Abstract
The coronavirus disease 2019 (COVID-19) pandemic has created major challenges for all countries around the globe. Retrospective studies have identified hypertension, cardiovascular disease, diabetes and older age as risk factors for high morbidity and mortality from COVID-19. There is a general concern that patients with immune-mediated kidney diseases, namely those on immunosuppressive therapies and/or those with more advanced kidney failure, could particularly be at risk for adverse outcomes due to a compromised antiviral immunity. Uncertainties exist on how management routines should be reorganized to minimize the risk of severe acute respiratory syndrome coronavirus 2 infection and what measures are necessary for infected patients. The aim of the present review of the Immunonephrology Working Group of the European Renal Association–European Dialysis and Transplant Association is to provide recommendations for the management of patients with immune-mediated kidney diseases based on the available evidence, similar circumstances with other infectious organisms and expert opinions from across Europe. Such recommendations may help to minimize the risk of encountering COVID-19 or developing complications during COVID-19 in patients with immune-mediated kidney disease.
Keywords: COVID-19, glomerulonephritis, lupus, renal vasculitis, steroids

INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new member of the coronavirus family, first described in the city of Wuhan, China in December 2019. Within months the virus spread around the globe and a pandemic was declared by the World Health Organization in early March 2020. SARS-CoV-2 causes a febrile infection of the respiratory tract, with viral pneumonia and respiratory failure being the most common organ complications leading to hospitalization and frequently requiring ventilation [1, 2]. Cytokine storm, disseminated coagulation and multiorgan failure represent a turning point in the course towards a lethal outcome. Data from China and Italy, the first European country encountering large numbers for COVID-19 morbidity and mortality, indicate that mortality progressively increases from the fifth decade of life and is associated with the presence of comorbid conditions, namely hypertension, cardiovascular disease, diabetes and chronic kidney disease (CKD) [3, 4].
The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) and many of its affiliated national societies of nephrology provide up-to-date information on the risk for COVID-19 on their websites (Box 1) or via selected publications [5]. However, few of these specifically address the concerns and needs of patients with immune-mediated kidney diseases. There is a general concern that patients with immune-mediated kidney diseases could face a lower accessibility to necessary care and drugs during the pandemic, could be particularly susceptible to infection with SARS-CoV-2 or, once infected, would have a higher risk for complications, including a possible fatal outcome (Table 1). Board members of the Immunonephrology Working Group of the ERA-EDTA collected all information available by mid-April 2020. The recommendations are not definite and will be updated as new information and scientific evidence becomes available on the ERA-EDTA website (https://www.era-edta.org/en/covid-19-news-and-information/).
Box 2.
Some of the key recommendations for the management of patients with immune-mediated kidney disease
|
Table 1.
Some of the concerns regarding COVID-19 in patients with immune-mediated kidney disease
|
Susceptibility to infection
|
Worse prognosis once infected
|
DEFINITIONS
For clarity, we define the terms used in this article as follows:
Patients with immune-mediated kidney diseases include:
All acute or chronic forms of glomerulonephritis, including immunoglobulin A (IgA) nephropathy, membranous nephropathy, C3 glomerulonephritis and others
Systemic autoimmune disorders affecting the kidney, including systemic lupus erythematosus, anti-neutrophil cytoplasmic antibody vasculitis, progressive scleroderma and others
All non-infectious forms of interstitial nephritis, excluding obstructive nephropathy
All thrombotic microangiopathies, even if of a known genetic cause
All non-infectious forms of podocytopathies with present or a history of massive proteinuria or nephrotic syndrome, even if of a known genetic cause.
Not included in this definition are kidney transplant recipients and dialysis patients who may require a set of different considerations, published elsewhere [6, 7].
Immunosuppressive treatments include the following:
Glucocorticosteroids, budesonide or other steroid agonists
Any calcineurin inhibitor
Inhibitors of the mammalian target of rapamycin
Antimetabolites such as azathioprine, mycophenolic acid and mycophenolate mofetil
Any alkylating agent
Immune modulators such as hydroxychloroquine
Any biological drug depleting immune cell subsets or interfering with co-stimulatory molecules, complement factors, interleukins or cytokines.
RECOMMENDATIONS
Risk categories
- Patients with immune-mediated kidney disease should be regarded as at risk to experience a more severe disease course of COVID-19, because
- Many of them are elderly and many have comorbidities known to enhance risk of adverse outcomes of COVID-19 [8].
- The medication used to control autoimmune kidney disease also suppresses host defence mechanisms needed to combat viral infections.
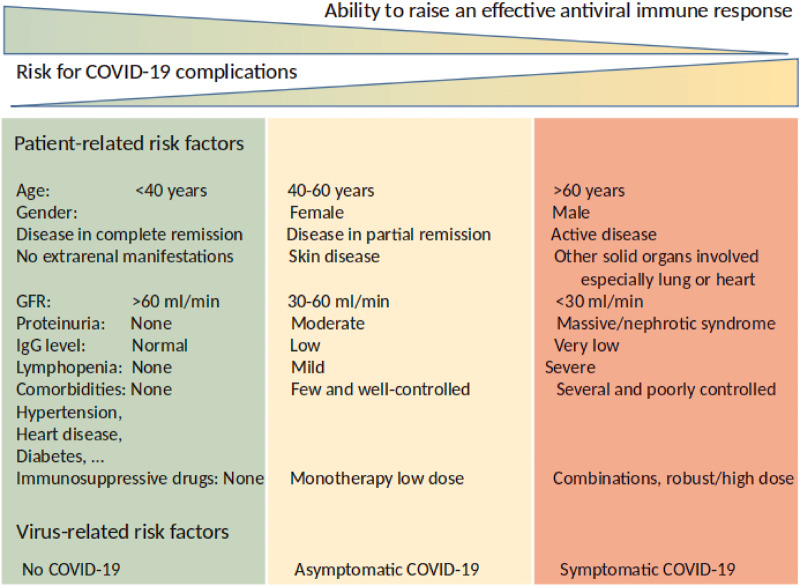
Individual risk assessment can assist decision-making for more or less rigid preventive measures or disease-related interventions (Figure 1).
Physical distancing is an essential preventive measure for the general population, but especially for high-risk populations, and needs to be guaranteed at work and on the way to work. If this is not feasible, working from home or being home on sick leave are important preventive measures to reduce individual risks and, on a population level, the burden of hospitalizations.
Patients with comorbidities, such as kidney disease, that require immunosuppression will most probably consume more healthcare resources when infected.
FIGURE 1.
Risk stratification of patients with immune-mediated kidney disease during the COVID-19 pandemic. Not all patients with immune-mediated kidney diseases are at the same risk for unfortunate outcomes of COVID-19. Patient- and virus-related risk factors allow stratifying patients for different levels of preventive measures before or during COVID-19 infection.
Areas with no known community transmission of SARS-CoV-2 (containment phase)
Instruct the patient on basic hygiene measures and secure transportation options; discourage travel or contact with travellers.
Update the vaccination status of each patient.
Provide patients with a sufficient stock of maintenance drugs.
Reschedule visits for patients with mild disease to make room for consultations with patients on immunosuppressive drugs [13].
Prioritize among patients with an indication for kidney biopsy, and postpone biopsies that can wait.
Do individual risk–benefit assessments and consider for instance postponing cytotoxic drugs and rituximab treatment given as maintenance therapy for immune-mediated kidney diseases, keeping in mind that relapses of disease can be detrimental.
Explore telemedicine options for maintaining patients' accessibility to regular care.
Secure sufficient amounts of personal protection equipment early.
Consider COVID-19 infection when a patient with immune-mediated kidney disease presents with a 'flare’. As fever or other classical symptoms may be mitigated, have virus testing performed immediately or, if not readily available, a thorax computed tomography scan can support diagnostic assessment [14, 15].
Areas with community transmission of SARS-CoV-2
Follow the evolving scientific information and be aware that clinical advice may change rapidly as experience grows.
Re-instruct patients on basic hygiene measures and safe transportation, encourage strict physical distancing and the consideration that everyone else may be contagious (discourage travel, attending events or contact with people with many contacts).
Replace office visits with telephone consultations. Avoid video communication if data safety is a concern. Recommend your patients have drugs and food delivered to their homes by relatives or courier services.
Reschedule clinic visits for patients with milder kidney disease to prioritize phone visits for patients on immunosuppressive drugs to be able to inform them about the virus and also ensure that they adhere to their treatment whether changed or not. Some healthcare systems have provided guidance on how to stratify patients with particular autoimmune diseases for care during the COVID-19 pandemic (https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/clinical-guide-rheumatology-patients-v1-19-march-2020.pdf).
Patients in high-risk situations (medical staff, employees with many contacts) may require a letter from their healthcare provider explaining why they require reassignment of duties or to facilitate working from home.
Kidney biopsies should only be performed in urgent cases.
Newly diagnosed patients require an individual risk–benefit assessment of whether to start an immunosuppressive treatment regimen based on disease progress, biopsy findings, kidney function, level of proteinuria and comorbidities during the pandemic. Viral screening before initiating immunosuppressive therapy at least excludes silent COVID-19 [16]. Cases with a slow progression rate, normal or near-normal kidney function and asymptomatic proteinuria may tolerate mitigation with renin–angiotensin system (RAS) inhibitors, blood pressure control and salt restriction until the risk of SARS-CoV-2 infection has declined.
Individual risk–benefit assessments should consider the factors listed in Figure 1.
Individual risk–benefit assessments should further consider that the scientific evidence for the efficacy of immunosuppressive therapy is poor for the control of chronic IgA nephropathy and focal segmental glomerulosclerosis without nephrosis, while the scientific evidence is strong for the control of systemic vasculitis, lupus nephritis and steroid-sensitive nephrotic syndrome.
Consider that a relapse of severe nephrotic syndrome implies further impairment of host defences via hypogammaglobulinaemia, thrombophilia and volume overload, all likely to increase the risk for COVID-19 complications.
Consider tapering the dose of immunosuppressive drugs in patients in sustained remission for some time, especially when limited data are available suggesting long-term immunosuppression.
Switch intravenous belimumab to subcutaneous dosing to reduce the frequency of office visits [13].
Consider increasing intervals or postponing scheduled infusions with biological drugs for maintenance therapy; for example, rituximab for systemic vasculitis in systemic lupus erythematosus [17].
Do not stop hydroxychloroquine maintenance therapy for lupus nephritis, as it prevents flares and reduces mortality in patients with lupus nephritis. In contrast, there is currently no sufficient scientific evidence to advocate the use of this drug for the prevention or treatment of COVID-19. Rather, lack of regular accessibility to refills of hydroxychloroquine puts patients with lupus nephritis at risk and therefore we recommend that in case of an imminent hydroxychloroquine shortage, consider switching to chloroquine or reducing the daily dose so that prescribed pills last longer [13].
Based on the current scientific evidence, do not stop treatment with RAS inhibitors [18].
Be aware of the psychosocial implications of the pandemic on patients with immune-mediated kidney diseases. Fearing acute illness and potential consequences for long-term health or loss of loved ones, home violence, financial problems, job loss and social distancing all add to the usual stress and burden of patients with chronic diseases [19]. This may affect mood, social ties and drug adherence. Actively inquire about compensation strategies and provide support, if possible.
Patients exposed to SARS-CoV-2 but clinically no COVID-19
Consider the patient as potentially contagious and insist on isolation at home. Patients on immunosuppressive drugs may shed the virus longer, even if asymptomatic.
Insist on swab testing, for example, in a drive-through diagnostic set-up or within the healthcare system.
Reduce steroids to the equivalent of a prednisolone dose of ~0.2 mg/kg/day, if clinically justified.
In case of leucopoenia/lymphopaenia, reduce the dose of cytotoxic drugs until the lymphocyte count recovers. Consider lymphopaenia as a possible sign of active COVID-19.
If patients have hypogammaglobulinaemia, intravenous IgG can be considered since this might also protect against secondary infections.
If apheresis is indicated, use fresh frozen plasma, not albumin, for replacement.
Based on current evidence, do not stop treatment with RAS inhibitors [18].
Mild COVID-19 in patients with immune-mediated kidney disease
Communicate the importance of physical distancing and personal hygiene.
Consider stopping or reducing treatment with antimetabolites. Do not stop corticosteroids abruptly without hydrocortisone replacement. Reduce prednisolone to ~0.2 mg/kg/day [17]. Low doses of calcineurin inhibitors might reduce replication of coronaviruses, as has been shown in in vitro data mainly for cyclosporin [20, 21]. Discuss the case with an infection medicine specialist, if available.
Postpone planned bolus doses of methylprednisolone, cyclophosphamide or biological drugs [17], if clinically justified. Plan for regular follow-up after recovery from infection for essential intravenous immunosuppressive therapy.
There is little evidence that complement inhibitors impair antiviral immunity, but direct experience in COVID-19 is lacking.
Consider hospitalization based on symptoms and the individual risk. Most patients can remain at home as long as symptoms are mild to moderate. Consider following up the development by phone every 24–48 h. Commercially available finger-clip pulse oximetry devices can help to detect subclinical hypoxia in home settings. Inform the patient to be observant of progressive symptoms with difficulty breathing or a high temperature that does not respond to antipyretic treatment.
In hospital settings, assess plasma levels of immunosuppressive drugs and other markers of the immune system (leucocyte count, immunoglobulins, CD19+ B cell and T-cell counts).
If patients have hypogammaglobulinaemia, intravenous immunoglobulin can be considered since this might also protect against secondary infections.
Based on current evidence, do not stop treatment with RAS inhibitors [18].
In patients with nephrotic syndrome, especially when severe, should consider the possibility of low-weight molecular heparin at prophylactic doses.
Severe COVID-19 in patients with immune-mediated kidney disease
Hospitalization to a special COVID-19 unit is necessary.
Recommendations regarding a reduction or avoidance of immunosuppressive therapy mentioned earlier apply here as well. Be aware of possible interactions between calcineurin inhibitors and treatment for COVID-19 (hydroxychloroquine and antiviral drugs). If patients have to be treated with calcineurin inhibitors, closely monitor calcineurin inhibitor plasma levels and perform an electrocardiogram every 48 h as long as COVID-19 treatment is maintained.
Treat COVID-19 according to local standard operating procedures and according to the literature [22]. In the case of cytokine storm and severe pulmonary inflammation immunosuppressive drugs, some selective biological drugs may even be beneficial [17, 23].
Consider dose adjustments for kidney excretory function and consult online tools informing about possible drug interactions in settings of polypharmacy.
Substitute stress-dose hydrocortisone in patients previously on glucocorticosteroids.
Consider antimicrobial prophylaxis.
Consider drug- or kidney function–related related defects in humoral and cellular immunity and consider immunoglobulin substitution in case of hypogammaglobulinaemia.
Consider that organ manifestations such as pulmonary infiltrates or kidney failure may relate either to the acute infection, which may also target the kidney [24], or may be due to the underlying autoimmune disorder.
CONCLUSIONS
The COVID-19 pandemic is a global challenge. Many situations require decisions in the absence of robust scientific evidence. Routine procedures and management algorithms are disrupted as priorities change and regulations are revised, sometimes on a daily base. In this setting, patients and doctors face unprecedented uncertainties that may cause harm to patients. The listed recommendations represent a first attempt to address these uncertainties based on available experience in similar scenarios and on the information available as of early April 2020 (Box 2). The consensus was achieved among the board members from all across Europe. We admit that many of these recommendations do not meet the highest standards of evidence-based medicine. As new information becomes available, updates will be published in open access on the dedicated website of the ERA-EDTA (https://www.era-edta.org/en/covid-19-news-and-information/). We hope this information will help to minimize the risks of patients with immune-mediated kidney disease to encounter SARS-CoV-2 or, once infected, to minimize the risks for unfortunate outcomes.
Box 1.
COVID-19-related information provided online open access by ERA-EDTA and many of its affiliated national societies of nephrology
ACKNOWLEDGEMENTS
The Immunonephrology Working Group receives financial and logistic support from ERA-EDTA. H.-J.A. was supported by the Deutsche Forschungsgemeinschaft (AN372/24-1).
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; doi:10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; pii: S0085-2538(20)30255-6. doi:10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naicker S, Yang CW, Hwang SJ. et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; pii: S0085-2538(20)30251-9. doi: 10.1016/j.kint.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basile C, Combe C, Pizzarelli F. et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 2020; pii: gfaa069. doi: 10.1093/ndt/gfaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alberici F, Delbarba E, Manenti C. et al. Management of patients on dialysis and with kidney transplant during COVID-19 coronavirus infection. Kidney Int Rep 2020; doi: 10.1016/j.ekir.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rombola G, Heidempergher M, Pedrini L. et al. Practical indications for the prevention and management of SARS-CoV-2 in ambulatory dialysis patients: lessons from the first phase of the epidemics in Lombardy. J Nephrol 2020; 33: 193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madjid M, Safavi-Naeini P, Solomon SD. et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020; doi:10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 9. Anders HJ, Andersen K, Stecher B.. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 2013; 83: 1010–1016 [DOI] [PubMed] [Google Scholar]
- 10. Syed-Ahmed M, Narayanan M.. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis 2019; 26: 8–15 [DOI] [PubMed] [Google Scholar]
- 11. Jardel S, Puechal XL, Quellec A. et al. Mortality in systemic necrotizing vasculitides: a retrospective analysis of the French Vasculitis Study Group registry. Autoimmun Rev 2018; 17: 653–659 [DOI] [PubMed] [Google Scholar]
- 12. Teh CL, Wan SA, Ling GR.. Severe infections in systemic lupus erythematosus: disease pattern and predictors of infection-related mortality. Clin Rheumatol 2018; 37: 2081–2086 [DOI] [PubMed] [Google Scholar]
- 13. Sawalha AH, Manzi S.. Coronavirus disease-2019: implication for the care and management of patients with systemic lupus erythematosus. Eur J Rheum 2020; 7: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ai T, Yang Z, Hou H. et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long C, Xu H, Shen Q. et al. Diagnosis of the coronavirus disease (COVID-19): RRT-PCR or CT? Eur J Radiol 2020; 126: 108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zingone F, Savarino EV.. Viral screening before initiation of biologics in patients with inflammatory bowel disease during the COVID-19 outbreak. Lancet Gastroenterol Hepatol 2020; pii: S2468-1253(20)30085-6. doi:10.1016/S2468-1253(20)30085-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ceribelli A, Motta F, De Santis M. et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun 2020; 109: 102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaduganathan M, Vardeny O, Michel T. et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brooks SK, Webster RK, Smith LE. et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020; 395: 912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carbajo-Lozoya J, Ma-Lauer Y, Malesevic M. et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including alisporivir. Virus Res 2014; 184: 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfefferle S, Schopf J, Kogl M. et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog 2011; 7: e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phua J, Weng L, Ling L. et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020; pii: S2213-2600(20)30161-2. doi: 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarzi-Puttini P, Giorgi V, Sirotti S. et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 2020; 38: 337–342 [PubMed] [Google Scholar]
- 24. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020;doi:10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]