Abstract
As coronavirus disease 2019 cases and deaths continue to expand globally, there is an urgent need to develop, test, and approve effective antiviral therapies. Currently, a majority of clinical trials are evaluating therapies in patients who are already hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) infection. Given that the median time between development of symptoms and need for hospitalization is 1 week, a golden opportunity to intervene early is being missed. Indeed, for many other viral infections, early treatment soon after development of symptoms is associated with decreased mortality, lower hospitalization rates, and lower likelihood of transmission to others. In this study, we advocate for randomized, double-blind, placebo controlled, clinical trials to evaluate promising agents early during SARS CoV-2 infection.
Keywords: antiviral therapy, COVID-19, SARS CoV-2
In this paper, we make the scientific argument for an increase in clinical trials treating SARS Co-V-2 early during infection. Early treatment of SARS-CoV-2 could disproportionately lower the mortality rate, but also lower hospitalization and decrease ongoing transmission of virus.
The global community is enduring the horrific first wave of coronavirus disease 2019 (COVID-19). In many cities, emergency departments are inundated, and the pandemic continues to have a disproportionate effect on the elderly [1–3]. The only policy option is physical distancing to “flatten the curve” and decrease incident cases [4]. What follows the first wave will be a difficult balancing act. Full relaxation of physical distancing will allow a rapid surge of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) infections [5]. Yet, the economy and supply chain may not tolerate sustained or recurrent shutdowns. Until there is widespread implementation of an effective vaccine, continued COVID-19 cases are inevitable.
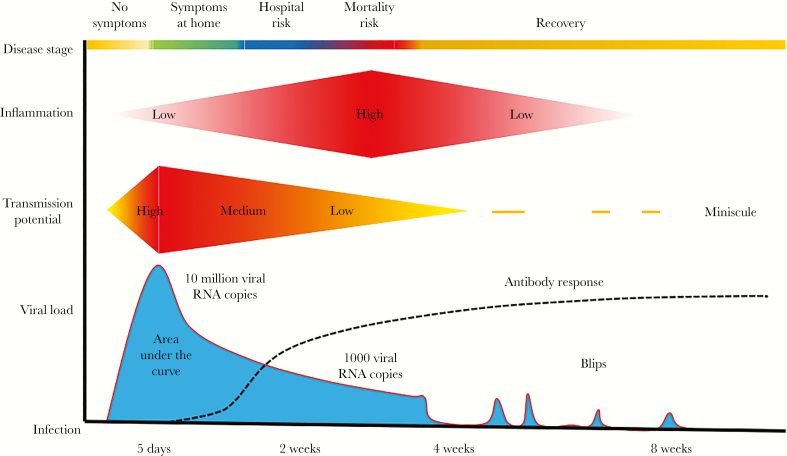
To manage the pandemic, effective strategies are needed to treat infection and lower its fatality rate. To this end, the biology of SARS CoV-2 provides a golden opportunity to diagnose and treat, before infection becomes uncontrollable. The average time between development of symptoms and requirement for hospitalization is 7 days [6], and average time between development of symptoms to death is 14–21 days [7]. Viral kinetic studies demonstrate that early infection consists of a 2- to 3-day surge in viral loads, followed by a slow decline that persists for 2–8 weeks [8–10]. Initial symptoms develop around the period of maximum viral load and represent an optimal time to intervene (Figure 1).
Figure 1.
Schematic of severe acute respiratory syndrome coronavirus 2 infection in a symptomatic person. RNA, ribonucleic acid.
Symptoms often start in the upper respiratory tract and progress to dyspnea [8]. As was demonstrated for influenza in immunocompromised hosts [11], elimination of viral replication during this early period may prevent progression to significant lung disease. If widely implemented in vulnerable populations such as the elderly, an early test-and-treat approach, applied at home, could lower case fatality and hospitalization rates.
Much like managing an epidemic is more challenging when a city has 10 000 cases rather than 10, managing a SARS CoV-2 lung infection is more difficult when the entire tissue is overrun with virus, rather than a small portion. There is a longstanding precedent that early treatment of viruses leads to better outcomes. In a trial of Ebola treatment, shorter symptom duration at presentation correlated with lower viral load, and it increased the likelihood that interventions would be lifesaving [12]. Early influenza treatment causes shorter duration of shedding and symptoms [13]. For human immunodeficiency virus (HIV), early treatment confers mortality benefits and lowers community transmission [14, 15].
The benefits of early test-and-treat methods may not be limited to the elderly. Life-threatening COVID-19 occurs in young, previously healthy people, particularly healthcare staff and transit workers who may be exposed to high viral inoculums [16]. Even among those who recover, symptoms can persist for weeks leading to multiple lost workdays [17]. Early treatment could provide benefit to those not at risk for hospitalization.
Widespread early treatment could also mitigate local epidemics. Although a proportion of SARS CoV-2 transmission is attributed to asymptomatic and presymptomatic viral shedding, many transmissions result from coughing and sneezing leading to airborne droplets. Household viral load is a determinant of transmission for human herpesviruses (HSVs) [18]. Therapeutic reduction in shedding is linked with less transmission for herpes simplex virus-2 [19]. Lowering viral load is a mainstay of HIV prevention [20]. Even though shedding duration and transmissibility is shorter for influenza than SARS CoV-2, early oseltamivir initiation limits the secondary attack rate to household contacts [21].
Despite multiple advantages of treating early, almost all ongoing trials are focused on hospitalized patients. At this later stage of COVID-19, although viral replication may augment disease progression, cytokine storm is an increasingly relevant aspect of pathogenesis, which is reflected by the use of biologics in the critically ill [22], and incomplete efficacy of antiviral agents [23]. A rational therapeutic approach would prevent critical illness altogether.
There are barriers to performing outpatient trials that must be solved. First, to implement a scalable early treatment strategy, widely available testing is needed. Home testing with self-sampling allows rapid diagnosis within 1 day of developing symptoms and avoids the need for personal protective equipment (PPE) utilization by healthcare staff. The US food & drug administration recently granted approval for home testing. Given the public health benefits of isolating cases, studies are making this available to citizens in cities with high burden of infection [24]. Self-quarantine will be more likely if people know they are infected, particularly given the economic incentive to continue working despite mild symptoms.
Second, because only 15% of infected people develop the clinically relevant outcome of hospitalization, proof-of-concepts trials must enroll large numbers of participants to be adequately powered. Although such trials are required for licensure or to establish a new indication for a licensed agent, we propose smaller, rapid studies using viral shedding metrics and symptoms as surrogate endpoints for risk of progression to severe disease. Virologic endpoints are established surrogates of treatment efficacy for HIV, hepatitis B, hepatitis C, HSV-2, influenza, and cytomegalovirus [13, 25–28]. It is likely that a profound reduction in shedding early during SARS Co-V-2 infection will translate into prevention of oxygen need and hospitalization. We performed power calculations and identified that a 30-participant trial with blinded and equally sized placebo and treatment arms would have 80% power to detect a 5-day reduction in shedding or symptomatology. Once test-of-concept studies show antiviral and clinical potential, adaptive trials with clinical outcomes of hospitalization can be initiated. This approach is of special importance in high-risk populations such as senior centers and cancer centers.
Smaller and less expensive studies will allow a more diverse portfolio of agents to be rapidly screened. We propose urgent double-blind, placebo-controlled, clinical trials testing agents with biologic activity against SARS CoV-2 in outpatients. Several small molecular agents demonstrate potency in vitro and in nonrandomized studies in humans, including remdesivir, favipiravir, and selinexor [29]. Drug screening revealed many other US Food and Druge Administration licensed agents that could be assessed [30]. Neutralizing antibody formulations are being developed and may have prophylactic value. Ideally, parallel studies would be conducted with each of these agents to provide clinicians with comparative efficacy, toxicity, and dosing convenience data to optimize early treatment.
Third, effective therapy, once discovered, must be delivered to an infected person’s home. In the age of Amazon.com, this goal is achievable. Indeed, the Seattle flu study recently pioneered home delivery of baloxavir for early influenza treatment in a clinical trial [31]. Alternatively, a specialized clinic staffed by well trained providers with optimal PPE, as well as those seroimmune to infection, could be established for intravenous infusions. The ongoing Antibody Mediated Prevention study for HIV, in which more than 40 000 doses of a neutralizing antibody or placebo have been infused in 4 continents, highlights the plausibility of such an intervention [32].
Finally, trials must specifically target minority populations, particularly those in underserved communities, who have been disproportionately impacted by the COVID-19 pandemic.
CONCLUSIONS
In Italy, overwhelmed physicians made a public plea for other countries to develop plans to keep patients at home [33]. Because the management of severe COVID-19 requires oxygen, ventilators, daily laboratory tests, and radiology, the goal should be to avoid severe infection. To lower hospitalization rates during the inevitable second and third waves of the pandemic, incremental policies to flatten the curve will be necessary. We propose an adjunctive measure: make COVID-19 less fatal. We also suggest to diagnose early and treat early, which may lower the hospitalization rate and lower the death rate.
Acknowledgments
Financial support. J.T.S. receives COVID-19 research funding from a Fred Hutchinson Cancer Research Center internal grant.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020; 323(18):1775–6. [DOI] [PubMed] [Google Scholar]
- 2. Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci 2020; 35:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McMichael TM, Clark S, Pogosjans S, et al. ; Public Health – Seattle & King County, EvergreenHealth, and CDC COVID-19 Investigation Team COVID-19 in a long-term care facility - King County, Washington, February 27-March 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patrick GT, Walker CW, Oliver W, et al. The Global Impact of COVID-19 and Strategies for Mitigation and Suppression. WHO Collaborating Centre for Infectious Disease Modelling, MRC Centre for Global Infectious Disease Analysis: Abdul Latif Jameel Institute for Disease and Emergency Analytics, Imperial College London, 2020. [Google Scholar]
- 5. Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020; 368:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8(5):475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; 323(15):1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581(7809):465–9. [DOI] [PubMed] [Google Scholar]
- 11. Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood 2011; 117:5050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulangu S, Dodd LE, Davey RT Jr, et al. ; PALM Writing Group; PALM Consortium Study Team A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019; 381:2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 14. Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One 2010; 5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382(19):1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mayer BT, Krantz EM, Wald A, et al. Estimating the risk of human herpesvirus 6 and cytomegalovirus transmission to Ugandan infants from viral shedding in saliva by household contacts. Viruses 2020; 12(2):171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corey L, Wald A, Patel R, et al. ; Valacyclovir HSV Transmission Study Group Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11–20. [DOI] [PubMed] [Google Scholar]
- 20. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fry AM, Goswami D, Nahar K, et al. Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo-controlled trial. Lancet Infect Dis 2015; 15:654–62. [DOI] [PubMed] [Google Scholar]
- 22. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395(10236):1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Available at: https://scanpublichealth.org.
- 25. Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology 2013; 144:1450–5.e2. [DOI] [PubMed] [Google Scholar]
- 26. Murray JS, Elashoff MR, Iacono-Connors LC, et al. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS 1999; 13:797–804. [DOI] [PubMed] [Google Scholar]
- 27. Natori Y, Alghamdi A, Tazari M, et al. ; CMV Consensus Forum Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis. Clin Infect Dis 2018; 66:617–31. [DOI] [PubMed] [Google Scholar]
- 28. Agyemang E, Magaret AS, Selke S, et al. Herpes simplex virus shedding rate: surrogate outcome for genital herpes recurrence frequency and lesion rates, and phase 2 clinical trials end point for evaluating efficacy of antivirals. J Infect Dis 2018; 218:1691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez MA. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob Agents Chemother 2020; 64(5):e00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing [published online ahead of print, April 30, 2020]. Nature 2020. doi: 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chu H, Boeckh M, Englund J, et al. The Seattle Flu Study: a community-based study of influenza. OFID 2019; 6:S1002. [Google Scholar]
- 32. Gilbert PB, Juraska M, deCamp AC, et al. Basis and statistical design of the passive HIV-1 antibody mediated prevention (AMP) test-of-concept efficacy trials. Stat Commun Infect Dis 2017; 9(1):20160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nacoti M, Ciocca A, Giupponi A, et al. At the epicenter of the Covid-19 pandemic and humanitarian crises in Italy: changing perspectives on preparation and mitigation. NEJM Catalyst 2020: doi: 10.1056/CAT.20.0080. [DOI] [Google Scholar]