had to obtain consent to deliver recalls, evaluation of those strategies is potentially limited by selection bias and may not be as generalizable. Also, the follow-up survey administered to Active Comparison parents who declined at index (component 2) may have served as a prompt and undermined group differences. There were clinic differences in terms of 3-dose coverage rates (lower for clinic 4 than clinic 1). Although we did not notice differences in HPV vaccination policies, future multisite trials should monitor clinic-level factors that may affect intervention implementation. Our study may have limited external generalizability, as it was conducted in an urban safety-net system serving mainly uninsured, poor, predominantly minority patients. Future studies should examine if interventions like ours are effective in other populations and settings (eg, higher socioeconomic status patients in independent pediatric clinics). Finally, the 12-month study period limited our ability to examine if intervention strategies had a delayed effect on coverage patterns.
Conclusions
Our randomized controlled trial, by measuring effects of a parent-targeted, multicomponent intervention on vaccine coverage, adds to the growing HPV intervention literature. We found that HPV-specific materials were effective at increasing 1-dose coverage among Hispanic but not black individuals. Recalls for doses 2 and 3 were effective for both racial/ethnic groups. Recalls to parents who declined the HPV vaccine was not effective and many parents did not choose to vaccinate their daughters (∼58%) despite an opportunity and supportive clinic policies. 3 Intervention research on what motivates parents to start the HPV vaccine series is urgently needed.
Acknowledgments
The authors acknowledge the support and cooperation received from the following individuals at Parkland Health and Hospital System, Dallas, TX: Deborah Crawford, RN (implementing recalls); Robert McDermott (data extraction); Susan Partridge, RN, Anna Barden, RN, Aletheia Miller (administrative support for conducting research); and Eric Walker, Kerrie Roberts Watterson, Albert Esparsen, Consuelo Cabrera, Shelia White-Jackson, MD, Urmesh Shah, MD, Cesar Termulo, MD, Levet Hamilton, RN (clinic site administrators, lead physicians, and lead nurse). These contributors received no compensation for their work other than their usual salary. They have no conflicts of interest relevant to this article.
Glossary
- AOR
adjusted odds ratio
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EHR
electronic health record
- HPV
human papillomavirus
- RA
research assistant
Footnotes
Dr Tiro conceptualized and designed the study, interpreted analyses, and drafted the initial manuscript; Ms Sanders carried out analyses and helped draft the initial manuscript; Dr. Pruitt assisted in analyses of the study, and critically reviewed the manuscript; Ms Stevens and Ms Bishop assisted in design of study, designed data collection instruments, coordinated and supervised data collection, and critically reviewed the manuscript; Dr Skinner assisted in design of study and critically reviewed the manuscript; Drs Persaud and Fuller assisted in design of study, facilitated data collection in the clinics, and critically reviewed the manuscript; and all authors approved the final submitted manuscript.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01729429).
FUNDING: All phases of this study were supported by a Cancer Prevention and Research Institute of Texas grant (PI: Tiro; PP 100047). In addition, language translation and educational brochure production was supported by cores of the UT Southwestern Harold C. Simmons Cancer Center Support Grant P30 (CA142543) and UT Southwestern Center for Translational Medicine grant (1U54AI108323).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Tiro JA , Saraiya M , Jain N , et al. Human papillomavirus and cervical cancer behavioral surveillance in the US. Cancer. 2008;113(suppl 10):3013–3030 [DOI] [PubMed] [Google Scholar]
- 2. Downs LS Jr , Scarinci I , Einstein MH , Collins Y , Flowers L . Overcoming the barriers to HPV vaccination in high-risk populations in the US. Gynecol Oncol. 2010;117(3):486–490 [DOI] [PubMed] [Google Scholar]
- 3. Tiro JA , Pruitt SL , Bruce CM , et al. Multilevel correlates for human papillomavirus vaccination of adolescent girls attending safety net clinics. Vaccine. 2012;30(13):2368–2375 [DOI] [PubMed] [Google Scholar]
- 4. Tsui J , Singhal R , Rodriguez HP , Gee GC , Glenn BA , Bastani R . Proximity to safety-net clinics and HPV vaccine uptake among low-income, ethnic minority girls. Vaccine. 2013;31(16):2028–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Humiston SG , Rosenthal SL . Challenges to vaccinating adolescents: vaccine implementation issues. Pediatr Infect Dis J. 2005;24(suppl 6):S134–S140 [DOI] [PubMed] [Google Scholar]
- 6. Ford CA , English A , Davenport AF , Stinnett AJ . Increasing adolescent vaccination: barriers and strategies in the context of policy, legal, and financial issues. J Adolesc Health. 2009;44(6):568–574 [DOI] [PubMed] [Google Scholar]
- 7. Rand CM , Humiston SG , Schaffer SJ , et al. Parent and adolescent perspectives about adolescent vaccine delivery: practical considerations for vaccine communication. Vaccine. 2011;29(44):7651–7658 [DOI] [PubMed] [Google Scholar]
- 8. Middleman AB . Adolescent immunizations: policies to provide a shot in the arm for adolescents. J Adolesc Health. 2007;41(2):109–118 [DOI] [PubMed] [Google Scholar]
- 9. Roberts JR , Thompson D , Rogacki B , et al. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine. 2015;33(14):1748–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Markowitz LE , Dunne EF , Saraiya M , et al. Centers for Disease Control and Prevention (CDC) . Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30 [PubMed] [Google Scholar]
- 11.Bloom B, Jones LI, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2012. Vital Health Stat 10. 2013(258):181 [PubMed] [Google Scholar]
- 12. Bartlett JA , Peterson JA . The uptake of human papillomavirus (HPV) vaccine among adolescent females in the United States: a review of the literature. J Sch Nurs. 2011;27(6):434–446 [DOI] [PubMed] [Google Scholar]
- 13. Brewer NT , Fazekas KI . Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med. 2007;45(2–3):107–114 [DOI] [PubMed] [Google Scholar]
- 14. Holman DM , Benard V , Roland KB , Watson M , Liddon N , Stokley S . Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szilagyi PG , Rand CM , McLaurin J , et al. Working Group on Adolescent Vaccination in the Medical Home . Delivering adolescent vaccinations in the medical home: a new era? Pediatrics. 2008;121(suppl 1):S15–S24 [DOI] [PubMed] [Google Scholar]
- 16. Rand CM , Shone LP , Albertin C , Auinger P , Klein JD , Szilagyi PG . National health care visit patterns of adolescents: implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161(3):252–259 [DOI] [PubMed] [Google Scholar]
- 17. Zimet GD . Health care professionals and adolescent vaccination. A call for intervention research. Hum Vaccin Immunother. 2014;10(9):2629–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Briss PA , Rodewald LE , Hinman AR , et al. The Task Force on Community Preventive Services . Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):97–140 [DOI] [PubMed] [Google Scholar]
- 19. Jacobson Vann JC , Szilagyi P . Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szilagyi PG , Humiston SG , Gallivan S , Albertin C , Sandler M , Blumkin A . Effectiveness of a citywide patient immunization navigator program on improving adolescent immunizations and preventive care visit rates. Arch Pediatr Adolesc Med. 2011;165(6):547–553 [DOI] [PubMed] [Google Scholar]
- 21. Szilagyi PG , Albertin C , Humiston SG , et al. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13(3):204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayne SL , duRivage NE , Feemster KA , Localio AR , Grundmeier RW , Fiks AG . Effect of decision support on missed opportunities for human papillomavirus vaccination. Am J Prev Med. 2014;47(6):734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiks AG , Grundmeier RW , Mayne S , et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics. 2013;131(6):1114–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cassidy B , Braxter B , Charron-Prochownik D , Schlenk EA . A quality improvement initiative to increase HPV vaccine rates using an educational and reminder strategy with parents of preteen girls. J Pediatr Health Care. 2014;28(2):155–164 [DOI] [PubMed] [Google Scholar]
- 25. Esernio-Jenssen D , Turow V . Parents’ understanding of the CDC’s vaccine information material. Am J Public Health. 1996;86(11):1648–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lieu TA , Glauber JH , Fuentes-Afflick E , Lo B . Effects of vaccine information pamphlets on parents’ attitudes. Arch Pediatr Adolesc Med. 1994;148(9):921–925 [DOI] [PubMed] [Google Scholar]
- 27. St-Amour M , Guay M , Perron L , et al. Are vaccination information leaflets useful for vaccinators and parents? Vaccine. 2006;24(14):2491–2496 [DOI] [PubMed] [Google Scholar]
- 28. Davis TC , Fredrickson DD , Arnold CL , et al. Childhood vaccine risk/benefit communication in private practice office settings: a national survey. Pediatrics. 2001;107(2). Available at: www.pediatrics.org/cgi/content/full/107/2/E17 [DOI] [PubMed] [Google Scholar]
- 29. Humiston SG , Albertin C , Schaffer S , et al. Health care provider attitudes and practices regarding adolescent immunizations: a qualitative study. Patient Educ Couns. 2009;75(1):121–127 [DOI] [PubMed] [Google Scholar]
- 30. Lechuga J , Swain GR , Weinhardt LS . The cross-cultural variation of predictors of human papillomavirus vaccination intentions. J Womens Health (Larchmt). 2011;20(2):225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stokley S , Jeyarajah J , Yankey D , et al. Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC Centers for Disease Control and Prevention (CDC) . Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624 [PMC free article] [PubMed] [Google Scholar]
- 32. Keenan K , Hipwell A , Stepp S . Race and sexual behavior predict uptake of the human papillomavirus vaccine. Health Psychol. 2012;31(1):31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nyhan B , Reifler J , Richey S , Freed GL . Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4). Available at: www.pediatrics.org/cgi/content/full/133/4/e835 [DOI] [PubMed] [Google Scholar]
- 34. Saslow D , Castle PE , Cox JT , et al. Gynecologic Cancer Advisory Group . American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57(1):7–28 [DOI] [PubMed] [Google Scholar]
- 35. Weinstein ND . The precaution adoption process. Health Psychol. 1988;7(4):355–386 [DOI] [PubMed] [Google Scholar]
- 36. Weinstein ND , Sandman PM . A model of the precaution adoption process: evidence from home radon testing. Health Psychol. 1992;11(3):170–180 [DOI] [PubMed] [Google Scholar]
- 37. Allen JD , Othus MK , Shelton RC , et al. Parental decision making about the HPV vaccine. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2187–2198 [DOI] [PubMed] [Google Scholar]
- 38. Fu LY , Bonhomme LA , Cooper SC , Joseph JG , Zimet GD . Educational interventions to increase HPV vaccination acceptance: a systematic review. Vaccine. 2014;32(17):1901–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Basu P , Mittal S . Acceptability of human papillomavirus vaccine among the urban, affluent and educated parents of young girls residing in Kolkata, Eastern India. J Obstet Gynaecol Res. 2011;37(5):393–401 [DOI] [PubMed] [Google Scholar]
- 40. Chan SS , Cheung TH , Lo WK , Chung TK . Women’s attitudes on human papillomavirus vaccination to their daughters. J Adolesc Health. 2007;41(2):204–207 [DOI] [PubMed] [Google Scholar]
- 41. Dempsey AF , Zimet GD , Davis RL , Koutsky L . Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486–1493 [DOI] [PubMed] [Google Scholar]
- 42. Kennedy A , Sapsis KF , Stokley S , Curtis CR , Gust D . Parental attitudes toward human papillomavirus vaccination: evaluation of an educational intervention, 2008. J Health Commun. 2011;16(3):300–313 [DOI] [PubMed] [Google Scholar]
- 43. Cox DS , Cox AD , Sturm L , Zimet G . Behavioral interventions to increase HPV vaccination acceptability among mothers of young girls. Health Psychol. 2010;29(1):29–39 [DOI] [PubMed] [Google Scholar]
- 44. Davis K , Dickman ED , Ferris D , Dias JK . Human papillomavirus vaccine acceptability among parents of 10- to 15-year-old adolescents. J Low Genit Tract Dis. 2004;8(3):188–194 [DOI] [PubMed] [Google Scholar]
- 45. Thorpe KE , Zwarenstein M , Oxman AD , et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ. 2009;180(10):E47–E57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zimet GD , Rosberger Z , Fisher WA , Perez S , Stupiansky NW . Beliefs, behaviors and HPV vaccine: correcting the myths and the misinformation. Prev Med. 2013;57(5):414–418 [DOI] [PubMed] [Google Scholar]
- 47. Scarinci IC , Garcés-Palacio IC , Partridge EE . An examination of acceptability of HPV vaccination among African American women and Latina immigrants. J Womens Health (Larchmt). 2007;16(8):1224–1233 [DOI] [PubMed] [Google Scholar]
- 48. Stokley S , Curtis CR , Jeyarajah J , Harrington T , Gee J , Markowitz L Centers for Disease Control and Prevention (CDC) . Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62(29):591–595 [PMC free article] [PubMed] [Google Scholar]
- 49. Mikulak AK . Parents’ vaccination concerns are about more than risk and benefit. Hum Vaccin. 2011;7(6):597–599 [DOI] [PubMed] [Google Scholar]
- 50. Stevens CE , Caughy MO , Lee SC , Bishop WP , Tiro JA . Does language moderate the influence of information scanning and seeking on HPV knowledge and vaccine awareness and initiation among Hispanics? Ethn Dis. 2013;23(1):95–102 [PMC free article] [PubMed] [Google Scholar]
- 51. Freed GL , Clark SJ , Butchart AT , Singer DC , Davis MM . Sources and perceived credibility of vaccine-safety information for parents. Pediatrics. 2011;127(suppl 1):S107–S112 [DOI] [PubMed] [Google Scholar]
- 52. Shui IM , Weintraub ES , Gust DA . Parents concerned about vaccine safety: differences in race/ethnicity and attitudes. Am J Prev Med. 2006;31(3):244–251 [DOI] [PubMed] [Google Scholar]
- 53. Hull PC , Williams EA , Khabele D , Dean C , Bond B , Sanderson M . HPV vaccine use among African American girls: qualitative formative research using a participatory social marketing approach. Gynecol Oncol. 2014;132(suppl 1):S13–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jeudin P , Liveright E , del Carmen MG , Perkins RB . Race, ethnicity and income as factors for HPV vaccine acceptance and use. Hum Vaccin Immunother. 2013;9(7):1413–1420 [DOI] [PubMed] [Google Scholar]
- 55. Sadaf A , Richards JL , Glanz J , Salmon DA , Omer SB . A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31(40):4293–4304 [DOI] [PubMed] [Google Scholar]
- 56. Jarrett C , Wilson R , O’Leary M , Eckersberger E , Larson HJ SAGE Working Group on Vaccine Hesitancy . Strategies for addressing vaccine hesitancy—a systematic review. Vaccine. 2015;33(34):4180–4190 [DOI] [PubMed] [Google Scholar]
- 57. Szilagyi PG , Schaffer S , Barth R , et al. Effect of telephone reminder/recall on adolescent immunization and preventive visits: results from a randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160(2):157–163 [DOI] [PubMed] [Google Scholar]
- 58. Suh CA , Saville A , Daley MF , et al. Effectiveness and net cost of reminder/recall for adolescent immunizations. Pediatrics. 2012;129(6). Available at: www.pediatrics.org/cgi/content/full/129/6/e1437 [DOI] [PubMed] [Google Scholar]
- 59. Schaffer SJ , Humiston SG , Shone LP , Averhoff FM , Szilagyi PG . Adolescent immunization practices: a national survey of US physicians. Arch Pediatr Adolesc Med. 2001;155(5):566–571 [DOI] [PubMed] [Google Scholar]
- 60. Tierney CD , Yusuf H , McMahon SR , et al. Adoption of reminder and recall messages for immunizations by pediatricians and public health clinics. Pediatrics. 2003;112(5):1076–1082 [DOI] [PubMed] [Google Scholar]