Abstract
Secondary bacterial infections occurred in 13.9% (5 of 36) of critical ill patients with coronavirus disease 2019. All 5 patients had been admitted to intensive care unit and received mechanical ventilation before developing bacterial infection. Active surveillance of culture should be performed for critically ill patients. Prevention of nosocomial infection should to be taken seriously.
Keywords: antibiotic prophylaxis, bacterial infection, COVID-19, critical ill, Gram-negative bacilli
In December 2019, a new strain of coronavirus caused a pneumonia outbreak in the Wuhan, Hubei province of China. Due to its ability to effectively transmit between humans, the novel virus, now called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread throughout China as well as other parts of the world. Secondary bacterial infection is an important complication of virus infection and is associated with serious outcomes, especially in influenza [1]. Until now, although several studies have investigated the epidemiological and clinical characteristics of coronavirus disease 2019 (COVID-19), information regarding secondary bacterial infections were limited [2, 3]. In this study, we report 5 cases of SARS-CoV-2 pneumonia complicated by secondary bacterial coinfection.
METHODS
Zhijiang Medical Center is a newly built campus of The First Affiliated Hospital, College of Medicine, Zhejiang University in Hangzhou, Zhejiang Province, China. It was completed in October 2019, its outpatient clinic opened in November 2019, and 3 internal medicine wards opened in January 2020. After the outbreak of COVID-19, the campus was exclusively used to treat patients with SARS-CoV-2, including 2 newly opened medical intensive care units (MICUs) with 29 beds each.
The microbiological and clinical data were retrospectively collected from electronic medical records. The definition of confirmed human infection with SARS-CoV-2 is in accordance with the World Health Organization interim guidance [4]. The diagnosis of COVID-19 was confirmed by positive result of real-time reverse-transcription polymerase chain reaction for respiratory specimens (rhinopharyngeal swab, sputum, or bronchoalveolar lavage fluid).
Secondary bacterial coinfection was defined as positive bacterial cultures accompanied by corresponding clinical manifestations, such as fever/hypothermia, purulent respiratory secretions, new or progressive pulmonary infiltrates in chest radiograph, and elevated peripheral white blood cell/C-reaction protein/procalcitonin.
The data was deidentified, hence informed consent was waived. This study was approved by the Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University.
RESULTS
By February 29, 2020, 101 patients with confirmed COVID-19 were admitted to the Zhijiang Medical Center including 36 patients in the ICU. Thirty-nine patients were subjected to microbial culture according to the doctors’ judgment. In total, 5 patients in the ICU (5.0%, 5 of 101 for all patients; 13.9%, 5 of 36 for patients in the ICU) were diagnosed with SARS-CoV-2 and secondary bacterial infection.
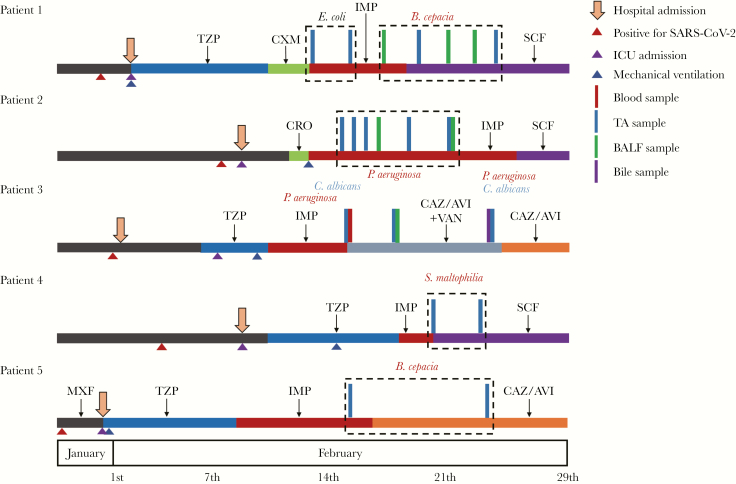
All 5 patients were aged ≥55 years, hospitalized in ICU, and received invasive mechanical ventilation (MV) before bacterial coinfection onset. Four patients were male with a Charlson’s comorbidity index of ≥4. The average time from ICU admission and MV to bacterial coinfection onset was 11 days and 8.6 days, respectively (Supplementary Table S1). All patients were given antibiotics before admission to the ICU (Figure 1).
Figure 1.
Timelines of the 5 patients with coronavirus disease 2019 and secondary bacterial coinfection. Vertical bars and colored texts indicate isolated times, samples, and organisms. A. fumigatus, Aspergillus fumigatus; B. cepacia, Burkholderia cepacia; BALF, bronchoalveolar lavage fluid; C. albicans, Candida albicans; CAZ/AVI, ceftazidime/avibactam; CRO, ceftriaxone; CXM, cefuroxime; E. coli, Escherichia coli; IMP, imipenem; K. pneumoniae, Klebsiella pneumoniae; MXF, moxifloxacin; P. aeruginosa, Pseudomonas aeruginosa; S. maltophilia, Stenotrophomonas maltophilia; SCF, cefoperazone/sulbactam; TA, tracheal aspirate; TZP, piperacillin/tazobactam; VAN, vancomycin.
Patient 1 was the only female patient without any underlying disease. Initial culture of tracheal aspirate (TA) yielded Escherichia coli displaying good susceptibility to antibiotics. Then, the pathogen shifted to Burkholderia cepacia, yielded repeatedly from TA and bronchoalveolar lavage fluid (BALF) samples 5 days after imipenem usage. Patient 2 was an elderly male who had multiple comorbidities (coronary heart disease, fatty liver, and chronic kindey disease). For cultures, both of TA and BALF yielded extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Patient 3 underwent liver transplantation for cirrhosis 3 years ago and used tacrolimus for antirejection. Candida albicans and Pseudomonas aeruginosa were initially isolated from blood and TA, respectively. Ten days later, both bile and TA samples yielded C albicans and P aeruginosa. In addition, Aspergillus fumigatus was cultured from TA. In patients 4 and 5, the pathogens responsible for secondary bacterial infection were Stenotrophomonas maltophilia and B cepacia, respectively, isolated from TA samples.
All 5 patients received appropriated definitive therapy. By April 8, 2020, patient 3 had died of liver failure, although SARS-Cov-2 test conversion to negative. Patients 1 and 4 received lung transplantation and were hospitalized in the ICU for rehabilitation. Patients 2 and 5 were still on MV, implying a poor outcome. The timelines and clinical characteristics of the 5 cases are displayed in Figure 1 and Supplementary Table S1.
DISCUSSION
In the present study, we reported 5 cases of SARS-CoV-2 pneumonia complicated with secondary bacterial infection. The newly opened campus and ICU provided a special environment without colonization of common multidrug-resistant nosocomial pathogens such as Acinetobacter baumannii, K pneumoniae, and Staphylococcus aureus. Thus, the present study provided relative true conditions of secondary bacterial infection in patients with COVID-19, especially for critical ill patients in the ICU.
The percentage of ICU patients was high in this study (35.6%, 36 of 101). The main cause was different tasks of hospitals during the outbreak of COVID-19 in Zhejiang Province. Our institution is located in the regional medical center of Zhejiang Province and has advantages in treating infectious diseases and providing critical care medicine. It was designated as the special hospital for severe and critical ill patients, and thus patients with severe illness were transferred from other parts of Zhejiang Province to our hospital.
Our results showed that 5.0% (5 of 101) of all patients developed to bacterial coinfection, which was slightly higher than that reported by Chen et al [2] (1%). It was believed that bacterial complications tended to present later in virus infection. The study by Chen et al [2] was conducted in the initial stage of the outbreak, so that was likely an underestimation of bacterial coinfection rate. Moreover, the occurrence of secondary bacterial infections is affected by the severity of COVID-19. In this study, all 5 patients were critically ill and in the ICU, and they received MV before getting secondary bacterial infections. For 65 non-ICU patients, only 3 patients were subjected to bacterial culture. The main reason was less likelihood of bacterial infection for these patients according to the doctors’ judgments, because of low procalcitonin level, nonproductive cough, or nonpurulent sputum. Based on our experience, the incidence of secondary bacterial infection in COVID-19 is low, especially for patients with mild or moderate symptoms and those hospitalized in the general care unit, suggesting usage of antibiotic prophylaxis with caution or prompt discontinuation of antibiotics if procalcitonin level is normal.
In this study, the prevalence of secondary bacterial infection was 13.9% (5 of 36) in ICU patients. The source of organisms may be microbiota of the respiratory tract carried by the patients before they developed COVID-19, especially for those with underlying diseases, eg, chronic kidney disease or solid organ transplant recipients. Another definite source is nosocomial environment. It was believed that the new and clean ICU environment in our institution was crucial for the relative low coinfection. Almost all intubated patients received antibiotics before cultures; however, the influence of previous antibiotics usage on secondary bacterial infection remains unclear. Gram-negative nonfermenting bacilli, including B cepacia, S maltophilia, and P aeruginosa, was the most common cause of coinfection. Studies focused on bacterial coinfection in influenza had shown that patients admitted to ICU and requiring MV were at high risk of this complication [1, 5]. However, S aureus and Streptococcus pneumoniae were the most common organisms identified in patients with influenza [5, 6], which usually happened in the early stage of the disease. The phenomenon indicated different pathogenic characteristics between SARS-CoV-19 and influenza. According to this study, the secondary bacterial infection in COVID-19 was associated with MV, which disrupted airway barriers and facilitated the invasion of opportunistic pathogens. In addition, previous broad-spectrum antibiotics altered mucosal microbiota and selected some naturally resistant bacterial such as carbapenem-resistant B cepacia and S maltophilia. It suggests that broad spectrum antibiotics prophylaxis should be viewed with great caution and culture surveillance is important during the course.
It is notable that nosocomial clone dissemination of multidrug-resistant organisms, eg, A baumannii and K pneumoniae, did not occur in the newly opened ICU in our hospital. On the contrary, during the outbreak of avian influenza A (H7N9) in 2003, although the same infection prevention and control policy was implemented in the main campus of the hospital, A baumannii and K pneumoniae were found to be the most common causes of bacterial infection, accounting for 90.7%, and was strongly associated with fatality [7]. It was proved that the contaminated environment was a significant source of nosocomial infection [8]. Our previous studies also reported long-term nosocomial prevalence of carbapenem-resistant A baumannii and K pneumoniae in the main campus of the hospital [9, 10], implying probable environmental colonization. Hence, strict cleanliness and disinfection of the hospital environment are an indispensable part in management of critical ill patients with COVID-19.
There are limitations of our study. First, the main disadvantage is the small number patients in the cohort, which only comprised 5 COVID-19 patients with bacterial coinfection, and these patients represented a severe population, so the conclusions here should be viewed with caution. Second, only the culture method was used to detect pathogen. Culture-independent techniques, such as next-generation sequencing, are high sensitive to identify potential pathogens.
CONCLUSIONS
Overall, the incidence of secondary bacterial infections in COVID-19 is low. It does not support common antibiotic prophylaxis, especially broad-spectrum drugs. Active disinfection of environmental surfaces is significant in reducing the risk of infection caused by multidrug-resistant nosocomial pathogens.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Table S1. Clinical Characteristics of Patients With COVID-19 and Secondary Bacterial Coinfection.
Acknowledgments
We thank all of the doctors and medical staff fighting coronavirus disease 2019 (COVID-19) in The First Affiliated Hospital, College of Medicine, Zhejiang University.
Financial support. This work was funded by the National Natural Science Foundation (No. 81301459) and the Jiaxing Science and Technology Bureau (No. 2020GZ30001).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. MacIntyre CR, Chughtai AA, Barnes M, et al. . The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis 2018; 18:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. . Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 28 January 2020.
- 5. Rice TW, Rubinson L, Uyeki TM, et al. . Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med 2012; 40:1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee EH, Wu C, Lee EU, et al. . Fatalities associated with the 2009 H1N1 influenza A virus in New York city. Clin Infect Dis 2010; 50:1498–504. [DOI] [PubMed] [Google Scholar]
- 7. Zheng S, Zou Q, Wang X, et al. . Factors associated with fatality due to avian influenza A(H7N9) infection in China. Clin Infect Dis 2019. doi: 10.1093/cid/ciz779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doll M, Stevens M, Bearman G. Environmental cleaning and disinfection of patient areas. Int J Infect Dis 2018; 67:52–7. [DOI] [PubMed] [Google Scholar]
- 9. Xu M, Fu Y, Kong H, et al. . Bloodstream infections caused by Klebsiella pneumoniae: prevalence of blaKPC, virulence factors and their impacts on clinical outcome. BMC Infect Dis 2018; 18:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou H, Yao Y, Zhu B, et al. . Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: a retrospective study from a Chinese hospital. Medicine (Baltimore) 2019; 98:e14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.