Abstract
The opioid epidemic has led to a serious examination of the use of opioids for the treatment of pain. Opioid drugs are effective due to the expression of opioid receptors throughout the body. These receptors respond to endogenous opioid peptides that are expressed as polypeptide hormones that are processed by proteolytic cleavage. Endogenous opioids are expressed throughout the peripheral and central nervous system and regulate many different neuronal circuits and functions. One of the key functions of endogenous opioid peptides is to modulate our responses to pain. This review will focus on the descending pain modulatory circuit which consists of the ventrolateral periaqueductal gray (PAG) projections to the rostral ventromedial medulla (RVM). RVM projections modulate incoming nociceptive afferents at the level of the spinal cord. Stimulation within either the PAG or RVM results in analgesia and this circuit has been studied in detail in terms of the actions of exogenous opioids, such as morphine and fentanyl. Further emphasis on understanding the complex regulation of endogenous opioids will help to make rational decisions with regard to the use of opioids for pain. We also include a discussion of the actions of endogenous opioids in the amygdala, an upstream brain structure that has reciprocal connections to the PAG that contribute to the brain’s response to pain.
Keywords: periaqueductal gray, rostral ventromedial medulla, amygdala, pain, opioid, enkephalin, beta-endorphin
1. Introduction
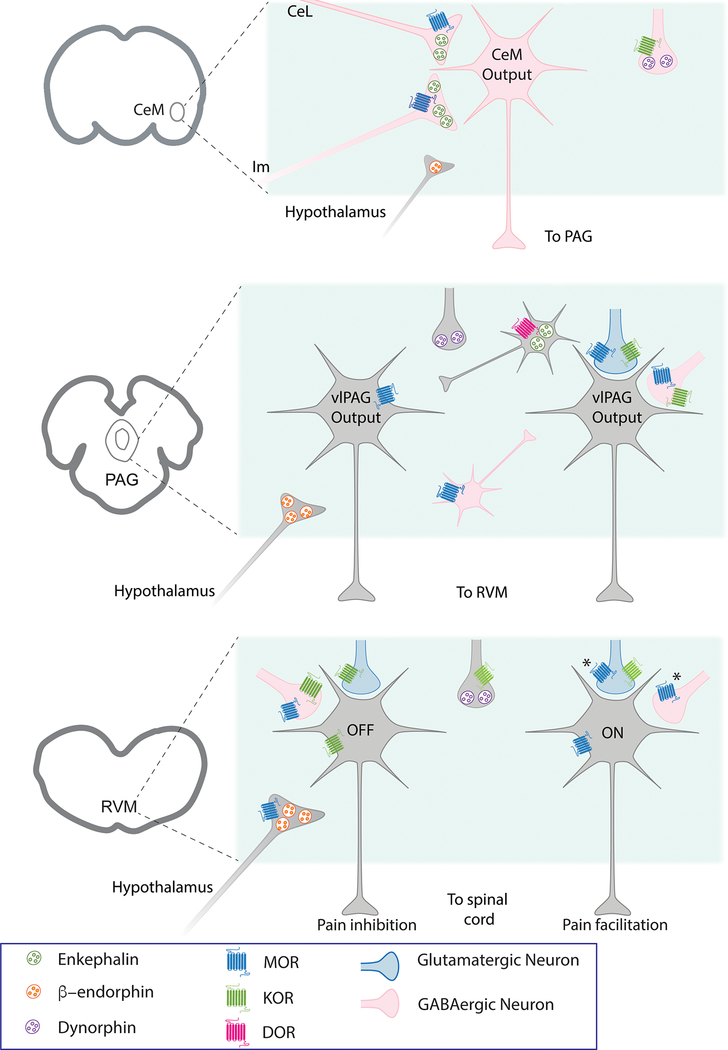
The opioid epidemic has thrust our dependence on opioids for the treatment of pain into the media spotlight. Despite decades of research to identify new therapeutics for pain management, opioids are still the gold standard for pain therapy. The analgesic effects of opioid drugs are due to their binding and activation of opioid receptors throughout the body. These receptors respond to endogenous opioid peptides, which are expressed as inactive polypeptide hormones that are activated through proteolytic cleavage. Endogenous opioids are expressed throughout the peripheral and central nervous system and regulate many different circuits and functions. One of the key functions of endogenous opioid peptides is to modulate our response to pain. This review will focus on the descending pain modulatory circuit which consists of the ventrolateral periaqueductal gray (PAG) projections to the rostral ventromedial medulla (RVM) that modulate incoming nociceptive afferents at the level of the spinal cord. Figure 1 shows our current understanding of the endogenous opioid peptides and receptors in the adult rat amygdala, PAG and RVM.
Figure 1.
Schematic depicting known endogenous opioids and opioid receptors based on function in the amygdala, PAG and RVM in naïve adult rats. AMYGDALA: Endogenous opioid peptides are released in the medial CeA (CeM). Enkephalin is likely released from lateral CeA (CeL) neurons and the main island of the intercalated cells (Im). While β-endorphin is release from hypothalamic terminals the source of dynorphin is not defined. The predominant opioid receptor expressed in the CeM is the mu opioid receptor (MOR) whose activation inhibits GABA release in the CeM. Kappa opioid receptors (KOR) are also expressed on GABA terminals. PAG: Endogenous opioid peptides are released in the PAG from terminals arising from the amygdala and the hypothalamus. The predominant opioid receptor in the PAG is MOR which is expressed on neurons in the PAG. MORs hyperpolarize neurons via activation of G protein-coupled potassium channels (GIRKs). MORs are also expressed on presynaptic glutamate and GABA terminals arising from outside the PAG and inhibit neurotransmitter release. KOR are also expressed on presynaptic terminals. Delta opioid receptors (DOR) have been observed on enkephalin terminals within the PAG using immunohistochemistry. Output neurons (gray) are both glutamatergic and GABAergic with heterogeneous sensitivity to opioids. RVM: In the RVM, OFF- and ON-cells are defined functionally with in vivo recordings but have been distinguished in in vitro studies as primary cells (OFF-like) that are opioid-insensitive and secondary cells (ON-like) that are directly hyperpolarized by MOR agonists. A proportion of primary cells are inhibited by KOR agonists. Both MOR and KOR receptors inhibit presynaptic glutamate and GABA release onto both cell types. It should be noted that DOR-mediated functions in both the PAG and RVM are increased with chronic morphine treatment or chronic pain. The functions of various endogenous opioids with respect to the spatial and temporal release and heterogeneous expression of opioid receptors in the descending pain circuit are not understood.
Electrical stimulation within the PAG or RVM typically results in analgesia (Barbaro, 1988; Fardin et al., 1984; Heinricher and Ingram, 2008; Hosobuchi, 1980; Mayer, 1984). Neurons in the caudal ventrolateral PAG contribute to stimulation-induced analgesia (Adams, 1976a; Akil et al., 1976; Bach and Yaksh, 1995; Hosobuchi et al., 1977). Opioids microinjected into the PAG produce potent antinociception (Heinricher and Morgan, 1999) and the behavioral antinociception produced by these agents is mediated by projections to the RVM (Sandkuhler and Gebhart, 1984; Tortorici and Morgan, 2002). These early experiments suggested that the PAG to RVM circuit was an “analgesia” circuit. In support of this, human imaging studies find activation of this circuit is associated with opioid analgesia, reward, and placebo responses (Eippert et al., 2009; Pecina et al., 2013; Wanigasekera et al., 2012). However, substantial evidence has been mounting that this circuit provides bidirectional modulation of pain and that facilitation of pain is also an important function of the circuit (Heinricher, 2003; Porreca et al., 2002). Opto- and chemogenetic circuit mapping strategies have identified a circuit from the amygdala through the PAG-RVM that contributes to hyperalgesia in neuropathic pain states (Huang et al., 2019). The PAG also mediates hyperalgesia associated with alcohol withdrawal (Avegno et al., 2018). Functional imaging studies in humans have implicated dysfunction in descending control through the PAG-RVM circuit in the etiologies of chronic pain syndromes (Bushnell et al., 2013; Yarnitsky, 2015).
As will become apparent throughout this review, anatomical distribution of endogenous opioids was the focus of early studies following the discovery of the endogenous opioid peptide gene family in the 1970s. Subsequent characterization of the receptors for these peptides, as well as for morphine, led to an intense focus on exogenous opioid regulation of the opioid receptor family of G protein-coupled receptors. Although these studies have characterized myriad physiological functions of opioids, there are many basic questions about endogenous opioids in the descending pain modulatory circuit that have yet to be answered. These questions include what are the relevant stimuli that induce their release, what are the temporal and spatial constraints on their release, and how chronic pain states alter the release of endogenous opioids. New methods are being developed and adopted to measure endogenous opioids (Al-Hasani et al., 2018) and define specific neuronal circuits (Miller, 2006) that are modulated by opioids. Several transgenic tools have been developed to study the neurons expressing endogenous opioids. These tools include knock-outs and Cre-recombinase lines for proopiomelanocortin (POMC), preproenkephalin, enkephalin and dynorphin. Additionally, tools to study the role of opioid receptors include mu opioid receptor (MOR) (Matthes et al., 1996), delta opioid receptor (DOR) (Filliol et al., 2000), and kappa opioid receptor (KOR) (Simonin et al., 1998) knockout mouse lines. Early studies defining the behavioural consequences of removing opioid peptides and receptors have been carefully reviewed (Corder et al., 2018; Kieffer and Gaveriaux-Ruff, 2002), including discussion of developmental adaptations in peptides and receptors in each knockout line. In brief, enkephalin knockout mice have deficits in supraspinal analgesia and inflammatory pain (Konig et al., 1996), β-endorphin knockouts show abnormal MOR regulation after chronic pain (Narita et al., 2013; Petraschka et al., 2007), and dynorphin knockouts display disrupted stress-induced behavioural responses (McLaughlin et al., 2003). Additional tools include a MOR-knockout rat line (Arttamangkul et al., 2019) and various opioid receptor Cre- and floxed-Cre mouse lines (Ehrich et al., 2015; Okunomiya et al., 2020; Reiss et al., 2017; Weibel et al., 2013) that should help to increase our knowledge of specific circuits that depend on endogenous opioid actions. Recent studies in the amygdala have begun to precisely define the release and actions of endogenous opioids in neural circuits activated by nociceptive stimuli (Winters et al., 2017), so we have included the amygdala, a brain structure that coordinates emotional aspects of pain with sensory information, in our discussion of the descending pain modulatory circuit.
2. PAG
The PAG integrates information from cortical and subcortical areas to modulate many different behaviors, including defensive responses to pain, threat and stress (Bandler and Keay, 1996; Keay and Bandler, 2001), as well as cardiovascular control (Carrive et al., 1987), and control of respiration (Sessle et al., 1981), lactation (Lonstein and Stern, 1997) and feeding (Sukikara et al., 2006). Heterogenous cell populations within the PAG surround the cerebral aqueduct and are organized in rostral-caudal columns that mediate distinct functions (Bandler and Shipley, 1994; Silva and McNaughton, 2019). Stimulation of the ventrolateral column of the PAG elicits analgesia in humans and antinociception in rats (Adams, 1976a; Akil et al., 1976; Bach and Yaksh, 1995; Barbaro, 1988; Hosobuchi et al., 1977) that is sensitive to naloxone (Adams, 1976b; Akil et al., 1976; Hosobuchi et al., 1977) indicating that stimulation of the ventrolateral PAG induces release of endogenous opioids. The term antinociception is used to describe opioid effects in rats because we can only measure their response to noxious stimuli, not their perception of pain. The behavioral antinociception produced by opioids is mediated by activation of PAG output neurons projecting to the RVM (Gebhart et al., 1984; Tortorici and Morgan, 2002).
It should be noted that the descending pain modulatory circuit has been shown to be sexually dimorphic (Loyd and Murphy, 2009, 2014). Males rats have significantly higher levels of the MOR in the ventrolateral PAG than cycling females and selective lesions of MOR disrupt morphine analgesia in males, but not females (Loyd et al., 2008). Female rats have a higher number of PAG to RVM projections but a lower percentage of these are activated by morphine (Loyd et al., 2007) suggesting that there are fundamental sex-dependent differences in circuitry. Indeed, differential responses to GABA were noted in the PAG in female compared to male rats (Tonsfeldt et al., 2016). The majority of early studies on endogenous opioid peptide localization and function used male rats so there is still a large gap in knowledge about endogenous opioid peptides in this circuit in females.
2.1. Endogenous opioids in PAG
Endogenous opioids, including met- and leu-enkephalin, and β-endorphin are widely expressed in the brain (Bodnar, 2013; Gu et al., 2017), including the descending pain modulatory circuit (Table 1 and Fig. 1). Enkephalin-containing terminals are observed throughout the PAG but are notably most dense in the ventrolateral PAG. These terminals are apposed to both GABA and non-GABA-containing dendrites, as well as PAG output neurons that project to the RVM (Williams and Beitz, 1990). A portion of the PAG-RVM projection neurons express mu opioid receptors (MOR) and delta opioid receptors (DOR) (Osborne et al., 1996; Wang and Wessendorf, 2002) indicating that endogenous opioids directly inhibit some PAG-RVM output neurons. The source of enkephalin terminals is not completely characterized but the enkephalin-expressing neurons are distributed in discrete populations throughout the PAG (Moss et al., 1983; Williams and Dockray, 1983). Enkephalin staining in the monkey PAG resembles that of rat (Haber and Elde, 1982a, b). Interestingly, some of the enkephalin-containing neurons in the PAG send projections to the amygdala (Li et al., 1990a) and the nucleus accumbens (Li et al., 1990b) indicating that opioid release in these areas may help to coordinate the response to pain in higher structures.
Table 1.
Endogenous opioid peptides in the descending pain modulatory pathway. References are listed chronologically with key details and findings.
| Reference | Year | Species | Age | Brain area | Endogeous opioids | Technique | Key findings |
|---|---|---|---|---|---|---|---|
| Hokfelt, et al. (1977) | 1977 | rat | adolescent | PAG, RVM, Lamina I and II spinal cord | IHC | Met-enkephalin immunoreactivity observed throughout the descending pain modulatory pathway. | |
| Hokfet, et al. (1979) | 1979 | rat | adolescent | RVM | enkephalin | IHC | Enkephalin-containing neurons project to spinal cord |
| Finley, et al. (1981) | 1981 | rat | ? | PAG | beta-endorphin | IHC | Beta-endorphin immunoreactivity in PAG terminals arising from hypothalamus. |
| Moss, et al. (1983) | 1983 | cat | adult | PAG | enkephalin | IHC | Enkephalin-containing neurons are found throughout the entire rostral-caudal extent of the PAG. |
| del Rio, et al. (1983) | 1983 | rat | adult | PAG | met-enkephalin | RIA from slice | Ca2+-dependent met-enkephalin release in response to Substance P superfusion over PAG slices. |
| Khachaturian, et al. (1983) | 1983 | rat | adult | PAG, RVM | Leu-enkephalin | IHC | Leu-enkephalin immunoreactivity in many brain areas. |
| Williams and Dockray (1983) | 1983 | rat | adolescent | RVM | met-enkephalin | IHC | Met-enkephalin immunoreactivity observed in many brain areas. |
| Guthrie and Basbaum (1984) | 1984 | rat | ? | RVM | met-enkephalin and dynorphin | IHC | Observed co-localization of opioid peptides in many cell populations in the medulla |
| Gray, et al. (1984) | 1984 | rat | ? | amygdala | met- and leu-enkephalin and beta-endorphin | IHC | Differential distribution of beta-endorphin and enkephalins in CeA and other subdivisions of the amygdala suggests different functions. |
| Williams, et al. (1995) | 1985 | rat | adult | PAG | met-enkephalin | Microdialysis | CFA inflammation-induced release of met-enkephalin in the PAG |
| Fallon and Leslie (1986) | 1986 | rat | adult | PAG, RVM, spinal cord | dynorphin | IHC | Dynorphin-containing cell bodies localized to many brain areas including the descending pain modulatory pathway. |
| Menetrey and Basbaum (1987) | 1987 | rat | ? | RVM | met-enkephalin and dynorphin | IHC with retrograde labeling | Localization of Substance P, met-enkephalin and dynorphin in the rat medulla. Substance P and met-enkephalin labeled medulla projecting neurons to the spinal cord, but dynorphin did not. |
| Kulling, et al. (1989) | 1989 | mouse | adult | PAG | beta-endorphin | ELISA | Mild social stress increased tail-flick latency and beta-endorphin immunoreactivity levels in PAG. |
| William and Beitz (1990) | 1990 | rat | adult | PAG | enkephalin | EM with retrograde labeling from RVM | In ventolateral PAG, 22% of enkephalin immunoreactive terminals were adjacent to PAG-RVM projection neurons |
| Li, et al. (1990a) | 1990 | rat | ? | PAG | leu-enkephalin | IHC with retrograde labeling | Enkephalin-containing neurons project to the NAc. |
| Li, et al. (1990b) | 1990 | rat | adolescent | PAG | leu-enkephalin | IHC with retrograde labeling | Enkephalin-containing neurons in PAG project to ipsilateral CeA. |
| Arvidsson, et al. (1992) | 1992 | monkey, cat | adult | RVM and spinal cord | met-enkephalin | IHC | Enkephalin-immmunoreactivity in cat and monkey is colocalized with 5-HT in a small proportion of bulbospinal neurons, similar to rat. |
| Bach and Yaksh (1995) | 1995 | rat | adult | PAG | beta-endorphin; met-enkephalin | RIA and HPLC | Stimulation of arcuate nucleus of hypothalamus release beta-endorphin. Stimulation of PAG release met-enkephalin. |
| Budai and Fields (1998) | 1998 | rat | adult | PAG and spinal cord | enkephalins | in vivo recording and iontophoresis | PAG manipulations modulate spinal dorsal horn neurons in a mu opioid receptor-dependent manner (blocked by MOR antagonists) |
| Martin-Schild, et al. (1999) | 1999 | rat, guinea pig, mouse | adult | PAG, spinal cord, amygdala | endomorphins | IHC | Endomorphin immunoreactivity widely distributed with some differences between endorphin 1 and 2. |
| Jacobsen, et al. (2006) | 2006 | rat | ? | amygdala | met-enkephalin and beta-endorphin | IHC | Mismatch between MOR1 staining and beta-endorphin and enkephalin-immunoreactive terminals |
| Poulin, et al. (2006) | 2006 | rat | ? | amygdala | met- and leu-enkephalin and beta-endorphin | IHC | Enkephalin-containing afferent to CeA from BNST, hypothalamus and parabrachial area, as well as intra-amygdaloid projections. |
| Gu and Wessendorf (2007) | 2007 | rat | adolescent | RVM | endomorphins | IHC with retrograde labeling | Endomorphin-2 containing fibers oppose 5-HT-containing neurons in the RVM. Some of the 5-HT-containing RVM neurons project to the dorsal horn of the spinal cord. |
| Wager, et al. (2007) | 2007 | human | adult | PAG, amygdala | endogenous opioids | [11C]carfentanil PET | Placebo-induced release of endogenous opioids displaced carfentanil in PAG and amygdala, among other brain structures |
| Nakamura, et al. (2013) | 2013 | rat | adult | PAG | beta-endorphin and dynorphin | IHC | Electrical stimulation of CeA results in increased dynorphin levels in PAG. Formalin injury increases beta-endorphin in PAG. |
| Winters, et al. (2017) | 2017 | rat | adult | amygdala | met-enkephalin | IHC and EM | Low level stimulation releases enkephalin that modulate neurotransmitter release onto intercalated cells of CeA as well as activated potassium channels on these neurons. |
| Sims-Williams, et al. (2017) | 2017 | human | adult | PAG | endogenous opioids | [11C]diprenorphine (DPN) PET | Deep brain stimulation induces the release of endogenous opioids in PAG. |
| Haber and Elde (1982) | 1982a | monkey | adult | brain, spinal cord | enkephalin | IHC | Distribution of enkephalin-containing fibers and terminals throughout the brain and spinal cord. Similar staining in PAG compared to rat. |
| Haber and Elde (1982) | 1982b | monkey | adult | PAG, RVM, spinal cord | enkephalin | IHC | Distribution of enkephalin-containing cell bodies were similar to rat. |
Abbreviations: CeA, central nucleus of the amygdala; CeL, lateral portion of the CeA; CFA, complete Freud’s adjuvant; EM, electron microscopy; HPLC, High-performance liquid chromatography; IHC, immunohistochemistry; NAc, nucleus accumbens; PAG, periaqueductal gray; PET, positron emission tomography; RIA, radioimmunoassay; RVM, rostral ventromedial medulla; 5-HT, 5 hydroxytryptamine.{Hokfelt, 1977 #27803}
β-endorphin-containing fibers from the arcuate nucleus of the hypothalamus project heavily to the PAG (Finley et al., 1981; Ibata et al., 1985; Sim and Joseph, 1991). Stimulation of the arcuate nucleus increases the release of β-endorphin in the PAG (Bach and Yaksh, 1995; Sun et al., 2007) but stimulation of the PAG predominately increases release of met-enkephalin (Bach and Yaksh, 1995). Both met-enkephalin and β-endorphin are full agonists at MORs (Alt et al., 1998). Endomorphin-2 (Tyr-Pro-Phe-Phe-NH2) is potentially another endogenous opioid peptide (Zadina et al., 1997) that is found at high levels within the PAG (Martin-Schild et al., 1999). Endomorphin 2-containing neurons from the hypothalamus project to PAG (Chen et al., 2008) and RVM (Gu and Wessendorf, 2007). This peptide is a partial agonist at MORs in the PAG (Narita et al., 2000). However, the status of endomorphin as an endogenous opioid has not been fully established as the gene responsible for the production of endomorphin or precursor peptides has not be found.
Nociceptive stimulation increases the release of opioid peptides in the ventrolateral PAG (Del Rio et al., 1983; Williams et al., 1995). β-endorphin release in the PAG is associated with stress-induced analgesia (Kulling et al., 1989), as well as peripheral injury (Nakamura et al., 2013). Similar increases in endomorphin 2 levels were reported following neuropathic pain (Sun et al., 2001). In addition, stimulation of the amygdala induces release of the KOR agonist dynorphin in the PAG (Nakamura et al., 2013) but dynorphin does not elicit analgesia when microinjected into the PAG (Fang et al., 1989). Thus, the endogenous opioid system responds to painful situations by activating opioid receptors in the PAG, but the physiological roles of the different peptides are not clearly understood.
It should be noted that the majority of studies to date have used adult rats. In neonatal rats, activation of opioid receptors in the PAG actually elicit excitatory responses in the spinal cord that shifts to an inhibitory response in adulthood (Kwok et al., 2014) suggesting that endogenous opioids may play an important role in organizing the descending pain modulatory pathway. Neonatal injury enhances opioid tone which presents as hypoalgesia to painful stimuli in later life (Laprairie and Murphy, 2009) that is exacerbated in females (LaPrairie and Murphy, 2007) indicating that the descending pain modulatory pathway can undergo experience-dependent plasticity that can affect overall pain thresholds. These findings may extend to humans where pre-term infants with prior NICU experience have decreased pain responses as teenagers (Hermann et al., 2006). This plasticity may play an important role in individual differences in the development of chronic pain.
2.2. Opioid receptors in ventrolateral PAG
The MOR is the primary opioid receptor that mediates analgesia (Matthes et al., 1996). In addition to high levels of MOR, neurons in the PAG also express DOR and KOR (Fig. 1), as well as orphanin FQ (OFQ) receptors (Bobeck et al., 2016; Chiou et al., 2002; Gutstein et al., 1998; Kalyuzhny and Wessendorf, 1998; Mansour et al., 1995). DORs are localized to enkephalin-containing terminals (Commons et al., 2001) and may act as autoreceptors. DOR-labeled non-GABA terminals synapse onto GABAergic neurons in the vlPAG suggesting that they inhibit glutamate inputs onto GABAergic neurons (Commons et al., 2001). Thus, they could contribute to analgesia by decreasing excitation of intrinsic GABAergic neurons within the PAG.
Co-localization of MOR and KOR mRNA and protein is observed in both the PAG and RVM (Gutstein et al., 1998), although opioids microinjected into the PAG produce robust antinociception (Cheng et al., 1986; Morgan et al., 2006) primarily via the MOR (Bodnar et al., 1988; Fang et al., 1989; Ossipov et al., 1995; Smith et al., 1988). The physiological roles of KORs in the PAG are not currently understood.
MORs are expressed both pre- and postsynaptically in the ventrolateral PAG. Opioid binding to postsynaptic MORs activates GIRK channels that hyperpolarize a subpopulation of PAG neurons (Chieng and Christie, 1994a; Ingram et al., 2007; Vaughan et al., 2003), including some PAG to RVM output neurons (Osborne et al., 1996). Postsynaptic MORs also couple to calcium channels and inhibit their activation (Connor and Christie, 1998). In contrast, MOR inhibition of GABA and glutamate release is observed in all PAG neurons (Chieng and Christie, 1994b; Ingram et al., 1998). Opioid receptor expression is actually markedly different in rats and mice. In mice, agonists of MOR, DOR and KOR receptors activate GIRK channels, albeit in different subpopulations of neurons (Vaughan et al., 2003). KOR agonists inhibit presynaptic GABA release in both mouse and rat PAG (Lau et al., 2020; Vaughan et al., 2003). Opioid agonist inhibition of glutamate release has largely been ignored because it is difficult to reconcile with the disinhibition hypothesis (see below) but a recent study finds that MOR inhibition of GABA is more efficient than inhibition of glutamate onto PAG-RVM projections neurons (Lau et al., 2020). These results provide evidence that individual circuits and excitatory/inhibitory input balance are differentially affected by opioids. Further studies examining the spatial and temporal release of endogenous opioids in response to different stimuli will undoubtedly significantly enhance our understanding of these circuits.
3. Disinhibition hypothesis of descending pain modulation
PAG output neurons to the RVM are inhibited by GABA under normal conditions. Removal of this inhibition, termed disinhibition, results in activation of the descending pain modulatory circuit and analgesia (reviewed in (Heinricher and Ingram, 2008; Lau and Vaughan, 2014). This hypothesis is supported by studies showing that GABAA receptor antagonists increase firing of ~75% of PAG neurons in vivo (Behbehani et al., 1990) and direct injections of GABAA receptor antagonists or glutamate agonists into the PAG elicit antinociception (Bobeck et al., 2014; Moreau and Fields, 1986; Morgan et al., 2003). Inhibition of GABA release was shown to be the primary driver of PAG neuron excitability when compared with opioid-induced hyperpolarization (Chiou and Huang, 1999). In addition, met-enkephalin release is increased after an injection of morphine and the GABAA receptor antagonist bicuculline (Williams et al., 1995) providing evidence that intrinsic enkephalin-containing neurons in the PAG are disinhibited by morphine and increased endogenous opioid release in turn disinhibits PAG output neurons. These results were further solidified with chemogenetic studies in transgenic mice where selective activation of PAG glutamatergic neurons decreased nociceptive responses and activation of GABAergic neurons facilitated nociceptive responses (Samineni et al., 2017). Thus, disinhibition plays a key role in the net effect of opioids in the PAG. However, outstanding questions remain in regard to the role of endogenous opioids in the PAG, including the role for endogenous opioids in regulating glutamatergic inputs and the temporal and spatial distribution of the release.
In the rat, microinjections of opioids directly into the PAG elicit antinociception (Siuciak and Advokat, 1987; Tortorici and Morgan, 2002; Tortorici et al., 2001; Yaksh et al., 1976) through activation of MORs, not KORs (Fang et al., 1989). It is clear that activation of PAG output neurons leads to antinociception but the exact circuitry between these PAG output neurons and well-described populations of RVM neurons, namely RVM ON- and OFF-cells (Heinricher and Ingram, 2008), is not understood. A simple model has been proposed in which opioids inhibit PAG GABAergic interneurons to disinhibit glutamatergic PAG projections to the RVM (Basbaum and Fields, 1984; Behbehani and Fields, 1979; Lau and Vaughan, 2014; Samineni et al., 2017). However, there is evidence that the circuit is much more complex due to the bi-directional nature of the descending pain modulatory circuit (Barbaro et al., 1986, 1989; Burgess et al., 2002; Porreca et al., 2002; Urban and Gebhart, 1999; Wei and Pertovaara, 1999). Anatomical studies in the rat describe both glutamatergic and GABAergic projections from the PAG to the RVM (Morgan et al., 2008). The GABAergic inputs predominately impinge on GABAergic RVM neurons that project to the spinal cord. Morphine microinjections into the PAG block glutamate activation of RVM neurons supporting the anatomical data that there is an inhibitory connection between PAG and RVM (Morgan et al., 1992). Thus, PAG to RVM circuitry is more complicated than simply disinhibition of excitatory descending projections and probably reflects the existence of parallel circuits contributing to the bidirectional control of pain mediated by the RVM (Lau and Vaughan, 2014; Williams and Beitz, 1990). In addition, PAG neurons project to the locus coeruleus and this circuit can also modulate nociceptive responses at the level of the spinal cord (Kim et al., 2018) suggesting that there are multiple reciprocal circuits engaged in response to pain.
A key issue in the field is potential species differences between rats and mice that may make the use of transgenic mouse lines problematic for further delineation of PAG to RVM circuitry. A retrograde labelling study from the RVM in a GAD67-GFP transgenic mouse line that labels GABAergic neurons found no overlap in the PAG between retrogradely labelled PAG-RVM projection neurons and GABAergic neurons (Park et al., 2010). These results suggest that there are no GABAergic projections from PAG to RVM in mice. This is certainly not the case in rats (Morgan et al., 2008). It is possible that specific GABAergic neuron markers label only a specific subpopulation of GABAergic neurons and this explains the difference in anatomical data between rats and mice. However, significant differences in activity of opioid receptors are also observed in mouse and rat (Chieng and Christie, 1994a; Vaughan et al., 2003; Vaughan and Christie, 1997) as detailed in the sections above. Given that the majority of studies characterizing the descending pain modulatory pathway have used rats and that in vivo characterization of RVM neurons in mice with respect to responses to nociceptive stimuli is limited (Hellman et al., 2007), further studies examining basic PAG and RVM physiology should be done in mice prior to use of transgenic mouse models. In vivo and in vitro studies are ongoing in rat models to further define this circuitry (Chen, et al., 2017), although the genetic tools to study circuits in rats lag behind the plethora of transgenic mouse models.
4. RVM
The PAG sends a dense projection to the RVM which is a brain structure that also integrates information from both cortical and subcortical areas of the brain. The RVM provides the predominant output from the descending pain modulatory circuit to the spinal cord (Heinricher and Fields, 2013a; Heinricher and Ingram, 2008). RVM neurons respond to nociceptive input through an ascending relay from the parabrachial area (Chen et al., 2017). There are two cell classes identified via their responses to noxious stimuli: OFF-cells that pause firing and ON-cells that fire a burst of action potentials in reponse to noxious input (Fig. 1). Opioids in the RVM elicit antinociception (Azami et al., 1982; Dickenson et al., 1979; Heinricher et al., 2001) via reducing the pause in RVM OFF-cells (Heinricher et al., 1992; Heinricher et al., 1994). ON-cell firing is correlated with hyperalgesia and systemic morphine reduces ON-cell firing (Barbaro et al., 1986; Heinricher et al., 1992) consistent with the inhibition of ON-cell firing by iontophoresis of MOR agonists directly onto ON-cells in vivo (Heinricher and Neubert, 2004; Neubert et al., 2004). Dense enkephalin-containing fibers impinge on RVM ON-cells (Mason et al., 1992) providing an anatomical substrate for the modulation of ON-cells by endogenous opioid peptides.
4.1. Endogenous opioids in RVM
Similar to observations in the PAG, enkephalins are also prevalent in RVM (Guthrie and Basbaum, 1984; Khachaturian et al., 1983; Williams and Dockray, 1983)(Table 1). Additionally, endomorphin fibers in the PAG impinge on serotonergic neurons expressing MOR (Gu and Wessendorf, 2007). Neurons in the RVM express MOR, DOR and KOR at lower levels than in the PAG (Drake et al., 2007; Gutstein et al., 1998; Kalyuzhny et al., 1996; Wang and Wessendorf, 1999). Activation of these receptors results in both pre- and postsynaptic actions in the RVM in vitro (Pan and Fields, 1996; Pan et al., 1990) supporting the in vivo electrophysiological studies showing direct inhibition of RVM ON-cells (Heinricher and Neubert, 2004; Neubert et al., 2004) and indirect activation of OFF-cells (Cheng et al., 1986; Heinricher et al., 1989; Heinricher et al., 1987; Morgan et al., 1992). There is evidence for interactions between MOR and DOR in the RVM (Marinelli et al., 2005), especially in chronic pain states (Hurley and Hammond, 2000, 2001).
Recent work has used elegant retrograde tracing combined with Cre-drivers in transgenic mouse lines to identify descending inputs from RVM to spinal cord (Cai et al., 2014; Francois et al., 2017; Zhang et al., 2015). Selective expression of markers and opsins in enkephalin-expressing RVM neurons find heterogeneous descending RVM circuits that are involved in modulating responses to nociceptive stimuli. Some of the RVM neurons co-expressed GABA and enkephalin and removing these neurons increased hypersensitivity to nociceptive stimuli in the mice (Zhang et al., 2015). MOR and DOR are expressed on a subpopulation of RVM neurons that project to the spinal cord in the rat (Kalyuzhny et al., 1996; Wang and Wessendorf, 1999) but it is unclear what contributions these neurons have to descending pain control.
The actions of KOR in the RVM are controversial. KOR agonists inhibit neurotransmitter release and activate GIRK currents in a subpopulation of RVM neurons (Ackley et al., 2001; Bie and Pan, 2003; Marinelli et al., 2002; Pan et al., 1997). The endogenous KOR agonist dynorphin is found in some terminals in the RVM (Fallon and Leslie, 1986; Menetrey and Basbaum, 1987). Although direct injections of KOR agonists into the RVM do not elicit antinociception (Meng et al., 2005), antagonists or loss of the either the dynorphin or KOR genes all result in hyperalgesia indicating there is a role for endogenous kappa agonists in the RVM in the modulation of pain (Schepers et al., 2008a; Schepers et al., 2008b).
5. Amygdala
The central nucleus of the amygdala (CeA), through its dense synaptic outputs to the PAG, plays a role in the “top-down” modulation of pain. The PAG sends projections to the lateral and medial subregions of the central nucleus of the amygdala (Li and Sheets, 2018), and both the lateral CeA (CeL) and the medial CeA (CeM) subdivisions project to the PAG, with the strongest projection to the ventrolateral PAG from the CeM (Oka et al., 2008; Sun et al., 2019) (Fig. 1). Thus, there is often particular interest in opioid actions in the CeM. However, given the strong synaptic inputs from the CeL to the CeA (Grove, 1988; McDonald, 1982) actions in both subdivisions of the CeA may ultimately influence the descending analgesic pathway. Where possible the subdivision of CeA will be identified but in many studies the subdivision is not specifically targeted or specified.
Electrical stimulation of the CeA produces analgesia (Oliveira and Prado, 2001). In addition, opioid analgesia requires an intact CeA in rodents and primates (Manning, 1998; Manning et al., 2001) and injection of MOR and DOR agonists into the CeA, such as morphine (Helmstetter et al., 1993; Pavlovic et al., 1996a) or β-endorphin (Pavlovic et al., 1996a) produce analgesia. Endogenous opioids acting in the CeA produce moderate analgesia (Valverde et al., 1996). Both the analgesia resulting from electrical stimulation of the CeA and opioid actions in the amygdala result from the CeA output neurons stimulating opioid release in the PAG (Oliveira and Prado, 2001; Pavlovic et al., 1996b).
5.1. Nociceptive activation of the amygdala
The amygdala is activated by acute (Bornhovd et al., 2002) and chronic pain (Baliki et al., 2006). It receives nociceptive information through multiple pathways. First, the amygdala receives nociceptive or threat information through a synaptic input to the capsular division of the CeA (Padilla et al., 2018; Palmiter, 2018). This information is delivered by synaptic inputs from the external lateral region of the parabrachial nucleus (PBel) that relays nociceptive information received from the spinal cord (Bernard et al., 1993). This capsular subdivision of the CeA has been termed the ‘nociceptive amygdala’ as it is preferentially activated by noxious stimuli (Neugebauer and Li, 2002). Further, nociceptive inputs are potentiated after acute (Kissiwaa and Bagley, 2018) and chronic (Fu and Neugebauer, 2008) noxious stimuli. Second, the amygdala receives polymodal sensory information, including nociceptive information, from multiple brain regions such as the thalamus (Moga et al., 1995) and cortex (McDonald and Mascagni, 1997). This polymodal nociceptive information is delivered to the basolateral amygdala (BLA) and likely results in the activation of a sub-population of BLA pyramidal neurons (Corder et al., 2019). The BLA may also receive purely nociceptive information as there is a population of neurons that are selectively activated by nociceptive stimuli (Corder et al., 2019). Third, the intercalated cells receive sensory, including nociceptive, information from the thalamus and sensory cortices (Asede et al., 2015; Bienvenu et al., 2015). Thus, the BLA neurons that code for negative valence, the capsular CeA neurons which are activated by nociceptive stimuli, and the intercalated cells receiving noxious sensory information, all make synaptic connections with the medial and lateral CeA (Beyeler et al., 2016; Cassell et al., 1999)
5.2. Endogenous opioids in the amygdala
The level of activity of the CeA neurons that project to the PAG is determined by both their activation by glutamatergic synaptic inputs and their inhibition by GABAergic synaptic inputs. Activation of MOR inhibits GABA release onto ~ 60% of CeA neurons projecting to the PAG whilst leaving glutamate release mostly unchecked, with inhibitory effects observed in only 23% of cells (Finnegan et al., 2005). This suggests that, similar to in the PAG (Vaughan and Christie, 1997), MOR agonists activate the descending pain modulatory pathway through disinhibition of CeA neurons projecting to the PAG.
The question then arises, how do endogenous opioids activate this descending pathway? Both enkephalin and β-endorphin are expressed in the amygdala. Very modest levels of β-endorphin positive fibres, likely from hypothalamic inputs, are found throughout the amygdala (Gray et al., 1984). Given that β-endorphin is released from neurons in the arcuate nucleus of the hypothalamus, this peptide may participate in stress-induced analgesia. Enkephalin is expressed at high levels in the CeA, with higher expression in the CeL, (Gray et al., 1984, Poulin et al., 2006) and one of its major input zones, the intercalated cells of the amygdala (Gray et al., 1984; Jacobsen et al., 2006; Poulin et al., 2006). It is possible that either endogenous opioid could participate in activation of the descending pathway. However, two lines of evidence suggest that enkephalin is more important for activating CeA-mediated projections to the PAG. First, there is significantly greater expression of enkephalin in the CeA and the intercalated cells. Second, endogenous analgesia in the CeA is potentiated by preventing peptidase activity and enkephalin is much more sensitive to peptidase degradation than β-endorphin (McKnight et al., 1982). Endogenous opioids, presumably dynorphin, also inhibit GABAergic inhibition onto CeM neurons through their actions at the KOR (Gilpin et al., 2014) although it is unknown whether these CeM neurons project to the PAG as only the MOR sensitivity of this projection was determined (Finnegan et al., 2005).
The endogenous opioids which produce analgesia in the CeA could be released from either CeA cells themselves or intercalated neurons. When the opioid sensitivity of GABAergic inputs onto CeA-PAG neurons was assessed, GABA release was stimulated indirectly by BLA stimulation (Finnegan et al., 2005). Finnegan and colleagues suggested that the source of this GABA could be from the intercalated neurons which are activated by the BLA neurons (Winters et al., 2017) that send a strong GABAergic projection to the CeA (Gregoriou et al., 2019). Likewise, endogenous opioid control of feedforward inhibition plasticity in the CeM was suggested to rely on intercalated neurons (Blaesse et al., 2015). Given this suggestion and the fact that endogenous opioids have been shown to be readily released from intercalated cells (Winters et al., 2017), one possible scenario is that intercalated cells release enkephalin which disinhibits CeA neurons that project to the PAG and activates the descending pain modulatory pathway.
5.3. Endogenous opioids in the intercalated cells
The intercalated neurons express high levels of enkephalin (Jacobsen et al., 2006; Poulin et al., 2006) which is packed into dense core vesicles ready for release (Winters et al., 2017). The intercalated cells receive a dense glutamatergic input from the BLA and pairs of electrical stimuli in the BLA excite the intercalated neurons sufficiently to release low levels of enkephalin (Winters et al., 2017). Moderate trains of 5 stimuli in the BLA stimulate enough enkephalin release to overcome the activity of peptidases and produce multiple cellular effects. The released enkephalin acts as a retrograde neuromodulator and inhibits release of glutamate from BLA terminals through activation of DOR (Figure 2, effect 1). Of particular note is that whilst exogenously applied enkephalin inhibits glutamate release at this synapse through activation of DOR or MOR, endogenously released enkephalin only acts via DOR. This is an important distinction that highlights the complex actions of opioids in circuits and suggests care should be taken when inferring the actions of endogenous opioids from studies using application of exogenous agonists. Additionally, a DOR positive allosteric modulator enhanced opioid inhibition at this synapse, but only at lower levels of opioid release (Winters et al., 2017). This is consistent with the positive allosteric modulators enhancing the affinity for agonists at the receptor and suggests that opioid positive allosteric modulators may be useful in pathologies when endogenous opioid signalling is reduced. The released enkephalin also acts via MOR on intercalated cells themselves and inhibits their release of GABA (Figure 2, effect 2) and directly inhibits their excitability through activation of a G protein-coupled inward rectifier potassium (GIRK) channel (Winters et al., 2017). This combination of reduced glutamatergic drive and direct inhibition would be expected to produce an overall inhibition of intercalated cell activity and their release of GABA in their target zones, including the CeA. Consistent with this, intercalated cell-mediated GABAergic inhibition of CeA neurons is reduced by exogenous enkephalin (Gregoriou et al., 2019). It would be very interesting to know whether intercalated neurons directly inhibit the CeA neurons projecting to the PAG. Thus, it is possible that nociceptive activation of the BLA produces feedforward activation of the intercalated cells to stimulate endogenous opioid release which then reduces intercalated cell-mediated GABAergic inhibition of the CeA neurons projecting to the PAG.
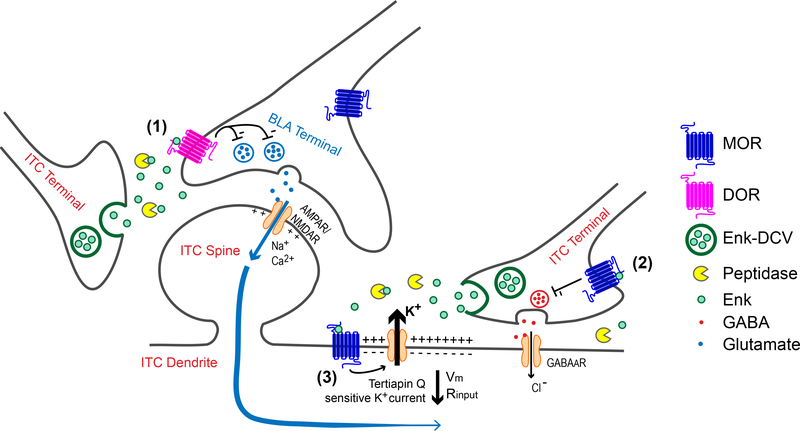
Figure 2.
Endogenous opioid actions in the the intercalated cells (ITC) of the amygdala.
(1) Low to moderate stimuli at BLA-Intercalated (ITC) synapses promote release of endogenous enkephalins from dense core vesicles (DCV) contained within ITC neurons. Sufficient peptide release through moderate stimulation is required to overcome peptidases and allow enkephalin signaling through DOR. Enkephalin reduces BLA-ITC synaptic activity by decreasing presynaptic glutamate release. (2) Moderate stimuli, together with peptidase inhibition, are required to overcome potential microarchitectural constraints to allow enkephalin-induced MOR activation which reduces presynaptic GABA release at local ITC-ITC synapses. (3) Activation of postsynaptic MORs by endogenously released opioids activates G protein-coupled potassium (GIRK) channels. The resulting efflux of K+ ions through these teriaptin Q-sensitive GIRK channels hyperpolarizes ITCs, reducing their excitability. Coincident synaptic activity could also be shunted (e.g. blue arrows) due to decreased input resistance. Both outcomes are expected to reduce total ITC activity and limit feedforward inhibition from the ITC, thus disinhibiting CeA outputs to regions, including the PAG. (Figure adapted from Winters, et al., 2017).
Although endogenous opioids could act at other sites in the amygdala, there are a couple of factors that suggest that endogenous opioids are strong regulators of the intercalated cells. Enkephalin is released in response to no or low levels of electrical stimulation. This is distinct from other sites of peptide release where endogenous opioid actions at other synapses require intense stimulation paradigms (Iremonger and Bains, 2009; Wagner et al., 1993; Weisskopf et al., 1993). It is possible that release of dense core vesicles are differently regulated in the intercalated cells, perhaps through differences in release machinery or intracellular calcium handling. Alternatively, we may be able to detect low concentrations of peptide that is released in response to low stimulation due to the high expression of opioid receptors in the intercalated cells (Poulin et al., 2006).
6. Role of endogenous opioids in cortical processing of pain
Top-down afferents arise from a variety of cortical and subcortical brain regions, including the anterior cingulate (ACC) and amygdala (Heinricher and Fields, 2013b; Keay and Bandler, 2001; Silva and McNaughton, 2019). Changes in connectivity between the ACC and the PAG are prominent in fMRI studies in chronic pain patients (Kong et al., 2010; Mainero et al., 2011; Mills et al., 2018; Truini et al., 2016). In addition, lesions of the ACC are generally agreed to reduce nociception in human patients (Davis et al., 1994; Foltz and White, 1962; Talbot et al., 1995). The prefrontal cortex (PFC)/ACC-amygdala-PAG circuit has been implicated in processing of aversive prediction-error signals (Roy et al., 2014). Inactivation of the PAG decreases the acquisition of fear conditioning and expectation blocks evoked responses due to aversive unconditioned stimuli in both the amygdala and PAG (Johansen et al., 2010). Interestingly, endogenous opioids have been shown to play important roles in conditioned fear for decades in studies utilizing naloxone to inhibit endogenous opioid signaling (Fanselow and Bolles, 1979; Helmstetter and Fanselow, 1987; McNally et al., 2011). Studies in humans find that naloxone induces sustained responses to aversive unconditioned stimuli indicating that endogenous opioids dampen acquisition of fear (Eippert et al., 2008). The use of systemic naloxone precludes determination of the sites of action of endogenous opioids in the human studies but MORs in the PAG are critical for fear learning (Cole and McNally, 2007; McNally and Cole, 2006). Collectively these results suggest that pain may be a teaching signal and endogenous opioids allow for reduced responses to expected pain (Eippert and Tracey, 2014).
More recently, additional projections from the PAG to the ventral tegmental area (VTA) (Omelchenko and Sesack, 2010; Suckow et al., 2013) have been implicated in avoidance behaviors in rodents associated with headache (Waung et al., 2019). Both glutamatergic and GABAergic projections from PAG (Waung et al., 2019) impinge on both DA and GABAergic neurons in VTA (Breton et al., 2019). Interestingly, although dopaminergic neurons comprise the majority of the neurons within the VTA and dopamine in the PAG modulates pain thresholds (Flores et al., 2004; Meyer et al., 2009), the VTA sends primarily GABAergic inputs to PAG (Ingram lab, unpublished observations). The role of endogenous opioids in modulating this circuit is yet to be established.
7. Conclusion
The study of endogenous opioids has been limited by the crude methods of stimulation of release and sensitivity of detection methods. Immunohistochemical methods have characterized cells that express opioid peptides and their projections, as well as release sites but there is little detailed information in terms of the temporal and spatial dimensions of endogenous opioid release. One of the key take-home messages of the recent work in the amygdala is that location of opioid receptors is important in determining their actions within circuits and that exogenous administration of opioids is a “hammer” that negates the intricate patterns of neural activity that are regulated by endogenous opioids. Further understanding of endogenous opioid release and actions within circuits will allow better manipulation of the circuits and novel therapies for pain.
Acknowledgements
This work was funded by National Institutes of Health under Award number R01DA042565 (SLI). The authors thank Dr. Laura Kozell and Courtney Bouchet for critical reading of the manuscript.
Footnotes
Declarations of Interest: None
Submission declaration and verification: This work has not been published previously.
References
- Ackley MA, Hurley RW, Virnich DE, and Hammond DL (2001). A cellular mechanism for the antinociceptive effect of a kappa opioid receptor agonist. Pain 91, 377–388. [DOI] [PubMed] [Google Scholar]
- Adams JE (1976a). Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 2, 161–166. [PubMed] [Google Scholar]
- Adams JE (1976b). Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 2, 161–166. [PubMed] [Google Scholar]
- Akil H, Mayer DJ, and Liebeskind JC (1976). Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science 191, 961–962. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, Wong JT, Mabrouk OS, McCall JG, Schmitz GP, Porter-Stransky KA, Aragona BJ, Kennedy RT, and Bruchas MR (2018). In vivo detection of optically-evoked opioid peptide release. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, and Woods JH (1998). Stimulation of guanosine-5’-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther 286, 282–288. [PubMed] [Google Scholar]
- Arttamangkul S, Plazek A, Platt EJ, Jin H, Murray TF, Birdsong WT, Rice KC, Farrens DL, and Williams JT (2019). Visualizing endogenous opioid receptors in living neurons using ligand-directed chemistry. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asede D, Bosch D, Luthi A, Ferraguti F, and Ehrlich I (2015). Sensory inputs to intercalated cells provide fear-learning modulated inhibition to the basolateral amygdala. Neuron 86, 541–554. [DOI] [PubMed] [Google Scholar]
- Avegno EM, Lobell TD, Itoga CA, Baynes BB, Whitaker AM, Weera MM, Edwards S, Middleton JW, and Gilpin NW (2018). Central Amygdala Circuits Mediate Hyperalgesia in Alcohol-Dependent Rats. J Neurosci 38, 7761–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azami J, Llewelyn MB, and Roberts MH (1982). The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain 12, 229–246. [DOI] [PubMed] [Google Scholar]
- Bach FW, and Yaksh TL (1995). Release into ventriculo-cisternal perfusate of ß-endorphin- and Met-enkephalin-immunoreactivity: effects of electrical stimulation in the arcuate nucleus and peripaqueductal gray of the rat. Brain Res 690, 167–176. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, and Apkarian AV (2006). Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci 26, 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, and Keay KA (1996). Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res 107, 285–300. [DOI] [PubMed] [Google Scholar]
- Bandler R, and Shipley MT (1994). Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? [published erratum appears in Trends Neurosci 1994 Nov;17(11):445] [see comments]. Trends Neurosci 17, 379–389. [DOI] [PubMed] [Google Scholar]
- Barbaro NM (1988). Studies of PAG/PVG stimulation for pain relief in humans. Prog Brain Res 77, 165–173. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, and Fields HL (1986). Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res 366, 203–210. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, and Fields HL (1989). Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res 6, 413–425. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, and Fields HL (1984). Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7, 309–338. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, and Fields HL (1979). Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res 170, 85–93. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Jiang MR, Chandler SD, and Ennis M (1990). The effect of GABA and its antagonists on midbrain periaqueductal gray neurons in the rat. Pain 40, 195–204. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, and Besson JM (1993). The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 329, 201–229. [DOI] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, and Tye KM (2016). Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron 90, 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie B, and Pan ZZ (2003). Presynaptic mechanism for anti-analgesic and anti-hyperalgesic actions of kappa-opioid receptors. J Neurosci 23, 7262–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu TC, Busti D, Micklem BR, Mansouri M, Magill PJ, Ferraguti F, and Capogna M (2015). Large intercalated neurons of amygdala relay noxious sensory information. J Neurosci 35, 2044–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Goedecke L, Bazelot M, Capogna M, Pape HC, and Jungling K (2015). mu-Opioid Receptor-Mediated Inhibition of Intercalated Neurons and Effect on Synaptic Transmission to the Central Amygdala. J Neurosci 35, 7317–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Chen Q, Morgan MM, and Ingram SL (2014). Contribution of adenylyl cyclase modulation of pre- and postsynaptic GABA neurotransmission to morphine antinociception and tolerance. Neuropsychopharmacology 39, 2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobeck EN, Ingram SL, Hermes SM, Aicher SA, and Morgan MM (2016). Ligand-biased activation of extracellular signal-regulated kinase 1/2 leads to differences in opioid induced antinociception and tolerance. Behav Brain Res 298, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ (2013). Endogenous opiates and behavior: 2012. Peptides 50, 55–95. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Williams CL, Lee SJ, and Pasternak GW (1988). Role of mu 1-opiate receptors in supraspinal opiate analgesia: a microinjection study. Brain Res 447, 25–34. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, and Buchel C (2002). Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain 125, 1326–1336. [DOI] [PubMed] [Google Scholar]
- Breton JM, Charbit AR, Snyder BJ, Fong PTK, Dias EV, Himmels P, Lock H, and Margolis EB (2019). Relative contributions and mapping of ventral tegmental area dopamine and GABA neurons by projection target in the rat. J Comp Neurol 527, 916–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP Jr., Vanderah TW, Lai J, and Porreca F (2002). Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci 22, 5129–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, and Low LA (2013). Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YQ, Wang W, Hou YY, and Pan ZZ (2014). Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol Pain 10, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, Dampney RA, and Bandler R (1987). Excitation of neurones in a restricted portion of the midbrain periaqueductal grey elicits both behavioural and cardiovascular components of the defence reaction in the unanaesthetised decerebrate cat. Neurosci Lett 81, 273–278. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, and Shi C (1999). The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci 877, 217–241. [DOI] [PubMed] [Google Scholar]
- Chen Q, Roeder Z, Li MH, Zhang Y, Ingram SL, and Heinricher MM (2017). Optogenetic Evidence for a Direct Circuit Linking Nociceptive Transmission through the Parabrachial Complex with Pain-Modulating Neurons of the Rostral Ventromedial Medulla (RVM). eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Hui R, Wang XL, Zhang T, Dong YX, and Li YQ (2008). Origins of endomorphin-immunoreactive fibers and terminals in different columns of the periaqueductal gray in the rat. J Comp Neurol 509, 72–87. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Fields HL, and Heinricher MM (1986). Morphine microinjected into the periaqueductal gray has differential effects on 3 classes of medullary neurons. Brain Res 375, 57–65. [DOI] [PubMed] [Google Scholar]
- Chieng B, and Christie MJ (1994a). Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol 113, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, and Christie MJ (1994b). Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol 113, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou LC, Fan SH, Guerrini R, and Calo G (2002). [Nphe(1)]N/OFQ-(1–13)-NH(2) is a competitive and selective antagonist at nociceptin/orphanin FQ receptors mediating K(+) channel activation in rat periaqueductal gray slices. Neuropharmacology 42, 246–252. [DOI] [PubMed] [Google Scholar]
- Chiou LC, and Huang LY (1999). Mechanism underlying increased neuronal activity in the rat ventrolateral periaqueductal grey by a mu-opioid. J Physiol 518 (Pt 2), 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, and McNally GP (2007). Opioid receptors mediate direct predictive fear learning: evidence from one-trial blocking. Learn Mem 14, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Beck SG, Rudoy C, and Van Bockstaele EJ (2001). Anatomical evidence for presynaptic modulation by the delta opioid receptor in the ventrolateral periaqueductal gray of the rat. J Comp Neurol 430, 200–208. [PubMed] [Google Scholar]
- Connor M, and Christie MJ (1998). Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J Physiol (Lond) 509, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, and Scherrer G (2019). An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363, 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, and Scherrer G (2018). Endogenous and Exogenous Opioids in Pain. Annu Rev Neurosci 41, 453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, and Dostrovsky JO (1994). Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain 59, 189–199. [DOI] [PubMed] [Google Scholar]
- Del Rio J, Naranjo JR, Yang HY, and Costa E (1983). Substance P-induced release of Met5-enkephalin from striatal and periaqueductal gray slices. Brain Res 279, 121–126. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Oliveras JL, and Besson JM (1979). Role of the nucleus raphe magnus in opiate analgesia as studied by the microinjection technique in the rat. Brain Res 170, 95–111. [DOI] [PubMed] [Google Scholar]
- Drake CT, De Oliveira AX, Harris JA, Connor DM, Winkler CW, and Aicher SA (2007). Kappa opioid receptors in the rostral ventromedial medulla of male and female rats. J Comp Neurol 500, 465–476. [DOI] [PubMed] [Google Scholar]
- Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PE, and Chavkin C (2015). Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J Neurosci 35, 12917–12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, and Buchel C (2008). Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. J Neurosci 28, 5465–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, and Buchel C (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543. [DOI] [PubMed] [Google Scholar]
- Eippert F, and Tracey I (2014). Pain and the PAG: learning from painful mistakes. Nat Neurosci 17, 1438–1439. [DOI] [PubMed] [Google Scholar]
- Fallon JH, and Leslie FM (1986). Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol 249, 293–336. [DOI] [PubMed] [Google Scholar]
- Fang FG, Haws CM, Drasner K, Williamson A, and Fields HL (1989). Opioid peptides (DAGO-enkephalin, dynorphin A(1–13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla. Brain Res 501, 116–128. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, and Bolles RC (1979). Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol 93, 736–744. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, and Besson JM (1984). A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res 306, 105–123. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, et al. (2000). Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25, 195–200. [DOI] [PubMed] [Google Scholar]
- Finley JC, Lindstrom P, and Petrusz P (1981). Immunocytochemical localization of beta-endorphin-containing neurons in the rat brain. Neuroendocrinology 33, 28–42. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, and Pan HL (2005). Effect of the {mu} opioid on excitatory and inhibitory synaptic inputs to periaqueductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther 312, 441–448. [DOI] [PubMed] [Google Scholar]
- Flores JA, El Banoua F, Galan-Rodriguez B, and Fernandez-Espejo E (2004). Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors. Pain 110, 205–214. [DOI] [PubMed] [Google Scholar]
- Foltz EL, and White LE Jr. (1962). Pain “relief” by frontal cingulumotomy. J Neurosurg 19, 89–100. [DOI] [PubMed] [Google Scholar]
- Francois A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, et al. (2017). A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839 e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, and Neugebauer V (2008). Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28, 3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart GF, Sandkuhler J, Thalhammer JG, and Zimmermann M (1984). Inhibition in spinal cord of nociceptive information by electrical stimulation and morphine microinjection at identical sites in midbrain of the cat. J Neurophysiol 51, 75–89. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M, Koob GF, and Schweitzer P (2014). Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology 77, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Cassell MD, and Kiss JZ (1984). Distribution of pro-opiomelanocortin-derived peptides and enkephalins in the rat central nucleus of the amygdala. Brain Res 306, 354–358. [DOI] [PubMed] [Google Scholar]
- Gregoriou GC, Kissiwaa SA, Patel SD, and Bagley EE (2019). Dopamine and opioids inhibit synaptic outputs of the main island of the intercalated neurons of the amygdala. Eur J Neurosci 50, 2065–2074. [DOI] [PubMed] [Google Scholar]
- Grove EA (1988). Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol 277, 315–346. [DOI] [PubMed] [Google Scholar]
- Gu M, and Wessendorf M (2007). Endomorphin-2-immunoreactive fibers selectively appose serotonergic neuronal somata in the rostral ventral medial medulla. J Comp Neurol 502, 701–713. [DOI] [PubMed] [Google Scholar]
- Gu ZH, Wang B, Kou ZZ, Bai Y, Chen T, Dong YL, Li H, and Li YQ (2017). Endomorphins: Promising Endogenous Opioid Peptides for the Development of Novel Analgesics. Neurosignals 25, 98–116. [DOI] [PubMed] [Google Scholar]
- Guthrie J, and Basbaum AI (1984). Colocalization of immunoreactive proenkephalin and prodynorphin products in medullary neurons of the rat. Neuropeptides 4, 437–445. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Mansour A, Watson SJ, Akil H, and Fields HL (1998). Mu and kappa opioid receptors in periaqueductal gray and rostral ventromedial medulla. Neuroreport 9, 1777–1781. [DOI] [PubMed] [Google Scholar]
- Haber S, and Elde R (1982a). The distribution of enkephalin immunoreactive fibers and terminals in the monkey central nervous system: an immunohistochemical study. Neuroscience 7, 1049–1095. [DOI] [PubMed] [Google Scholar]
- Haber S, and Elde R (1982b). The distribution of enkephalin immunoreactive neuronal cell bodies in the monkey brain: preliminary observations. Neurosci Lett 32, 247–252. [DOI] [PubMed] [Google Scholar]
- Heinricher MM (2003). Orphanin FQ/nociceptin: from neural circuitry to behavior. Life Sci 73, 813–822. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Barbaro NM, and Fields HL (1989). Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res 6, 427–439. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Cheng ZF, and Fields HL (1987). Evidence for two classes of nociceptive modulating neurons in the periaqueductal gray. J Neurosci 7, 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, and Fields HL, eds. (2013a). Central nervous system mechanisms of pain modulation. (London: Elsevier; ). [Google Scholar]
- Heinricher MM, and Fields HL (2013b). Central nervous system mechanisms of pain modulation In Wall and Melzack’s Textbook of Pain, 6th ed, McMahon S, Koltzenburg M, Tracey I, and Turk DC, eds. (London: Elsevier; ), pp. 129–142. [Google Scholar]
- Heinricher MM, and Ingram SL (2008). The brainstem and nociceptive modulation In The Senses: A Comprehensive Reference (San Diego, CA: Academic Press; ), pp. 593–626. [Google Scholar]
- Heinricher MM, and Morgan MM (1999). Supraspinal mechanisms of opioid analgesia In Opioids and Pain Control, Stein C, ed. (Cambridge: Cambridge University Press; ), pp. 46–69. [Google Scholar]
- Heinricher MM, Morgan MM, and Fields HL (1992). Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience 48, 533–543. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Tortorici V, and Fields HL (1994). Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 63, 279–288. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, and Neubert MJ (2004). Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol 92, 1982–1989. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, and Jobst EE (2001). Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain 92, 129–138. [DOI] [PubMed] [Google Scholar]
- Hellman KM, Brink TS, and Mason P (2007). Activity of murine raphe magnus cells predicts tachypnea and on-going nociceptive responsiveness. J Neurophysiol 98, 3121–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS, and Tershner SA (1993). Inhibition of the tail flick reflex following microinjection of morphine into the amygdala. Neuroreport 4, 471–474. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, and Fanselow MS (1987). Effects of naltrexone on learning and performance of conditional fear-induced freezing and opioid analgesia. Physiol Behav 39, 501–505. [DOI] [PubMed] [Google Scholar]
- Hermann C, Hohmeister J, Demirakca S, Zohsel K, and Flor H (2006). Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain 125, 278–285. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y (1980). The current status of analgesic brain stimulation. Acta Neurochir Suppl (Wien) 30, 219–227. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y, Adams JE, and Linchitz R (1977). Pain relief by electrical stimulation of the central gray matter in humans an dits reversal by naloxone. Science 197, 183–186. [DOI] [PubMed] [Google Scholar]
- Huang J, Gadotti VM, Chen L, Souza IA, Huang S, Wang D, Ramakrishnan C, Deisseroth K, Zhang Z, and Zamponi GW (2019). A neuronal circuit for activating descending modulation of neuropathic pain. Nat Neurosci 22, 1659–1668. [DOI] [PubMed] [Google Scholar]
- Hurley RW, and Hammond DL (2000). The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci 20, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, and Hammond DL (2001). Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. J Neurosci 21, 2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata Y, Kawakami F, Okamura H, Obata-Tsuto HL, Morimoto N, and Zimmerman EA (1985). Light and electron microscopic immunocytochemistry of beta-endorphin/beta-LPH-like immunoreactive neurons in the arcuate nucleus and surrounding areas of the rat hypothalamus. Brain Res 341, 233–242. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, and Morgan MM (2007). Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology 32, 600–606. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, and Christie MJ (1998). Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18, 10269–10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iremonger KJ, and Bains JS (2009). Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci 29, 7349–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KX, Hoistad M, Staines WA, and Fuxe K (2006). The distribution of dopamine D1 receptor and mu-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience 141, 2007–2018. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, and Blair HT (2010). Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci 13, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny AE, Arvidsson U, Wu W, and Wessendorf MW (1996). mu-Opioid and delta-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci 16, 6490–6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny AE, and Wessendorf MW (1998). Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol 392, 528–547. [PubMed] [Google Scholar]
- Keay KA, and Bandler R (2001). Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 25, 669–678. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, and Watson SJ (1983). Enkephalin systems in diencephalon and brainstem of the rat. J Comp Neurol 220, 310–320. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, and Gaveriaux-Ruff C (2002). Exploring the opioid system by gene knockout. Prog Neurobiol 66, 285–306. [DOI] [PubMed] [Google Scholar]
- Kim JH, Gangadharan G, Byun J, Choi EJ, Lee CJ, and Shin HS (2018). Yin-and-yang bifurcation of opioidergic circuits for descending analgesia at the midbrain of the mouse. Proc Natl Acad Sci U S A 115, 11078–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissiwaa SA, and Bagley EE (2018). Central sensitization of the spino-parabrachial-amygdala pathway that outlasts a brief nociceptive stimulus. J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, and Su TP (2010). Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res 211, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, and Zimmer A (1996). Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 383, 535–538. [DOI] [PubMed] [Google Scholar]
- Kulling P, Siegfried B, Frischknecht HR, Messiha FS, and Pasi A (1989). Beta-endorphin-like immunoreactivity levels in the hypothalamus, the periaqueductal grey and the pituitary of the DBA mouse: determination by ELISA and relationship to nociception. Physiol Behav 46, 25–28. [DOI] [PubMed] [Google Scholar]
- Kwok CH, Devonshire IM, Bennett AJ, and Hathway GJ (2014). Postnatal maturation of endogenous opioid systems within the periaqueductal grey and spinal dorsal horn of the rat. Pain 155, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPrairie JL, and Murphy AZ (2007). Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain 132 Suppl 1, S124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie JL, and Murphy AZ (2009). Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front Behav Neurosci 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, and Vaughan CW (2014). Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol 29, 159–164. [DOI] [PubMed] [Google Scholar]
- Lau BK, Winters BL, and Vaughan CW (2020). Opioid presynaptic disinhibition of the midbrain periaqueductal grey descending analgesic pathway. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JN, and Sheets PL (2018). The central amygdala to periaqueductal gray pathway comprises intrinsically distinct neurons differentially affected in a model of inflammatory pain. J Physiol 596, 6289–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Jia HG, Rao ZR, and Shi JW (1990a). Serotonin-, substance P- or leucine-enkephalin-containing neurons in the midbrain periaqueductal gray and nucleus raphe dorsalis send projection fibers to the central amygdaloid nucleus in the rat. Neurosci Lett 120, 124–127. [DOI] [PubMed] [Google Scholar]
- Li YQ, Rao ZR, and Shi JW (1990b). Midbrain periaqueductal gray neurons with substance P- or enkephalin-like immunoreactivity send projection fibers to the nucleus accumbens in the rat. Neurosci Lett 119, 269–271. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, and Stern JM (1997). Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci 17, 3364–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, and Murphy AZ (2007). Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience 147, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, and Murphy AZ (2009). The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plast 2009, 462879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, and Murphy AZ (2014). The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol 259, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Wang X, and Murphy AZ (2008). Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 28, 14007–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C, Boshyan J, and Hadjikhani N (2011). Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70, 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH (1998). A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J Neurosci 18, 9453–9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Merin NM, Meng ID, and Amaral DG (2001). Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J Neurosci 21, 8238–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, and Watson SJ (1995). Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat 8, 283–305. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Connor M, Schnell SA, Christie MJ, Wessendorf MW, and Vaughan CW (2005). delta-opioid receptor-mediated actions on rostral ventromedial medulla neurons. Neuroscience 132, 239–244. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Schnell SA, Wessendorf MW, and Christie MJ (2002). Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes. J Neurosci 22, 10847–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Schild S, Gerall AA, Kastin AJ, and Zadina JE (1999). Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405, 450–471. [PubMed] [Google Scholar]
- Mason P, Back SA, and Fields HL (1992). A confocal laser microscopic study of enkephalin-immunoreactive appositions onto physiologically identified neurons in the rostral ventromedial medulla. J Neurosci 12, 4023–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. (1996). Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823. [DOI] [PubMed] [Google Scholar]
- Mayer DJ (1984). Analgesia produced by electrical stimulation of the brain. Prog Neuropsychopharmacol Biol Psychiatry 8, 557–564. [DOI] [PubMed] [Google Scholar]
- McDonald AJ (1982). Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 208, 401–418. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, and Mascagni F (1997). Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 77, 445–459. [DOI] [PubMed] [Google Scholar]
- McKnight AT, Corbett AD, Paterson SJ, Magnan J, and Kosterlitz HW (1982). Comparison of in vitro potencies in pharmacological and binding assays after inhibition of peptidases reveals that dynorphin (1–9) is a potent kappa-agonist. Life Sci 31, 1725–1728. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, and Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23, 5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, and Cole S (2006). Opioid receptors in the midbrain periaqueductal gray regulate prediction errors during pavlovian fear conditioning. Behav Neurosci 120, 313–323. [DOI] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, and Blair HT (2011). Placing prediction into the fear circuit. Trends Neurosci 34, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrey D, and Basbaum AI (1987). The distribution of substance P-, enkephalin- and dynorphin-immunoreactive neurons in the medulla of the rat and their contribution to bulbospinal pathways. Neuroscience 23, 173–187. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP, Harasawa I, and Fields HL (2005). Kappa opioids inhibit physiologically identified medullary pain modulating neurons and reduce morphine antinociception. J Neurophysiol 93, 1138–1144. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Morgan MM, Kozell LB, and Ingram SL (2009). Contribution of dopamine receptors to periaqueductal gray-mediated antinociception. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G (2006). Optogenetics. Shining new light on neural circuits. Science 314, 1674–1676. [DOI] [PubMed] [Google Scholar]
- Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, and Henderson LA (2018). Brainstem Pain-Control Circuitry Connectivity in Chronic Neuropathic Pain. J Neurosci 38, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Weis RP, and Moore RY (1995). Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359, 221–238. [DOI] [PubMed] [Google Scholar]
- Moreau JL, and Fields HL (1986). Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 397, 37–46. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC, and Lane DA (2003). Behavioral evidence linking opioid-sensitive GABAergic neurons in the ventrolateral periaqueductal gray to morphine tolerance. Neuroscience 118, 227–232. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fossum EN, Levine CS, and Ingram SL (2006). Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav 85, 214–219. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Heinricher MM, and Fields HL (1992). Circuitry linking opioid-sensitive nociceptive modulatory systems in periaqueductal gray and spinal cord with rostral ventromedial medulla. Neuroscience 47, 863–871. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, and Aicher SA (2008). Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 140, 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss MS, Glazer EJ, and Basbaum AI (1983). The peptidergic organization of the cat periaqueductal gray. I. The distribution of immunoreactive enkephalin-containing neurons and terminals. J Neurosci 3, 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Tomida M, Yamamoto T, Ando H, Takamata T, Kondo E, Kurasawa I, and Asanuma N (2013). The endogenous opioids related with antinociceptive effects induced by electrical stimulation into the amygdala. Open Dent J 7, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Imai S, Nakamura A, Ozeki A, Asato M, Rahmadi M, Sudo Y, Hojo M, Uezono Y, Devi LA, et al. (2013). Possible involvement of prolonging spinal micro-opioid receptor desensitization in the development of antihyperalgesic tolerance to micro-opioids under a neuropathic pain-like state. Addict Biol 18, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Mizoguchi H, Dun NJ, Hwang BH, Endoh T, Suzuki T, Nagase H, and Tseng LF (2000). G protein activation by endomorphins in the mouse periaqueductal gray matter. J Biomed Sci 7, 221–225. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, and Heinricher MM (2004). Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain 110, 158–165. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, and Li W (2002). Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol 87, 103–112. [DOI] [PubMed] [Google Scholar]
- Oka T, Tsumori T, Yokota S, and Yasui Y (2008). Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neurosci Res 62, 286–298. [DOI] [PubMed] [Google Scholar]
- Okunomiya T, Hioki H, Nishimura C, Yawata S, Imayoshi I, Kageyama R, Takahashi R, and Watanabe D (2020). Generation of a MOR-CreER knock-in mouse line to study cells and neural circuits involved in mu opioid receptor signaling. Genesis 58, e23341. [DOI] [PubMed] [Google Scholar]
- Oliveira MA, and Prado WA (2001). Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Res Bull 54, 55–63. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, and Sesack SR (2010). Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J Neurosci Res 88, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, and Christie MJ (1996). Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol 4902, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Kovelowski CJ, Nichols ML, Hruby VJ, and Porreca F (1995). Characterization of supraspinal antinociceptive actions of opioid delta agonists in the rat. Pain 62, 287–293. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Johnson CW, Barker FD, Patterson MA, and Palmiter RD (2018). A Neural Circuit Underlying the Generation of Hot Flushes. Cell Rep 24, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD (2018). The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci 41, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZZ, and Fields HL (1996). Endogenous opioid-mediated inhibitions of putative pain-modulating neurons in rat rostral ventromedial medulla. Neuroscience 74, 855–862. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Tershner SA, and Fields HL (1997). Cellular mechanism for anti-analgesic action of agonists of the k-opioid receptor. Nature 389, 382–385. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, and Osborne PB (1990). Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol 427, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Kim JH, Yoon BE, Choi EJ, Lee CJ, and Shin HS (2010). T-type channels control the opioidergic descending analgesia at the low threshold-spiking GABAergic neurons in the periaqueductal gray. Proc Natl Acad Sci U S A 107, 14857–14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic ZW, Cooper ML, and Bodnar RJ (1996a). Opioid antagonists in the periaqueductal gray inhibit morphine and beta-endorphin analgesia elicited from the amygdala of rats. Brain Res 741, 13–26. [DOI] [PubMed] [Google Scholar]
- Pavlovic ZW, Cooper ML, and Bodnar RJ (1996b). Opioid antagonists in the periaqueductal gray inhibit morphine and ß-endorphin analgesia elicited from the amygdala of rats. Brain Res 741, 13–26. [DOI] [PubMed] [Google Scholar]
- Pecina M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, and Zubieta JK (2013). Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology 38, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraschka M, Li S, Gilbert TL, Westenbroek RE, Bruchas MR, Schreiber S, Lowe J, Low MJ, Pintar JE, and Chavkin C (2007). The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience 146, 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, and Gebhart GF (2002). Chronic pain and medullary descending facilitation. Trends Neurosci 25, 319–325. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Chevalier B, Laforest S, and Drolet G (2006). Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol 496, 859–876. [DOI] [PubMed] [Google Scholar]
- Reiss D, Ceredig RA, Secher T, Boue J, Barreau F, Dietrich G, and Gaveriaux-Ruff C (2017). Mu and delta opioid receptor knockout mice show increased colonic sensitivity. Eur J Pain 21, 623–634. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, and Wager TD (2014). Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci 17, 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samineni VK, Grajales-Reyes JG, Copits BA, O’Brien DE, Trigg SL, Gomez AM, Bruchas MR, and Gereau R.W.t. (2017). Divergent Modulation of Nociception by Glutamatergic and GABAergic Neuronal Subpopulations in the Periaqueductal Gray. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J, and Gebhart GF (1984). Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res 305, 77–87. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Gehrke BJ, and Shippenberg TS (2008a). Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain 138, 423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, and Shippenberg TS (2008b). Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain 136, 320–330. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Ball GJ, and Lucier GE (1981). Suppressive influences from periaqueductal gray and nucleus raphe magnus on respiration and related reflex activities and on solitary tract neurons, and effect of naloxone. Brain Res 216, 145–161. [DOI] [PubMed] [Google Scholar]
- Silva C, and McNaughton N (2019). Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog Neurobiol 177, 33–72. [DOI] [PubMed] [Google Scholar]
- Sim LJ, and Joseph SA (1991). Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat 4, 97–109. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, and Kieffer BL (1998). Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J 17, 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, and Advokat C (1987). Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res 424, 311–319. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Perrotti JM, Crisp T, Cabral ME, Long JT, and Scalzitti JM (1988). The mu opiate receptor is responsible for descending pain inhibition originating in the periaqueductal gray region of the rat brain. Eur J Pharmacol 156, 47–54. [DOI] [PubMed] [Google Scholar]
- Suckow SK, Deichsel EL, Ingram SL, Morgan MM, and Aicher SA (2013). Columnar distribution of catecholaminergic neurons in the ventrolateral periaqueductal gray and their relationship to efferent pathways. Synapse 67, 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, and Canteras NS (2006). A role for the periaqueductal gray in switching adaptive behavioral responses. J Neurosci 26, 2583–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Wang Y, Zhao CS, Chang JK, and Han JS (2001). Changes in brain content of nociceptin/orphanin FQ and endomorphin 2 in a rat model of neuropathic pain. Neurosci Lett 311, 13–16. [DOI] [PubMed] [Google Scholar]
- Sun Y, Blanco-Centurion C, Zou B, Bendell E, Shiromani PJ, and Liu M (2019). Amygdala GABA Neurons Project To vlPAG And mPFC. IBRO Rep 6, 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Gu XL, and Yu LC (2007). The neural pathway of galanin in the hypothalamic arcuate nucleus of rats: activation of beta-endorphinergic neurons projecting to periaqueductal gray matter. J Neurosci Res 85, 2400–2406. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Villemure JG, Bushnell MC, and Duncan GH (1995). Evaluation of pain perception after anterior capsulotomy: a case report. Somatosens Mot Res 12, 115–126. [DOI] [PubMed] [Google Scholar]
- Tonsfeldt KJ, Suchland KL, Beeson KA, Lowe JD, Li MH, and Ingram SL (2016). Sex Differences in GABAA Signaling in the Periaqueductal Gray Induced by Persistent Inflammation. J Neurosci 36, 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorici V, and Morgan MM (2002). Comparison of morphine and kainic acid microinjections into identical PAG sites on the activity of RVM neurons. J Neurophysiol 88, 1707–1715. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM, and Vanegas H (2001). Tolerance to repeated microinjection of morphine into the periaqueductal gray is associated with changes in the behavior of off- and on-cells in the rostral ventromedial medulla of rats. Pain 89, 237–244. [DOI] [PubMed] [Google Scholar]
- Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, La Cesa S, Tarsitani L, Mainero C, Sarzi-Puttini P, Cruccu G, et al. (2016). Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol 34, S129–133. [PubMed] [Google Scholar]
- Urban MO, and Gebhart GF (1999). Central mechanisms in pain. Med Clin North Am 83, 585–596. [DOI] [PubMed] [Google Scholar]
- Valverde O, Fournie-Zaluski MC, Roques BP, and Maldonado R (1996). Similar involvement of several brain areas in the antinociception of endogenous and exogenous opioids. Eur J Pharmacol 312, 15–25. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Bagley EE, Drew GM, Schuller A, Pintar JE, Hack SP, and Christie MJ (2003). Cellular actions of opioids on periaqueductal grey neurons from C57B16/J mice and mutant mice lacking MOR-1. Br J Pharmacol 139, 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, and Christie MJ (1997). Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol 4982, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, and Chavkin C (1993). Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature 363, 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, and Wessendorf MW (1999). Mu- and delta-opioid receptor mRNAs are expressed in spinally projecting serotonergic and nonserotonergic neurons of the rostral ventromedial medulla. J Comp Neurol 404, 183–196. [DOI] [PubMed] [Google Scholar]
- Wang H, and Wessendorf MW (2002). Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neurons projecting to the rostral ventromedial medulla. Neuroscience 109, 619–634. [DOI] [PubMed] [Google Scholar]
- Wanigasekera V, Lee MC, Rogers R, Kong Y, Leknes S, Andersson J, and Tracey I (2012). Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A 109, 17705–17710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Margolis EB, Charbit AR, and Fields HL (2019). A Midbrain Circuit that Mediates Headache Aversiveness in Rats. Cell Rep 28, 2739–2747 e2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, and Pertovaara A (1999). MK-801, an NMDA receptor antagonist, in the rostroventromedial medulla attenuates development of neuropathic symptoms in the rat. Neuroreport 10, 2933–2937. [DOI] [PubMed] [Google Scholar]
- Weibel R, Reiss D, Karchewski L, Gardon O, Matifas A, Filliol D, Becker JA, Wood JN, Kieffer BL, and Gaveriaux-Ruff C (2013). Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice. PLoS One 8, e74706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, and Nicoll RA (1993). The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature 365, 188. [DOI] [PubMed] [Google Scholar]
- Williams FG, and Beitz AJ (1990). Ultrastructural morphometric analysis of enkephalin-immunoreactive terminals in the ventrocaudal periaqueductal gray: Analysis of their relationship to periaqueductal gray-raphe magnus projection neurons. Neuroscience 38, 381–394. [DOI] [PubMed] [Google Scholar]
- Williams FG, Mullet MA, and Beitz AJ (1995). Basal release of Met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res 690, 207–216. [DOI] [PubMed] [Google Scholar]
- Williams RG, and Dockray GJ (1983). Distribution of enkephalin-related peptides in rat brain: immunohistochemical studies using antisera to met-enkephalin and met-enkephalin Arg6Phe7. Neuroscience 9, 563–586. [DOI] [PubMed] [Google Scholar]
- Winters BL, Gregoriou GC, Kissiwaa SA, Wells OA, Medagoda DI, Hermes SM, Burford NT, Alt A, Aicher SA, and Bagley EE (2017). Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat Commun 8, 14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, and Rudy TA (1976). Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114, 83–103. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D (2015). Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 156 Suppl 1, S24–31. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, and Kastin AJ (1997). A potent and selective endogenous agonist for the mu-opiate receptor. Nature 386, 499–502. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han BX, Zhou X, and Wang F (2015). Identifying local and descending inputs for primary sensory neurons. J Clin Invest 125, 3782–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]