Abstract
High postprandial blood glucose levels are associated with increased mortality, cardiovascular events and development of diabetes in the general population. Interventions targeting postprandial glucose have been shown to prevent both cardiovascular events and diabetes. This study evaluates the efficacy and safety of a novel nutritional supplement targeting postprandial glucose excursions in non-diabetic adults. Sixty overweight healthy male and female participants were recruited at two centers and randomized in a double-blind, placebo-controlled, crossover design. The supplement, a water-based drink containing 2.6g of amino acids (L-Leucine, L-Threonine, L-Lysine Monohydrochloride, L-Isoleucine, L-Valine) and 250 mcg of chromium picolinate, was consumed with a standardized carbohydrate-rich meal. The primary endpoint was the incremental area under the curve (iAUC) for venous blood glucose from 0 to 120 minutes. Secondary endpoints included glucose iAUC 0–180 minutes and the maximum glucose concentration (Cmax), for both venous and capillary blood glucose. In the intention-to-treat-analysis (n = 60) the supplement resulted in a decreased venous blood glucose iAUC0-120min compared to placebo, mean (SE) of 68.7 (6.6) versus 52.2 (6.8) respectively, a difference of -16.5 mmol/L•min (95% CI -3.1 to -30.0, p = 0.017). The Cmax for venous blood glucose for the supplement and placebo were 6.45 (0.12) versus 6.10 (<0.12), respectively, a difference of -0.35 mmol/L (95% CI -0.17 to -0.53, p<0.001). In the per protocol-analysis (n = 48), the supplement resulted in a decreased Cmax compared to placebo from 6.42 (0.14) to 6.12 (0.14), a difference of -0.29 mmol/L (95% CI -0.12 to -0.47, p = 0.002). No significant differences in capillary blood glucose were found, as measured by regular bed-side glucometers. The nutritional supplement drink containing amino acids and chromium improves the postprandial glucose homeostasis in overweight adults without diabetes. Future studies should clarify, whether regular consumption of the supplement improves markers of disease or could play a role in a diet aiming at preventing the development of diabetes.
Introduction
High postprandial glucose levels after an oral glucose load are associated with the development of type 2 diabetes (T2D), cardiovascular disease (CVD) as well as increased mortality even in absence of a pre-diabetic condition such as impaired fasting glucose (IFG) [1–8]. A causal relationship between high postprandial glucose levels and T2D or CVD appears likely since interventions targeting postprandial glycemia have been shown to prevent these clinical conditions. This has explicitly been shown for pharmacologic interventions with acarbose [9–11] and is robustly supported by studies on low glycemic index (GI) diets [12–17] as well as experimental data describing possible underlying mechanisms [18–21].
Prevention of T2D and CVD are highly relevant and urgent public health issues since rates of obesity and overweight as well as the age of populations increase [1]. At the same time carbohydrate-rich foods with a high GI and a high glycemic load (GL), which became widespread throughout the 20th century paralleled by an increasing prevalence of obesity and T2D [22], are very popular and commonly available to the consumer [23]. These foods bear a high potential of causing pronounced glucose excursions and insulin spikes, which in turn might contribute to the development of T2D and CVD.
The aim of this study was to show efficacy and safety of a novel nutritional supplement drink (hereinafter called “the supplement”)—a blend of five specific amino acids (5AA) and chromium picolinate (CrPic) in water, in a two center, double-blinded RCT. The 5AA were selected on basis of their rapid appearance in the blood stream after intake of whey protein [24] and chromium picolinate for its potential effect on insulin sensitivity [25]. The supplement has been developed to lower postprandial glycemia when consumed with a carbohydrate-rich meal and showed promising results in published [26] and unpublished pilot studies.
Methods
Study design
The randomized, double-blind, placebo-controlled, crossover study was performed by KGK Science Inc. at two centers (London ON, Canada and Orlando FL, US). Participants attended two single day periods separated by a 7-day washout period.
Ethics
This trial was registered at clinicaltrials.gov as NCT03152682. The registration was done with some delay with respect to patient recruitment, due to lack of clarity who was in charge of the submission. The authors confirm that all ongoing and related trials for this intervention are registered. The investigational product was reviewed by Health Canada and classified as a food. Issuance of a permit to conduct this clinical trial in Canada was therefore not required by Health Canada. Similarly, an Investigational New Drug permit was not required for conduct of the study in the USA. This study was approved for conduct at both the Canadian and the USA sites by the Institutional Review Board (IRB Services, Aurora, Ontario) on March 14, 2017. It was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki and its subsequent amendments. Amendments to the protocol were approved on June 14, 2017 and July 27, 2017. Written informed consent was obtained from all participants. The investigational products were produced in the USA and no permit for the import of the investigational product into Canada was required.
Participants
Individuals were recruited from Southwestern Ontario, Canada and Orlando, Florida, using the KGK Science Inc.’s internal participant database along with local electronic and physical advertisement devoid of gender or racial bias. Participants were required to be between 18 and 50 years of age, have a BMI between 25–29.9 kg/m2 and be in a good physical and mental condition according to their own perception (on a five point scale from ‘poor’ to ‘excellent’), medical history and laboratory results (full list of inclusion and exclusion criteria in S1 Table).
Test product and placebo
The supplement was a lightly carbonated drink containing a proprietary blend with molar ratios presented in [26] and a total of 2.6 g 5AA (L-Leucine, L-Threonine, L-Lysine Monohydrochloride, L-Isoleucine and L-Valine) as well as 250 mcg CrPic (Good Idea®, Good Idea, Inc., Larkspur, CA). Besides water and the active ingredients, the test product also contained citric acid, lemon natural flavor, sodium benzoate and potassium sorbate. The placebo product was identical to the test product with respect to all ingredients, but 5AA and CrPic. The investigational product and placebo were sealed in identically appearing bottles and matched in regard to color, taste and texture.
Intervention
After an overnight fast of 12h, the participants reported to the study facility in the morning. Upon confirmation of participant status and eligibility criteria, an intravenous cannula for repeated blood sampling was placed into the antecubital vein.
Participants started by consuming 175 ml of the supplement or placebo on an empty stomach (time 0), and 3 min later they began with the ingestion of a standardized breakfast meal, alternating between eating and drinking. Three portions of 50 ml each of the supplement or placebo were required to be consumed at min 3, 7 and 11 and the breakfast meal was to be completed by min 14. At the 14 min mark, the final 30 ml of test product or placebo were ingested. The total amount of supplement or placebo was 355 ml. The test meal consisted of white wheat bread (110 g without crust), butter (10 g) and ham (38 g). The calorie and macronutrient composition of the standardized breakfast meal at the London clinical site was: 377 kcal, 47.7g carbohydrates, 14.9 g proteins and 14.1 g fats. The corresponding figures for the Orlando site were: 350 kcal, 45.3 g carbohydrates, 13.1 g proteins and 13 g fats.
Blood samples were collected at 0, 15, 30, 45, 60, 90, 120 and 180 min for glucose (venous and capillary) and insulin (venous) analysis. Capillary blood glucose was measured by the CONTOUR® monitoring system (London site) and the OneTouch® Ultra 2 monitoring system (Orlando site). Quantitative determination of glucose in human serum was conducted via the enzymatic method with hexokinase utilizing the Roche Cobas e701 Analyzer (sensitivity limit 0.5 mmol/L, intra-assay coefficient of variation (CV) 2.7% at 3.1 mmol/L, and 1.4% at 19.8 mmol/L). Quantitative determination of insulin in human serum was conducted via electrochemiluminescence immunoassay utilizing the Roche Cobas e602 Analyzer (sensitivity limit 4 pmol/L, intra-assay CV 7.5% at 106 pmol/L, and 4.5% at 1 357 pmol/L).
Safety endpoints were analyzed in blood drawn at screening and both investigational visits (before starting the actual intervention and associated blood sampling) and included: blood count (hemoglobin, hematocrit, platelet count, red blood cell count, red cell indices, red cell distribution width, white blood cell count, and differentials (neutrophils, lymphocytes, monocytes, eosinophils, basophils)), liver function (alanine transaminase, aspartate transaminase, bilirubin), and kidney function (creatinine, electrolytes (Na, K, Cl)). Besides the blood parameters, also blood pressure and heart rate were monitored. Urine pregnancy tests (Biostrip HCG, Innovatek Medical Inc.) were conducted at both sites for participants of childbearing age during the screening and baseline visits.
Outcome measures
The pre-defined primary outcome variable was 120-minute incremental area under the curve (iAUC0-120min) for venous serum glucose. Time zero (0) indicates the time when the participant started eating the standardized meal and then repeated blood samples were taken until the last one at 120 min post meal start. The iAUC is the area under the curve, above the baseline levels. It corrects for variations in fasting plasma glucose levels within and between individuals and is thus optimal for comparing the effect of a food on blood glucose levels of individuals independently of their fasting plasma glucose. The iAUC is used, among others, for the calculation of the glycemic index [27]. Secondary outcome variables for venous sampling were: glucose iAUC0-180min; serum insulin iAUC0-120min and iAUC0-180min; peak glucose value in 120 minutes (Cmax 0-120min) and 180 minutes (Cmax 0-180min); time to peak glucose for 120 minutes (Tmax 0-120min) and 180 minutes (Tmax 0-180min); serum insulin Cmax 0-120min and Cmax 0-180min. Secondary outcome variables for capillary measurements were glucose iAUC0-120min and iAUC0-180min; Cmax 0-120min and Cmax 0-180min; Tmax 0-120min and Tmax 0-180min, as well as eating patterns as assessed by a 3-day food record for the weeks prior to days 0 and 8. The difference in each secondary outcome between supplement group and placebo group was tested separately by applying the method described in the Statistical analysis section.
Sample size calculation
A sample size of 30 participants per center (total of 60 participants) was calculated based on an expected iAUC of 66.09 mmol•min/L for the placebo and a pooled standard deviation of 61.50 mmol•min/L from a study by Lustig et al [28]. A mean difference of 36.8 mmol/L/min between the investigational product group and the placebo group is expected for the iAUC for each center, assuming a two-sided test with alpha equal to 5%, 80% power and a 20% drop-out rate from enrollment to final, post-baseline measurement.
Randomization
Randomization numbers were assigned by a blinded investigator per the order of a randomization list generated by www.randomization.com and allocated to their sequence in a decentralized manner in a 1:1 ratio, with a separate randomization list for each site. An un-blinded associate that was not involved in any data capture or study assessments labeled the investigational product. A randomization schedule was created and provided to the investigator indicating the order of randomization. Investigators, other site personnel and participants were blinded to the products.
Statistical analysis
All analyses were performed on the pooled data of the two centers. The following analytical populations were defined for the study: Safety population–all participants who received either product and on whom any post-randomization safety information was available; Intention-to-treat (ITT) population–all participants who received either product and on whom any post-randomization efficacy information was available; Per Protocol (PP) population–all participants who consumed 100% of the investigational products, did not have any major protocol violations, and completed all study visits and procedures connected with measurement of the primary variable.
The trapezoid rule was used for calculating iAUC0-120min and iAUC0-180min. The trapezoidal rule is a numerical integration method used to approximate the area under a curve. It is widely used to calculate the area under pharmacokinetic curves [29, 30]. Although simple, the trapezoidal rule is a reliable method for calculating iAUC, which has been shown to be more strongly correlated to glycemic response than total AUC [31, 32]. For the iAUC calculations, invalid data points in the ITT and PP analysis were handled by simple imputation methods–averaging values directly adjacent to the missing data point or using a participant’s corresponding value from the other study period (pre-ingestion). It was possible to calculate the primary endpoint for 57 participants. Calculations for all other outcome variables were performed on the original data set without imputation. The between group changes from pre-ingestion were analyzed by a mixed model repeated measures analysis of variance. The model included subject as a random effect, with fixed effects of group, sequence, and visit. The p-values for each change was derived by a linear contrast statement of this model. P-values ≤ 0.05 were considered statistically significant. All statistical analysis was completed using the R Statistical Software Package Version 3.2.2 (R Core Team, 2015) and SAS version 9.3 (Cary, North Carolina) for Microsoft Windows.
Results
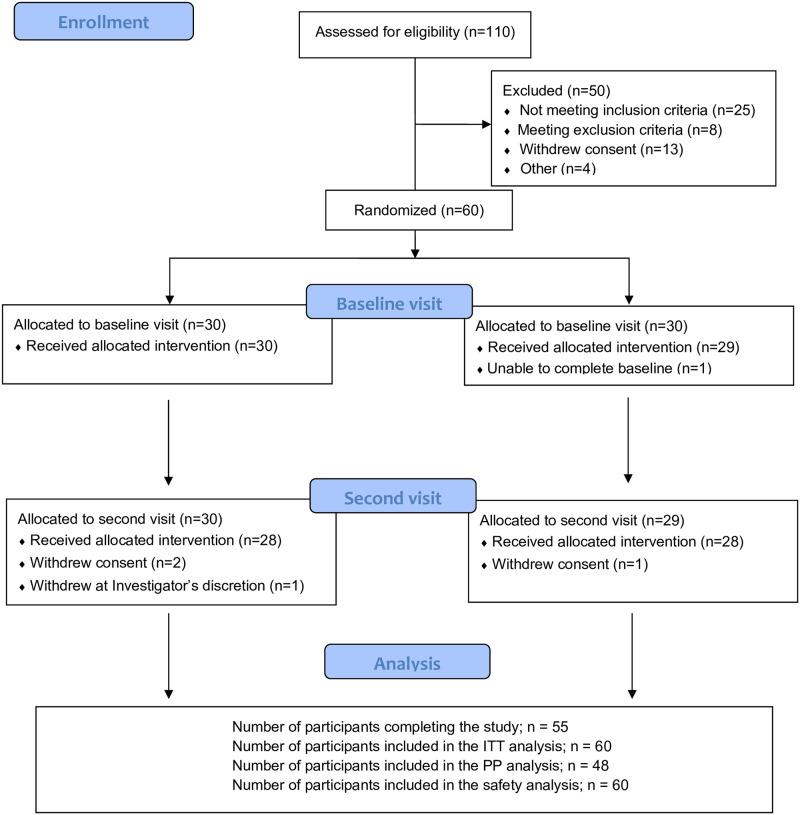
The participant recruitment began in March 2017 and the study was completed by August 2017. In order to recruit 60 men and women in equivalent numbers and according to pre-defined inclusion and exclusion criteria, a total of 110 candidates needed to be screened. The participant flow is outlined in Fig 1.
Fig 1. Participant flow.
The ITT population included 60 participants (32 female, 28 male) from which 12 were excluded, with a resulting PP population of 48 participants (23 female, 25 male). Baseline characteristics are presented in Table 1. Out of the 12 excluded participants, seven were from the supplement → placebo sequence and five from the placebo → supplement sequence. The reasons for the exclusions were: starting antibiotic treatment (n = 1), delayed or missing blood samples (n = 3), non-adherence to the protocol (n = 2), fainting (n = 1) and wish to terminate the participation before second trial day (n = 5).
Table 1. Participant characteristics at screening1.
| ITT population (n = 60) | PP population (n = 48) | |
|---|---|---|
| Gender, n (%) | ||
| Female | 32 (53%) | 23 (48%) |
| Male | 28 (47%) | 25 (52%) |
| Alcohol consumption status, n (%) | ||
| None | 21 (35%) | 17 (35%) |
| Occasional | 26 (43%) | 21 (44%) |
| Weekly | 13 (22%) | 10 (21%) |
| Smoking status, n (%) | ||
| Ex-smoker | 5 (8%) | 3 (6%) |
| No | 55 (92%) | 45 (94%) |
| Health questionnaire, n (%) | ||
| Excellent | 22 (37%) | 18 (38%) |
| Very good | 27 (45%) | 21 (44%) |
| Good | 11 (18%) | 9 (19%) |
| Race, n (%) | ||
| Western European white | 28 (47%) | 21 (44%) |
| Black or African American | 15 (25%) | 12 (25%) |
| Other | 17 (28%) | 15 (31%) |
| Age, yrs (SD) | 34.6 (10.4) | 35.5 (10.5) |
| Weight, kg (SD) | 79.7 (10.2) | 80.8 (10.7) |
| BMI, kg/m2 (SD) | 27.4 (1.59) | 27.5 (1.59) |
| Fasting venous blood glucose, mmol/L (SD) | 4.93 (0.42) | 4.95 (0.42) |
| HbA1c, % (SD) | 5.48 (0.33) | 5.47 (0.31) |
| Systolic blood pressure, mmHg (SD) | 119.8 (8.9) | 117.7 (9.0) |
| Diastolic blood pressure, mmHg (SD) | 76.2 (8.1) | 75.8 (8.7) |
| Heart rate, bpm (SD) | 69.0 (11.1) | 69.2 (11.2) |
1 Values are n (%), or means (SD)
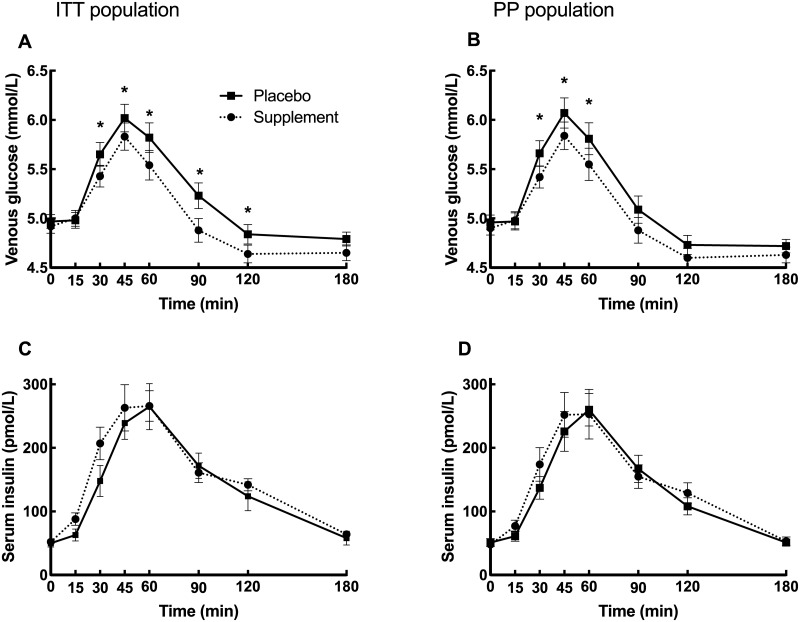
The glucose and insulin responses are presented in Fig 2 and Table 2.
Fig 2. Postprandial glucose and insulin responses.
Glucose and insulin responses for the ITT (n = 54–58, for different time points) and PP (n = 48) populations respectively after intake of a high carbohydrate sandwich meal with supplement or placebo. Data are expressed as means ± SE. Asterisc (*) indicates a significant difference between treatments (p<0.05) at the respective time point.
Table 2. Glucose and insulin responses for ITT and PP populations1.
| Supplement | Placebo | Group Difference | 95% CI | ||||||||
| ITT population | n | Mean | SE | n | Mean | SE | Mean | SE | Upper | Lower | p-value |
| Serum glucose | |||||||||||
| iAUC0-120 min, mmol/L•min | 54 | 52·20 | 6·79 | 58 | 68·72 | 6·62 | -16·54 | 6·71 | -3·08 | -30·01 | 0·017 |
| iAUC0-180 min, mmol/L•min | 54 | 62·44 | 8·42 | 58 | 79·07 | 8·19 | -16·63 | 8·56 | 0·55 | -33·81 | 0·058 |
| Cmax0-120 min, mmol/L | 54 | 6·10 | 0·12 | 58 | 6·45 | 0·12 | -0·35 | 0·09 | -0·17 | -0·53 | <0·001 |
| Tmax0-120 min, min | 54 | 51·04 | 2·49 | 58 | 52·29 | 2·85 | -1·25 | 5·32 | 5·32 | -7·82 | 0·704 |
| Serum insulin | |||||||||||
| iAUC0-120 min, pmol/L•min | 54 | 15 803 | 1 589 | 58 | 14 523 | 1 556 | 1 279 | 1 334 | 3 958 | -1 399 | 0·342 |
| iAUC0-180 min, pmol/L•min | 54 | 19 361 | 1 908 | 58 | 17 253 | 1 869 | 2 108 | 1 589 | 5 298 | -1 083 | 0·191 |
| Cmax 0–120 min, pmol/L | 54 | 380·99 | 35·19 | 58 | 345·70 | 34·63 | 35·29 | 25·58 | 86·66 | -16·07 | 0·174 |
| Tmax 0–120 min, min | 54 | 59·38 | 3·43 | 58 | 58·78 | 3·33 | 0·60 | 3·76 | 8·16 | -6·95 | 0·873 |
| Supplement | Placebo | Group Difference | CI 95% | ||||||||
| PP population | n | Mean | SE | n | Mean | SE | Mean | SE | Upper | Lower | p-value |
| Serum glucose | |||||||||||
| iAUC0-120 min, mmol/L•min | 48 | 53·73 | 7·26 | 48 | 66·48 | 7·26 | -12·75 | 6·95 | 1·24 | -26·74 | 0·073 |
| iAUC0-180 min, mmol/L•min | 48 | 62·36 | 8·72 | 48 | 74·37 | 8·72 | -12·01 | 8·91 | 5·93 | -29·94 | 0·185 |
| Cmax 0–120 min, mmol/L | 48 | 6·12 | 0·14 | 48 | 6·42 | 0·14 | -0·29 | 0·09 | -0·12 | -0·47 | 0·002 |
| Tmax 0–120 min, min | 48 | 49·30 | 2·98 | 48 | 49·61 | 2·98 | -0·31 | 3·22 | 6·17 | -6·79 | 0·923 |
| Serum insulin | |||||||||||
| iAUC0-120 min, pmol/L•min | 48 | 14 833 | 1 607 | 48 | 13 546 | 1 607 | 1 268 | 1 416 | 4 119 | -1 582 | 0·375 |
| iAUC0-180 min, pmol/L•min | 48 | 17 737 | 1 791 | 48 | 15 713 | 1 791 | 2 024 | 1 719 | 5 483 | -1 436 | 0·245 |
| Cmax 0–120 min, pmol/L | 48 | 357·15 | 35·81 | 48 | 324·42 | 35·81 | 32·73 | 26·62 | 86·30 | -20·85 | 0·225 |
| Tmax 0–120 min, min | 48 | 59·50 | 3·48 | 48 | 58·17 | 3·48 | 1·33 | 3·96 | 9·29 | -6·64 | 0·739 |
In the ITT population, the supplement led to a 24% reduction in venous serum glucose iAUC0-120 min (p = 0.03) as the primary outcome measure as well as a 5.4% reduction in Cmax 0–120 (p<0.01) as compared to placebo. There were significant differences in serum glucose between placebo and the supplement at 30, 45, 60, 90 and 120 minutes (reduction by 4%, 3%, 5%, 6% and 4% respectively). No significant differences in the iAUCs for insulin, Tmax or iAUC for capillary measurements were observed.
In the PP population a 4.5% reduction in Cmax 0–120 (p<0.01) could be documented. There were significant reductions in serum glucose by the supplement compared with placebo at 30, 45 and 60 minutes (-4%, -4% and -5% respectively). No significant differences in the iAUCs for venous serum glucose or insulin as well as Tmax or iAUC for capillary measurements, were observed.
An analysis of subgroups was performed in the PP population. Pronounced effects were observed in the middle-aged group (40–50 years old, n = 23) with a 30% reduction in venous glucose iAUC0-120 min (p = 0.006) and a 5% reduction in Cmax (p = 0.004) after the supplement compared to placebo. The other subgroups (gender, race) did not show any significant changes in the primary endpoint.
There were no severe adverse events, as categorized by the Medical Dictionary for Regulatory Activities, version 17. Out of 19 mild to moderate adverse events (AE) recorded in 16 individuals experiencing these events, three events were categorized as possibly related to the supplement (one nervous system disorder and two gastrointestinal disorders) and one as possibly related to placebo (vascular disorder). All AEs were resolved by the end of the study without requiring medical treatment or hospitalization.
Discussion
In this controlled clinical trial, we show a significant reduction of the serum glucose iAUC in healthy overweight subjects after a mixed meal consumed together with the supplement, compared to placebo. There are different ways in which the supplement could beneficially influence the glucose metabolism and health in the target population of individuals that are not yet affected by cardiovascular disease or diabetes. From a GI point of view [33], the supplement was able to reduce the overall GI of the high glycemic test meal. Although there is still some controversy on the role of low GI food and meals [12, 34], most studies comparing diets with different GI´s favor the ones with a lower GI in regards to the development of T2D [12–17, 35, 36].
Elevated postprandial glucose has been identified as an important risk factor for developing T2D as well as CVD and even overall mortality in individuals without impaired glucose tolerance (IGT) or T2D. The factual endpoints studied in these epidemiological studies were the 60 and 120 minutes glucose values after a standardized glucose load varying between 50g and 100g of glucose [1–8]. This study showed significant differences for venous glucose at 30, 45, 60 and 90 minutes in favor of the supplement, thus showing a significant reduction of the surrogate risk factor 60-minute postprandial glucose. The use of a mixed meal as opposed to glucose solutions tests efficacy of the supplement closer to a real-life scenario.
There is ample evidence for a causal relationship between elevated postprandial glucose and the development of T2D and CVD. Experimental evidence on a cellular level shows that an induction of endothelial dysfunction by postprandial hyperglycemia is most likely a consequence of increased oxidative stress [18–21]. In human studies, postprandial glucose excursions have been shown to correlate with the extent of atherosclerosis [21]. Interventions targeting postprandial glycemia have been shown to prevent atherosclerosis but also the development of T2D [9–11] and possibly even cardiovascular events [9, 10].
Participants in this study were mostly overweight and the glucose lowering effect of the supplement was pronounced in the older subgroup (40–50 yrs) showing a significant and impressive 30% reduction of iAUC0-120 min glucose. Since overweight and increasing age are the main risk-factors for developing diabetes [1], efficacy of the supplement has been shown in a group, in which diabetes prevention is highly relevant.
The exact mechanisms leading to the observed effects of the supplement on postprandial glucose are not completely clear. Chromium is, however, known to play a role in glucose metabolism, possibly by potentiating insulin interaction with its receptor via binding of a low molecular chromium binding protein [37–39]. Yet, its role in improving glucose metabolism as a supplement is not well described in controlled clinical studies, most of which were performed in patients with T2D [40–43]. Milk and whey protein are known to stimulate insulin [44–50] and thus reduce the postprandial glucose response to a glucose solution [45, 47–51]. However, in the case of single amino acids, the insulinogenic effect is not attributable to all amino acids [52–55] and requires higher doses than the ones used here [52, 53, 56–58]. Hence, the iAUC for insulin was not significantly increased by the supplement compared to placebo and there was only an indication of a trend towards an earlier insulin response when looking at insulin iAUC´s. Studies looking at insulin and the development of T2D or IGT suggest that a pronounced first-phase insulin response compared to a late insulin increase could be beneficial [55, 59–61]. In addition it has been described, that proteins and amino acids have a distinct effect on gastric emptying, possibly delaying the glucose uptake [62–64]. A ‘priming’ of the stomach with the first sips of the supplement leading to a slowing of gastric emptying and a better insulin homeostasis could contribute to the effects on postprandial glucose observed. Further research will have to substantiate these hypotheses.
The limitations of this study include missing values due to the high number of blood draws required for each participant and thus the need for imputation of some results when performing the iAUC calculation in the ITT analysis. Another limitation is the lack of data on menstrual cycle for the female participants, since it is known that blood glucose regulation differs between the follicular and luteal phases [65]. Overall, there were no significant effects when looking at the capillary blood measurements. This can be explained by the use of patient glucometers, which are known for their imprecision [66, 67] and cannot be expected to produce reliable data for the calculation of differences in glucose iAUC. This is surely a systematic problem for the analysis of this secondary end point. However, in a previous study of an early version of the supplement, professional glucometers used for capillary blood glucose measurements showed significant reductions of postprandial glucose, in line with that shown for venous glucose in this study [26]. Another limitation of this study was the variability in brands of bread used for the standardized test meal between the Canadian and American sites. This resulted in a slight difference in fiber content between the meals, with the Canadian brand having 2 grams more dietary fiber than the US brand. However, the macronutrient and caloric content of the test meals remained relatively similar between sites and individuals at each center received the same brand in the supplement and placebo test meal.
In conclusion, the supplement drink tested in this study efficiently reduced the postprandial glucose excursions after a meal with a high carbohydrate content without causing any adverse events. Future studies will have to evaluate whether regular consumption of the supplement improves markers of disease or might even prevent the development of diabetes. Effects on satiety and food cravings will also have to be studied. Definition of the mechanisms involved in the observed effects will help to develop supplements that could not only be used for the prevention of diabetes but may also be an option for dietary interventions in metabolic disease.
Supporting information
(PDF)
(DOC)
(XLSX)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funder provided support in the form of salaries [EÖ, LHL, KA and RÖ] and consulting fees [starScience GmbH] for authors but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Federation ID. IDF Diabetes Atlas. Brussels, Belgium; 2017.
- 2.Kodama S, Saito K, Tanaka S, Horikawa C, Fujiwara K, Hirasawa R, et al. Fasting and post-challenge glucose as quantitative cardiovascular risk factors: a meta-analysis. J Atheroscler Thromb. 2012;19(4):385–396. 10.5551/jat.10975 [DOI] [PubMed] [Google Scholar]
- 3.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22(2):233–240. 10.2337/diacare.22.2.233 [DOI] [PubMed] [Google Scholar]
- 4.Einarson TR, Machado M, Henk Hemels ME. Blood glucose and subsequent cardiovascular disease: update of a meta-analysis. Curr Med Res Opin. 2011;27(11):2155–2163. 10.1185/03007995.2011.626760 [DOI] [PubMed] [Google Scholar]
- 5.Bergman M, Chetrit A, Roth J, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts mortality: observations from the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabet Med. 2016;33(8):1060–1066. 10.1111/dme.13116 [DOI] [PubMed] [Google Scholar]
- 6.Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 24year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Res Clin Pract. 2016;120:221–228. 10.1016/j.diabres.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 7.Ning F, Zhang L, Dekker JM, Onat A, Stehouwer CD, Yudkin JS, et al. Development of coronary heart disease and ischemic stroke in relation to fasting and 2-hour plasma glucose levels in the normal range. Cardiovasc Diabetol. 2012;11:76 10.1186/1475-2840-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahim B, De Bacquer D, De Backer G, Gyberg V, Kotseva K, Mellbin L, et al. The prognostic value of fasting plasma glucose, two-hour postload glucose, and HbA. Diabetes Care. 2017;40(9):1233–1240. 10.2337/dc17-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. The Lancet. 2002;359(9323):2072–2077. [DOI] [PubMed] [Google Scholar]
- 10.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494. 10.1001/jama.290.4.486 [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Coleman RL, Chan JCN, Chiasson JL, Feng H, Ge J, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(11):877–886. 10.1016/S2213-8587(17)30309-1 [DOI] [PubMed] [Google Scholar]
- 12.Augustin LS, Kendall CW, Jenkins DJ, Willett WC, Astrup A, Barclay AW, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis. 2015;25(9):795–815. 10.1016/j.numecd.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Schulze MB, Martinez-Gonzalez MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396 10.1136/bmj.k2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2017:1–20. [DOI] [PubMed] [Google Scholar]
- 15.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20(4):545–550. 10.2337/diacare.20.4.545 [DOI] [PubMed] [Google Scholar]
- 16.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. Journal of the American Medical Association. 1997;277(6):472–477. 10.1001/jama.1997.03540300040031 [DOI] [PubMed] [Google Scholar]
- 17.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. The American Journal of Clinical Nutrition. 2008;87(3):627–637. 10.1093/ajcn/87.3.627 [DOI] [PubMed] [Google Scholar]
- 18.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106(10):1211–1218. 10.1161/01.cir.0000027569.76671.a8 [DOI] [PubMed] [Google Scholar]
- 19.Humpert PM. Oxidative stress and glucose metabolism—is there a need to revisit effects of insulin treatment? Diabetologia. 2010;53(3):403–405. 10.1007/s00125-009-1652-9 [DOI] [PubMed] [Google Scholar]
- 20.Node K, Inoue T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc Diabetol. 2009;8:23 10.1186/1475-2840-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis. 1999;144(1):229–235. 10.1016/s0021-9150(99)00059-3 [DOI] [PubMed] [Google Scholar]
- 22.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. The American Journal of Clinical Nutrition. 2004;79(5):774–779. 10.1093/ajcn/79.5.774 [DOI] [PubMed] [Google Scholar]
- 23.Cust AE, Skilton MR, van Bakel MM, Halkjaer J, Olsen A, Agnoli C, et al. Total dietary carbohydrate, sugar, starch and fibre intakes in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 2009;63 Suppl 4:S37–60. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactoseequivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. American Journal of Clinical Nutrition. 2004;80(5):1246–1253. 10.1093/ajcn/80.5.1246 [DOI] [PubMed] [Google Scholar]
- 25.Cefalu WT, Bell-Farrow AD, Stegner J, Wang ZQ, King T, Morgan T, et al. Effect of chromium picolinate on insulin sensitivity in vivo. J Trace Elem Exp Med. 1999;12(2):71–83. [Google Scholar]
- 26.Ostman E, Forslund A, Oste R, Bjorck I. A drink containing amino acids and chromium picolinate improves postprandial glycemia at breakfast in healthy, overweight subjects. Functional Food in Health and Disease. 2017;7(2):88–97. [Google Scholar]
- 27.Cheng KC, Li Y, Cheng JT. Merit of incremental area under the curve (iAUC) in nutrition is varied in pharmacological assay—A review. Clinical Journal of Diabetes Care and Control. 2018;1(2):180008. [Google Scholar]
- 28.Lustig RH, Greenway F, Velasquez-Mieyer P, Heimburger D, Schumacher D, Smith D, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. International Journal of Obesity. 2006;20(2):331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill Jones N. Finding the area under a curve using JMP and a trapezoidal rule. JMPer Cable. 1997:9–11.
- 30.Yeh S-T. Using trapezoidal rule for the area under a curve calculation. NESUG 15. p. 1–5.
- 31.Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990;13(2):172–175. 10.2337/diacare.13.2.172 [DOI] [PubMed] [Google Scholar]
- 32.Brouns F, Björck I, Frayn KN, Gibbs AL, Lang V, Slama G, et al. Glycaemic index methodology. Nutrition Research Reviews. 2005;18:145–171. 10.1079/NRR2005100 [DOI] [PubMed] [Google Scholar]
- 33.Standardization I-IOf. ISO 26642:2010; Food products—Determination of the glycaemic index (GI) and recommendation for food classification. 2010.
- 34.Sluijs I, Beulens JW, van der Schouw YT, van der AD, Buckland G, Kuijsten A, et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr. 2013;143(1):93–99. 10.3945/jn.112.165605 [DOI] [PubMed] [Google Scholar]
- 35.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218–232. 10.3945/ajcn.113.079533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev. 2009(1):CD006296 10.1002/14651858.CD006296.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson RA. Nutritional factors influencing the glucose/insulin system: chromium. J Am Coll Nutr. 1997;16(5):404–410. 10.1080/07315724.1997.10718705 [DOI] [PubMed] [Google Scholar]
- 38.Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr. 1998;17(6):548–555. 10.1080/07315724.1998.10718802 [DOI] [PubMed] [Google Scholar]
- 39.Cefalu WT, Hu FB. Role of Chromium in Human Health and Diabetes. Diabetes Care. 2004;27(11):2741–2751. 10.2337/diacare.27.11.2741 [DOI] [PubMed] [Google Scholar]
- 40.Yin RV, Phung OJ. Effect of chromium supplementation on glycated hemoglobin and fasting plasma glucose in patients with diabetes mellitus. Nutrition Journal. 2015;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30(8):2154–2163. 10.2337/dc06-0996 [DOI] [PubMed] [Google Scholar]
- 42.Frauchiger MT, Wenk C, Colombani PC. Effects of acute chromium supplementation on postprandial metabolism in healthy young men. J Am Coll Nutr. 2004;23(4):351–357. 10.1080/07315724.2004.10719378 [DOI] [PubMed] [Google Scholar]
- 43.San Mauro-Martin I, Ruiz-Leon AM, Camina-Martin MA, Garicano-Vilar E, Collado-Yurrita L, Mateo-Silleras B, et al. [Chromium supplementation in patients with type 2 diabetes and high risk of type 2 diabetes: a meta-analysis of randomized controlled trials]. Nutr Hosp. 2016;33(1):27 10.20960/nh.v33i1.27 [DOI] [PubMed] [Google Scholar]
- 44.Power O, Hallihan A, Jakeman P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino acids. 2009;37(2):333–339. 10.1007/s00726-008-0156-0 [DOI] [PubMed] [Google Scholar]
- 45.Wildova E, Kraml P, Potockova J, Dlouhy P, Andel M. The assessment of the serum C-peptide and plasma glucose levels by orally administered whey proteins in type 2 diabetes mellitus. Physiol Res. 2017;66(6):993–999. 10.33549/physiolres.933477 [DOI] [PubMed] [Google Scholar]
- 46.Gannon MC, Nuttall FQ, Krezowski PA, Billington CJ, Parker S. The serum insulin and plasma glucose responses to milk and fruit products in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1986;29(11):784–791. 10.1007/BF00873217 [DOI] [PubMed] [Google Scholar]
- 47.Östman EM, Liljeberg Elmståhl HGM, Björck IME. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. American Journal of Clinical Nutrition. 2001;74:96–100. 10.1093/ajcn/74.1.96 [DOI] [PubMed] [Google Scholar]
- 48.Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr. 2010;91(4):966–975. 10.3945/ajcn.2009.28406 [DOI] [PubMed] [Google Scholar]
- 49.Hutchison AT, Feinle-Bisset C, Fitzgerald PC, Standfield S, Horowitz M, Clifton PM, et al. Comparative effects of intraduodenal whey protein hydrolysate on antropyloroduodenal motility, gut hormones, glycemia, appetite, and energy intake in lean and obese men. Am J Clin Nutr. 2015;102(6):1323–1331. 10.3945/ajcn.115.114538 [DOI] [PubMed] [Google Scholar]
- 50.Hutchison AT, Piscitelli D, Horowitz M, Jones KL, Clifton PM, Standfield S, et al. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am J Clin Nutr. 2015;102(6):1574–1584. 10.3945/ajcn.115.117556 [DOI] [PubMed] [Google Scholar]
- 51.Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V. A whey protein supplement decreases post-prandial glycemia. Nutr J. 2009;8:47 10.1186/1475-2891-8-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ullrich SS, Fitzgerald PC, Schober G, Steinert RE, Horowitz M, Feinle-Bisset C. Intragastric administration of leucine or isoleucine lowers the blood glucose response to a mixed-nutrient drink by different mechanisms in healthy, lean volunteers. Am J Clin Nutr. 2016;104(5):1274–1284. 10.3945/ajcn.116.140640 [DOI] [PubMed] [Google Scholar]
- 53.Kalogeropoulou D, LaFave L, Schweim K, Gannon MC, Nuttall FQ. Lysine ingestion markedly attenuates the glucose response to ingested glucose without a change in insulin response. The American Journal of Clinical Nutrition. 2009;90(2):314–320. 10.3945/ajcn.2008.27381 [DOI] [PubMed] [Google Scholar]
- 54.Chartrand D, Da Silva MS, Julien P, Rudkowska I. Influence of Amino Acids in Dairy Products on Glucose Homeostasis: The Clinical Evidence. Can J Diabetes. 2017;41(3):329–337. 10.1016/j.jcjd.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 55.Gannon MC, Nuttall JA, Nuttall FQ. Oral arginine does not stimulate an increase in insulin concentration but delays glucose disposal. The American Journal of Clinical Nutrition. 2002;76(5):1016–1022. 10.1093/ajcn/76.5.1016 [DOI] [PubMed] [Google Scholar]
- 56.Ullrich SS, Fitzgerald PC, Nkamba I, Steinert RE, Horowitz M, Feinle-Bisset C. Intragastric Lysine Lowers the Circulating Glucose and Insulin Responses to a Mixed-Nutrient Drink without Slowing Gastric Emptying in Healthy Adults. J Nutr. 2017;147(7):1275–1281. 10.3945/jn.117.252213 [DOI] [PubMed] [Google Scholar]
- 57.Fasching P, Ratheiser K, Nowotny P, Uurzemann S, Parzer S, Waldhausl W. Insulin production following intravenous glucose, arginine, and valine: different pattern in patients with impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Metabolism. 1994;43(3):385–389. 10.1016/0026-0495(94)90109-0 [DOI] [PubMed] [Google Scholar]
- 58.Kalogeropoulou D, Lafave L, Schweim K, Gannon MC, Nuttall FQ. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism. 2008;57(12):1747–1752. 10.1016/j.metabol.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 59.Bergstrom RW, Wahl PW, Leonetti DL, Fujimoto WY. Association of fasting glucose levels with a delayed secretion of insulin after oral glucose in subjects with glucose intolerance. The Journal of Clinical Endocrinology & Metabolism. 1990;71(6):1447–1453. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto K, Miyake S, Yano M, Ueki Y, Yamaguchi Y, Akazawa S, et al. Glucose tolerance, insulin secretion, and insulin sensitivity in nonobese and obese Japanese subjects. Diabetes Care. 1997;20(10):1562–1568. 10.2337/diacare.20.10.1562 [DOI] [PubMed] [Google Scholar]
- 61.Hayashi T, Boyko EJ, Sato KK, McNeely MJ, Leonetti DL, Kahn SE, et al. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care. 2013;36(5):1229–1235. 10.2337/dc12-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baruffol C, Jordi J, Camargo S, Radovic T, Herzog B, Fried M, et al. L-lysine dose dependently delays gastric emptying and increases intestinal fluid volume in humans and rats. Neurogastroenterol Motil. 2014;26(7):999–1009. 10.1111/nmo.12354 [DOI] [PubMed] [Google Scholar]
- 63.Stanstrup J, Schou SS, Holmer-Jensen J, Hermansen K, Dragsted LO. Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. Journal of proteome research. 2014;13(5):2396–2408. 10.1021/pr401214w [DOI] [PubMed] [Google Scholar]
- 64.Brun AC, Stordal K, Johannesdottir GB, Bentsen BS, Medhus AW. The effect of protein composition in liquid meals on gastric emptying rate in children with cerebral palsy. Clin Nutr. 2012;31(1):108–112. 10.1016/j.clnu.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 65.Diamond MP, Simonson DC, DeFronzo RA. Menstrual cyclicity has a profound effect on glucose homeostasis*†*Presented in part at the Forty-Fourth Annual Meeting of The American Fertility Society, October 10 to 13, 1988, Atlanta, Georgia.†Supported by grant no. 186280 from the Juvenile Diabetes Foundation to M.P.D. and by grant no. RR00125 from the General Clinical Research Center. Fertility and Sterility. 1989;52(2):204–208. [PubMed] [Google Scholar]
- 66.Rice M, Coursin D. Glucose meters: Here today, gone tomorrow? Critical care medicine. 2016;44(2):e97–100. 10.1097/CCM.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 67.Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6(5):1060–1075. 10.1177/193229681200600510 [DOI] [PMC free article] [PubMed] [Google Scholar]