The novel coronavirus 2019 or SARS-CoV-2 has spread worldwide since first being detected in China in December 2019. It has been declared a global health emergency by the World Health Organization [1], and public health measures have been applied, including social distancing, work restrictions and home-working promotion. As many countries have flattened the epidemic curve, they are now examining strategies to reopen their economies, requiring evidence-based strategies to return workers to their jobs in the safest way possible.
Occupational physicians can play key roles in monitoring workers’ health and developing effective return to work guidelines. Along with clinical presentation, laboratory tests provide added value to confirm the diagnosis and the stage of COVID-19.
Rapid tests based on viral antigen or antibody detection are often scarce [2]. The use of reverse transcriptase-polymerase chain reaction (RT-PCR), based on viral-RNA detection, may be limited to high-risk patients, healthcare and first-responder personnel. The Spanish Society of Infectious Diseases and Clinical Microbiology and other societies [3–5] have established that RT-PCR can remain positive for up to 1 month in patients who are no longer contagious [6]. RT-PCR is a useful diagnostic test in COVID-19, but used alone qualitatively (positive or negative), it may be inadequate to determine the end of a COVID-19-affected worker’s isolation. The combined use of SARS-CoV-2 viral-RNA detection and serological antibody determination could improve the management of COVID-19 patients, but timing is important. Doing tests too early may result in test repetition and waste of resources, whereas delaying tests may delay return to work.
The best strategy, preventing any contagious worker from entering/re-entering the workplace based on large-scale screening, is usually not available. Therefore, best practice for safe return to work after COVID-19 requires accurately identifying the final phases of the disease, where the worker is clinically recovered and no longer contagious. As laboratory tests are limited, we propose the combined use of:
Clinical parameters based on clinical evolution and days since exposure [7–9]. The isolated use of clinical criteria without laboratory support for return to work decisions would only be justified in circumstances where laboratory tests are unavailable [7,10,11].
Genomic tests (viral-RNA detection) have been the primary diagnostic and ‘proof of cure’ tests during the pandemic. A negative RT-PCR has been commonly used as a requirement for return to work, but it may remain positive for weeks after clinical recovery [4]. The Cycle threshold (Ct) value of the quantitative RT-PCR has been correlated with infectivity, suggesting that people with Ct values above 33–34 are no longer contagious because virus can no longer be grown in cell cultures from samples exceeding that cut-off [5]. More studies are needed to confirm this result and employ Ct as a criterion in clinical practice.
Serological tests (detection of antibodies) are an alternative approach based on the worker’s immune response to the viral infection. Positive IgM titres generally reflect acute infection, whereas positive IgG titres indicate convalescent or past disease. However, there are insufficient data to estimate the level of IgG titres required to be protective and the duration of immunity [6,12,13].
We conducted a literature review using the search terms ‘coronavirus’ and ‘workers’ and ‘return to work’ in PubMed for original publications written in English from 1 December 2019 to 15 April 2020. More than 180 publications were found but based on review of titles and abstracts, we found no articles specifically addressing return to work guidelines. Therefore, to develop evidence-based return to work guidelines, articles based on coronavirus diagnosis using genomic and serological testing and articles related to infectivity and immunity were reviewed with the same dates and criteria. Local European guidelines, and US_CDC reports were also consulted. A panel of experts was then convened by the Spanish Association of Occupational Medicine (AEEMT) to discuss and elaborate return to work guidelines.
Until a vaccine or herd immunity is established, we propose the following return to work strategies. All workers must remain isolated at home for the duration of any significant symptoms. Depending on the worker’s relative future risk of exposure to SARS-CoV-2 and persons at risk for infection, there are two different scenarios:
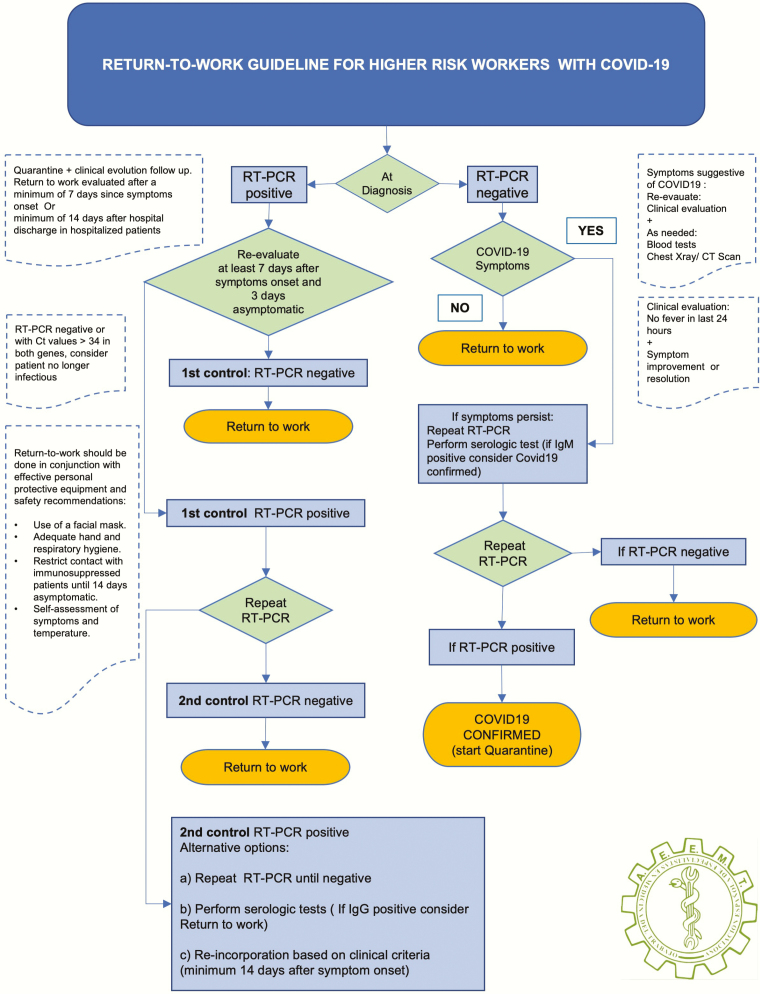
Workers at higher risk of exposure: existence of a double high-risk (high risk for the worker, and high risk from the worker to third parties), despite the proper use of personal protective equipment, contact with patients is possible. This group includes essential workers such as healthcare workers (physicians, nurses, hospital laboratory technicians and other healthcare workers) or public safety workers (police, fire and ambulance). In this group, we propose the algorithms summarized in Figure 1.
Figure 1.
Return to work guideline for higher risk workers with COVID-19.
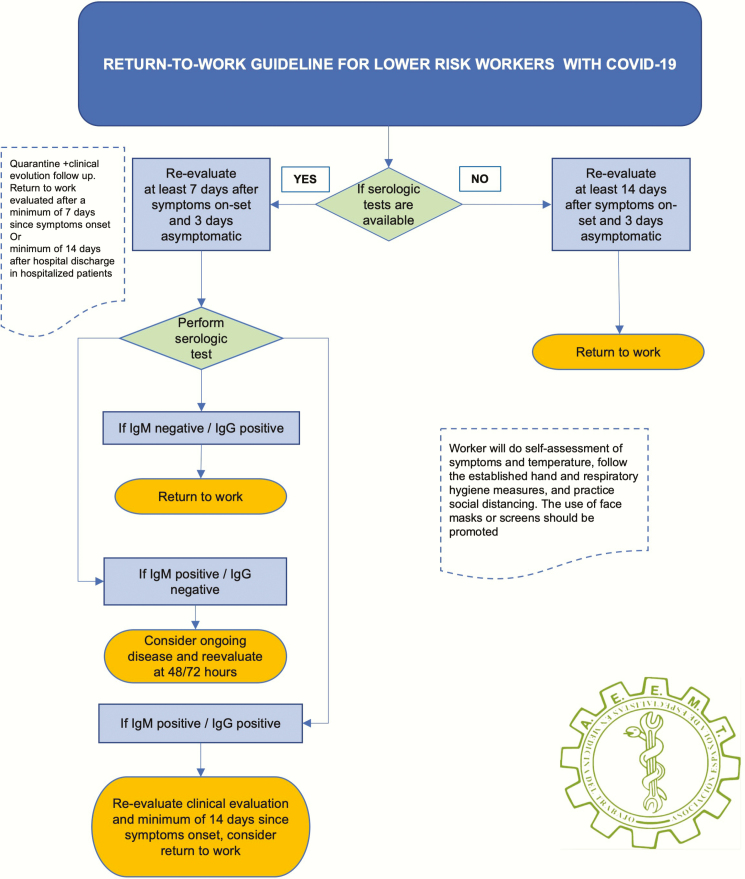
Workers with lower risk of exposure: activities that, with the use of general and collective protective equipment and social distancing, do not present a greater than average population risk of exposure. In this second occupational group, we propose the algorithms summarized in Figure 2.
Figure 2.
Return to work guideline for lower risk workers with COVID-19.
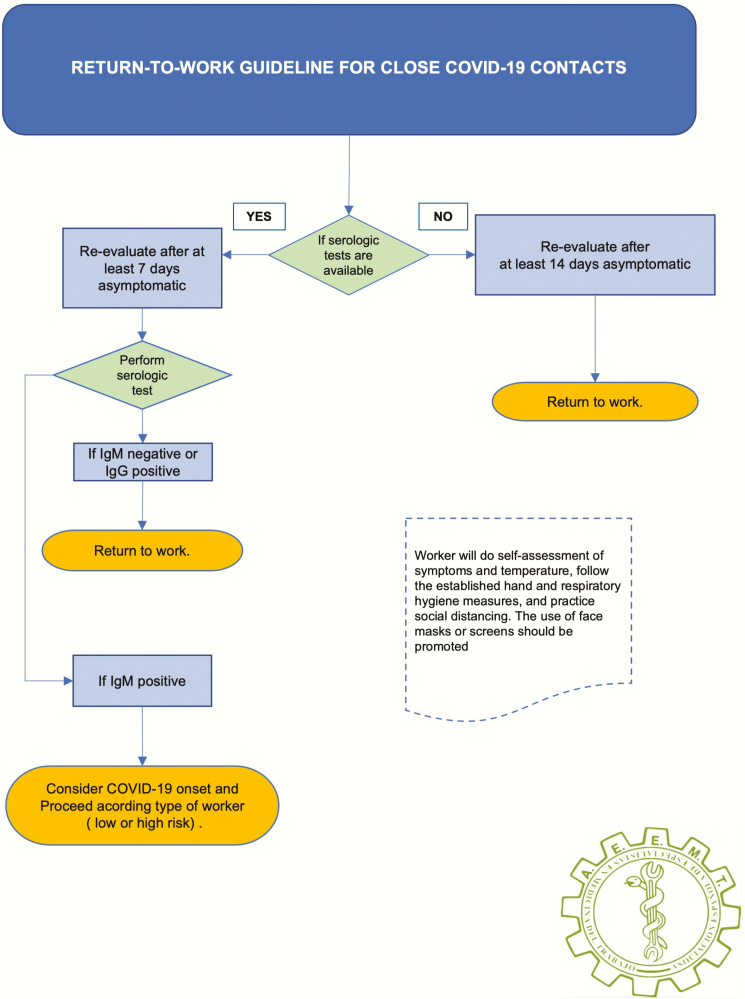
Employees who are household contacts of COVID-19 patients represent another unique group due to the potential incubation latency from initial exposure to secondary infections. For return to work of COVID-19 close contacts, we propose the algorithms summarized in Figure 3.
Figure 3.
Return to work guideline for close COVID-19 contacts.
A separate issue is the reintroduction of employees who have worked remotely during the pandemic to the physical workplace. For this group, we propose a gradual and staggered return to work [14]. Each organization should establish its own pace to progressively bring employees back according to each worker’s need to physically attend work, the strategic interests of the employer and the individual vulnerabilities of each worker [15]. According to COVID-19 susceptibility, home-workers could gradually return to the workplace in the following order: firstly, not particularly susceptible workers (employees <50 without underlying health conditions); secondly, workers from 50–60 years old, without underlying health conditions; next workers >60 without underlying health conditions; and lastly vulnerable workers. Close follow-up of the workforce upon return should be undertaken [16].
In conclusion, return to work guidelines in any pandemic will depend on the state of the local epidemic, the nature and conditions of each job and on the availability of testing. Guidelines need to be reviewed and updated over time as local epidemic status and supplies may change. In the current situation with a high rate of transmission and limited testing resources, it is important to differentiate between high- and low-risk workers. While low-risk workers’ guidelines may rely on clinical criteria, more specific testing-based strategies should be used for high-risk workers.
References
- 1. World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report – 95 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200424-sitrep-95-covid-19.pdf?sfvrsn=e8065831_4 (11 May 2020, date last accessed).
- 2. Li Z, Yi Y, Luo X et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol 2020. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Consideraciones de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica para dar de alta al personal sanitario por COVID19. 2020. https://seimc.org/contenidos/documentoscientificos/recomendaciones/seimc-rc-2020-alta_personal_sanitario_con_covid-19.pdf (11 May 2020, date last accessed).
- 4. Wölfel R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5. La Scola B, Le Bideau M, Andreani J et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis 2020; doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. To KK-W, Tsang OT-Y, Leung W-S et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC. Coronavirus Disease 2019 (COVID-19) Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html (11 May 2020, date last accessed). [Google Scholar]
- 8. Guan W-J, Ni Z-Y, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Prevention and Control. Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19. Stockholm: ECDC, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidance-discharge-and-ending-isolation-first%20update.pdf. (11 May 2020, date last accessed). [Google Scholar]
- 10. CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention. Criteria for return to work for healthcare personnel with suspected or confirmed COVID-19 (Interim Guidance). https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html (11 May 2020, date last accessed). [Google Scholar]
- 11.Prevention and Control (ECDC). Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – seventh update. Stockholm: ECDC, 2020. [Google Scholar]
- 12. NY STATE Protocols for Essential Personnel to Return to Work Following COVID-19 Exposure or Infection https://coronavirus.health.ny.gov/system/files/documents/2020/04/doh_covid19_essentialpersonnelre turntowork_rev2_033120.pdf (11 May 2020, date last accessed).
- 13. Zhao J, Yuan Q, Wang H et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu N, Li C, Ning SS et al. Epidemiological characteristics of COVID-19 in Shaanxi province. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:E034. [DOI] [PubMed] [Google Scholar]
- 15. Lopez J, Mitja O. Propuesta de intervenciones de salud pública para el control de la infección SARSCoV-2 en la comunidad. Salida coordinada del confinamiento. Control epidemiológico COVID19. 2020. https://gacetamedica.com/investigacion/covid-19-como-se-podria-realizar-una-salida-del-confinamiento-gradual/ (11 May 2020, date last accessed).
- 16. Roda WC, Varughese MB, Han D, Li MY. Why is it difficult to accurately predict the COVID-19 epidemic? Infect Dis Model 2020;5:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]