Abstract
BACKGROUND AND IMPORTANCE
Extracorporeal membrane oxygenation (ECMO) represents a life-saving therapy in cases of refractory hypoxia and has been utilized in patients suffering from the most severe forms of coronavirus disease 2019 (COVID-19). A strikingly high mortality rate of 94% was described in early reports of patients with COVID-19 transitioned to ECMO. Later case reports and series demonstrating successful recovery from COVID-19 after ECMO have revived interest in this therapeutic modality, including the recent approval of ECMO for COVID-19 patients by the Food and Drug Administration (FDA). Here, we present the first reports of devastating intracranial hemorrhage as a complication of veno-venous (VV) ECMO in two COVID-19 patients.
CLINICAL PRESENTATION
We performed a retrospective analysis of 2 cases of devastating intracranial hemorrhage in patients on VV-ECMO for the treatment of COVID-19. Collected data included clinical history, laboratory results, treatment, and review of all available imaging. Both patients demonstrated activated partial thromboplastin times (aPTT) within an appropriate therapeutic range. No risk factors that clearly predicted likelihood of this complication were identified.
CONCLUSION
Understanding the complications of ECMO in this cohort and developing therapeutic algorithms to aid in optimal patient selection will be critical in the limited resource setting experienced as a result of global pandemic. We propose the use of head computed tomography (CT) to identify devastating neurological complications as early as possible, aiding in the resource allocation of ECMO machines to the most appropriately selected patients.
Keywords: COVID-19, Extracorporeal membrane oxygenation, Intracranial hemorrhage, Intraparenchymal hemorrhage, Patient selection, Resource allocation, SARS-CoV-2
ABBREVIATIONS
- aPTT
activated partial thromboplastin times
- ARDS
acute respiratory distress syndrome
- CPAP
continuous positive airway pressure
- CT
computed tomography
- CTHs
head CTs
- ECMO
extracorporeal membrane oxygenation
- FDA
Food and Drug Administration
- ICH
intracranial hemorrhage
- IPH
intraparenchymal hemorrhage
- MICU
medical intensive care unit
- PCR
polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SLE
systemic lupus erythematosus
- OSA
obstructuve sleep apnea
- VV
veno-venous
The respiratory illness, COVID-19, caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a sudden onset and rapidly evolving pandemic. The natural progression for those developing the severe and life-threatening form of the disease has been described as clinically similar to acute respiratory distress syndrome (ARDS), often requiring intubation, lung-protective ventilation strategies, proning and extracorporal membrane oxygenation (ECMO).1 Over the last 3 decades, ECMO has gained increasing traction in the treatment of patients with severe ARDS who develop refractory hypoxia,2 and has thus been proposed in the treatment of patients with the most severe forms of coronavirus disease 2019 (COVID-19).3,4 The most common complications of ECMO are bleeding and thrombosis. Not only do patients on ECMO require therapeutic anticoagulation to maintain therapy, which predisposes patients to additional bleeding complications,5 but the bypass itself both consumes and damages platelets and dilutes clotting factors.6 Intracranial hemorrhage (ICH), in the setting of ECMO, is a recognized entity, with rates up to 21%.7 The most consistent factors that have been demonstrated to increase the risk of ECMO-related ICH have been prehospitalization anticoagulation therapy and thrombocytopenia.7,8 Here, we describe 2 patients who required ECMO for refractory hypoxia secondary to COVID-19 and developed neurologically devastating intraparenchymal hemorrhage (IPH) despite lacking the classical risk factors.
CLINICAL PRESENTATION
Per institutional guidelines, consent was not required and thus was not obtained or sought.
Case 1
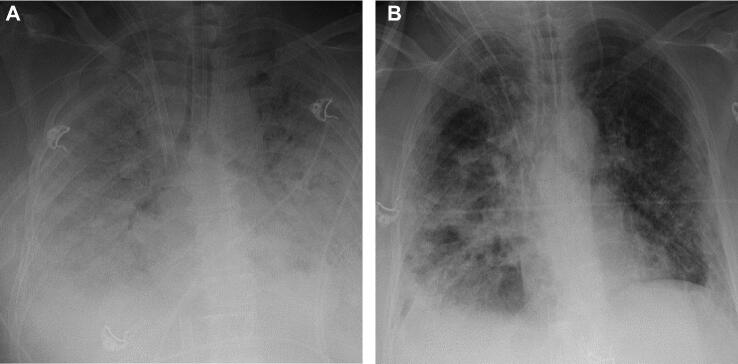
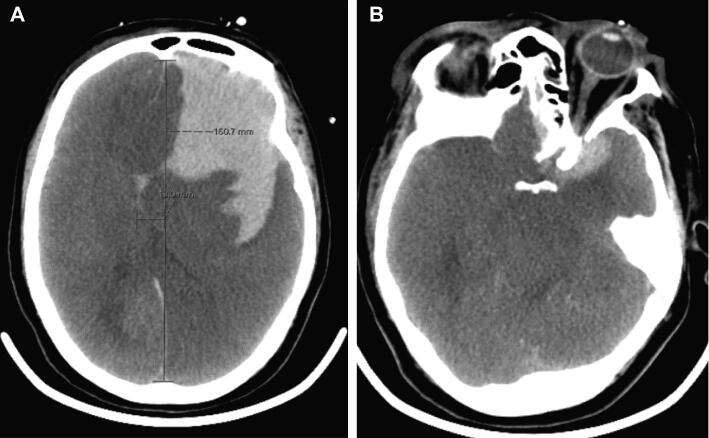
A 58-yr-old female with a past medical history of diabetes and systemic lupus erythematosus (SLE), who presented with 6 d of cough, fever, and shortness of breath, determined to have COVID-19 with positive SARS-CoV-2 polymerase chain reaction (PCR). The patient's respiratory illness had improved with prolonged ECMO, as evidenced by progressively increased areas of aeration on chest X-ray (CXR, Figure 1A and 1B). The patient had been intubated and placed on ECMO prior to transfer (hospital day 1) and was placed prone overnight for 16 h/d beginning on hospital day 16. On hospital day 19, she was noted to have a nonreactive right pupil after being returned supine in the morning. Head CT demonstrated a large volume (8.4 × 4.6 × 4.7 cm) left frontal IPH with a 1.3 cm left to right midline shift, as well as sulcal effacement, and uncal and subfalcine herniation (Figure 2A and 2B). She had been heparinized since the initiation of VV-ECMO with an aPTT of 60 at the time of hemorrhage. Patient was comfortably extubated on hospital day 20 and expired.
FIGURE 1.
Fifty-eight-year-old (F) patient with past medical history of diabetes and SLE, presented with COVID-19 on ECMO. CXR on day 1 A and day 19 B after transfer to the heart and vascular intensive care unit (HVICU).
FIGURE 2.
CTH on HVICU day 19, demonstrating a large-volume dominant hemisphere, frontal IPH, left to right midline shift, and near-complete effacement of the lateral ventricles A as well as obliteration of the basal cisterns B.
Case 2
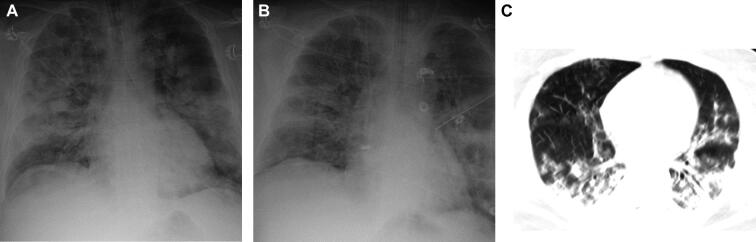
A 46-yr-old male with a past medical history of hypertension and obstructuve sleep apnea (OSA), on nasal continuous positive airway pressure (CPAP) at home. The patient presented with 7 d of cough, fever, and shortness of breath and was ultimately determined to have COVID-19 with positive SARS-CoV-2 PCR and characteristic infiltrates on lung imaging (Figure 3). He was transferred to the medical intensive care unit (MICU) for progressive respiratory distress and was intubated and transitioned to ECMO on day 6 of admission. On hospital day 13, he was noted to have a fixed and dilated left pupil. Head CT demonstrated a large (6.7 × 6.0 × 5.9 cm) left frontal IPH with a 1.4 cm left to right midline shift, as well as uncal and subfalcine herniation (Figure 4). He had been heparinized since the initiation of VV-ECMO with an aPTT of 71 at the time of hemorrhage. Patient was comfortably extubated and expired.
FIGURE 3.
Forty-six-year-old (M) patient with a past medical history of HTN and OSA, presenting with COVID-19 on ECMO. CXR on day 0 A and on day 5 B after transfer to the MICU. C, CT chest on MICU day 7.
FIGURE 4.
A, CTH on MICU day 7 with a large-volume frontoparietal IPH displacing the motor strip. B, CTH slice with the largest volume of IPH, demonstrating a fluid-fluid level. Also apparent are significant left-to-right midline shift C and uncal herniation D.
DISCUSSION
ICH is a well-described complication of ECMO, regardless of initial etiology.8 While it is generally accepted that the risk of bleeding complications on ECMO rises with marked derangements in the clotting cascade,9 no such alterations could be identified in the COVID-19 patients described here (Table). Patients on ECMO secondary to viral respiratory illnesses have previously been shown to have a high rate of devastating ICH in the setting of the 2009 H1N1 influenza.10,11 While the precise mechanisms underlying this phenomenon remain unclear, this and other series have suggested that microvascular thrombosis may underlie ischemia and subsequent hemorrhage in these patients, a finding that is particularly relevant in the case of COVID-19, for which microvascular thrombosis has been observed as a precipitator of respiratory pathology.12 In addition, COVID-19 has been hypothesized to cause a cytokine storm, which may result in platelet dysfunction, despite normal platelet counts, as observed here.
TABLE.
Laboratory Values of COVID-19 Patients on ECMO
| Laboratory value | Reference range | 46-yr-old (M) patient (admit) | 46-yr-old (M) patient (IPH) | 58-yr-old (F) patient (admit) | 58-yr-old (F) patient (IPH) |
|---|---|---|---|---|---|
| White blood cells | 4.0-11.0 | 32.6 | 41.3 | 5.5 | 15.6 |
| Hemoglobin | 13.5-17.5 | 13.2 | 9.0 | 13.5 | 7.4 |
| Hematocrit | 40-52 | 39 | 26 | 43.7 | 22 |
| Platelets | 150-400 | 351 | 197 | 188 | 139 |
| aPTT | 25.1-36.5 | 26.3 | 70.9 | 34.2 | 60.3 |
| PT | 9.4-12.5 | 15.7 | 14.4 | 18.1 | 17.8b |
| INR | 0.8-1.1 | 1.4 | 1.3 | 1.6 | 1.6b |
| d-dimer | 0.00-0.50 | Unavailable | 1.04b | Unavailable | 19.72c |
| Fibrinogen | 170-410 | 649 | 592 | 158a | 250a |
| AST | 15-41 | 51 | 112 | 32 | 29 |
| ALT | 17-63 | 48 | 51 | 10 | 22 |
| AP | 38-126 | 52 | 91 | 36 | 58 |
| Direct bilirubin | 0.1-0.5 | 1.0 | 0.8 | 0.1 | 0.2 |
| Indirect bilirubin | 0.2-0.7 | 0.4 | 0.9 | 0.2 | 0.4 |
| Total bilirubin | 0.3-1.2 | 1.4 | 1.7 | 0.3 | 0.6 |
aPTT = activated partial thromboplastin time, PT = prothrombin time, INR = international normalized ration, AST = aspartate amino transferase, ALT = alanine amino transferase, AP = alkaline phosphatase.
Laboratory values of COVID-19 patients on ECMO on admission (adenotes laboratory values obtained greater than 24 h after admission), or within 24 h prior to hemorrhage, unless otherwise noted by b(obtained more than 24 h prior to the identification of hemorrhage) or c(obtained after the identification of hemorrhage).
Lastly, the condition of a global pandemic has generated an unprecedented requirement for resource allocation and promotion of interventions that provide the highest value care—a function of quality, efficacy, safety, and cost. Lidegran et al13 conducted a retrospective review of head CTs (CTHs) performed on patients while on ECMO.13 Notably, they observed a rate of 37% of CTHs with ICH or cerebral infarction, and 13% of these with findings significant enough to motivate withdrawal of ECMO. Coupled with the 94.1% mortality of patients placed on ECMO for refractory hypoxia in the largest available cohort study of COVID-19 patients,14 a compelling argument for the utilization of CTH screening in patients on ECMO for COVID-19 can be made. Portable CT has the potential to identify patients who have suffered a brain injury that would otherwise be overlooked due to the poor reliability of coagulation derangements as a clinical predictor of hemorrhage in this cohort, as well as the inability to perform neurological assessments in the setting of paralysis and sedation. This could reduce the length of time that ventilators and ECMO machines are being utilized in patients who have unknowingly suffered devastating neurological injury, increasing the availability of these scare resources for patients who retain a high probability of meaningful recovery.
CONCLUSION
IPH is a well-described and important complication of ECMO. Despite occurring in the first 4 d on ECMO circuit,15 these intracranial complications are often devastating, detected in a delayed fashion, and generally a terminal event with no indication for intervention. CT screening could be utilized to identify patients who have suffered a brain injury that would otherwise be undetected due to the poor reliability of classic coagulation markers as accurate clinical predictors of hemorrhage in this cohort, as well as the inability to perform neurological assessments in the setting of paralysis, sedation, and proning.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;S2213-S2600(20):30127-30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel B, Chatterjee S, Davignon S, Herlihy JP. Extracorporeal membrane oxygenation as rescue therapy for severe hypoxemic respiratory failure. J Thorac Dis. 2019;11(Suppl 14):S1688-S1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhan WQ, Li MD, Xu M, Lu YB. Successful treatment of COVID-19 using extracorporeal membrane oxygenation, a case report. Eur Rev Med Pharmacol Sci. 2020;24(6):3385-3389. [DOI] [PubMed] [Google Scholar]
- 4. Li X, Guo Z, Li B et al.. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. 2020;66(5):475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas J, Kostousov V, Teruya J. Bleeding and thrombotic complications in the use of extracorporeal membrane oxygenation. Semin Thromb Hemost. 2018;44(1):20-29. [DOI] [PubMed] [Google Scholar]
- 6. Hampton CR, Verrier ED. Systemic consequences of ventricular assist devices: alterations of coagulation, immune function, inflammation, and the neuroendocrine system. Artif Organs. 2002;26(11):902-908. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher-Sandersjoo A, Thelin EP, Bartek J Jr, Elmi-Terander A, Broman M, Bellander BM. Management of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation (ECMO): an observational cohort study. PLoS One. 2017;12(12):e0190365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavayas YA, Del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion. 2018;33(1_Suppl):42-50. [DOI] [PubMed] [Google Scholar]
- 9. Chaves RCF, Rabello Filho R, Timenetsky KT et al.. Extracorporeal membrane oxygenation: a literature review. Rev Bras Ter Intensiva. 2019;31(3):410-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies A, Jones D, Bailey M et al.. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888-1895. [DOI] [PubMed] [Google Scholar]
- 11. Chow FC, Edlow BL, Frosch MP, Copen WA, Greer DM. Outcome in patients with H1N1 influenza and cerebrovascular injury treated with extracorporeal membrane oxygenation. Neurocrit Care. 2011;15(1):156-160. [DOI] [PubMed] [Google Scholar]
- 12. Luo W, Yu H, Gou J et al.. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints. published online: February 26, 2020; 2020020407. [Google Scholar]
- 13. Lidegran MK, Mosskin M, Ringertz HG, Frenckner BP, Linden VB. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: clinical benefits in diagnosis and treatment. Acad Radiol. 2007;14(1):62-71. [DOI] [PubMed] [Google Scholar]
- 14. Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taylor D, Wyncoll D. Intracranial hemorrhage occurs early in patients with severe respiratory failure requiring venovenous extracorporeal membrane oxygenation. Crit Care Med. 2020;48(4):e339-e340. [DOI] [PubMed] [Google Scholar]