Abstract
Background
Many countries have acquired antiviral stockpiles for pandemic influenza mitigation and a significant part of the stockpile may be focussed towards community-based treatment.
Methods
We developed a spreadsheet-based, decision tree model to assess outcomes averted and cost-effectiveness of antiviral treatment for outpatient use from the perspective of the healthcare payer in the UK. We defined five pandemic scenarios—one based on the 2009 A(H1N1) pandemic and four hypothetical scenarios varying in measures of transmissibility and severity.
Results
Community-based antiviral treatment was estimated to avert 14–23% of hospitalizations in an overall population of 62.28 million. Higher proportions of averted outcomes were seen in patients with high-risk conditions, when compared to non-high-risk patients. We found that antiviral treatment was cost-saving across pandemic scenarios for high-risk population groups, and cost-saving for the overall population in higher severity influenza pandemics. Antiviral effectiveness had the greatest influence on both the number of hospitalizations averted and on cost-effectiveness.
Conclusions
This analysis shows that across pandemic scenarios, antiviral treatment can be cost-saving for population groups at high risk of influenza-related complications.
Keywords: cost-effectiveness, decision tree, neuraminidase inhibitors, pandemic influenza
Introduction
Influenza pandemics are rare, unpredictable events with potentially serious consequences. They are considered to be important public health emergencies by the World Health Organization, and a number of countries, with many having specific pandemic preparedness plans.1–3 Neuraminidase inhibitors (NAI) often feature prominently in pandemic influenza preparedness plans2 and several high-income countries have acquired NAI stockpiles because pandemic specific vaccines may not be widely available for up to 6 months.4 Clinical trials show NAI effectiveness in modestly reducing duration of symptomatic illness in patients with uncomplicated seasonal influenza.5–14 However, these trials were under-powered to assess NAI impact on secondary outcomes such as hospitalizations.15–17 Two meta-analyses of the extant clinical trial data, examining outcomes based on the intention-to-treat-influenza infected (ITTI) approach, found that early NAI treatment (≤48 h of symptom onset) was associated with a risk reduction of 5918 and 63%19 for hospital admission in otherwise healthy patients with influenza. Other meta-analyses of trial data that evaluated all outpatients with influenza-like-illness (ILI) using the intention-to-treat (ITT) approach did not find a reduction in hospitalizations in those treated with NAIs.20,21
If a future pandemic is severe, hospital capacity may be exhausted and therefore reserved for the severely ill who are most likely to benefit.22 Countries may decide to focus a significant part of their pandemic response plan towards community treatment aimed at averting hospitalizations. Policy makers considering NAI stockpiling for a future pandemic of unknown severity will have to consider both number of hospitalizations averted and the cost-effectiveness of such an intervention. NAI treatment for pandemic influenza has generally been estimated to be cost-effective for higher-income countries.23–25 However, a review identified that previous health economic evaluations often neglected pandemic uncertainty by only evaluating singular, fixed pandemic scenarios.26 Moreover, few models have incorporated the increased risks of adverse pandemic influenza-related outcomes for patients with at-risk conditions. We present a spreadsheet-based decision tree model that evaluates the impact of community-based NAI treatment in terms of the averted influenza-related hospitalizations and associated cost-effectiveness in a range of pandemic scenarios.
Methods
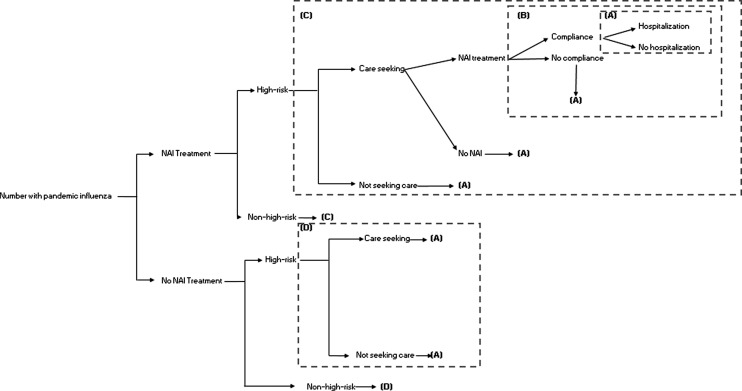
We built a decision tree model (Fig. 1) to calculate the impact of community-based NAI treatment for five pandemic scenarios. The first scenario is based on the UK’s A(H1N1)pdm09 experience, with a clinical attack rate (CAR) of 7% and a case hospitalization risk (CHR) of 0.3 and 1.5% among non-high-risk and high-risk patients, respectively (Table 1). The other four scenarios were based on hypothetical pandemics that varied the CAR (20 and 30%) and the CHR (1.05–4.0% for non-high-risk patients; 5–20% for high-risk patients) (Table 1). The hypothetical scenarios are based on a risk assessment framework developed by the CDC.27,28 A standardized risk space was defined based on previous influenza pandemics, and hypothetical pandemic scenarios were identified from this risk space to allow easy comparisons to future economic evaluations. The CHRs for the high-risk groups in these four hypothetical pandemics were assumed to be five times the CHR for the non-high-risk group of patients based on estimates from the 2009 A(H1N1) pandemic.29 We also assumed that the percentage of patients seeking outpatient/ambulatory care would increase with the CHR of the pandemic, ranging from 40% among non-high-risk patients in a 2009-type pandemic to ~81% among high-risk patients when the CHR is 20% (Table 1). We estimated the number of deaths averted through averting hospitalizations by multiplying the number of hospitalizations averted with an in-hospital mortality risk that was constant across the scenarios.
Fig. 1.
Decision analytical model tree comparing outcomes in ‘NAI treatment’ and ‘no NAI treatment’ groups for patients with symptomatic pandemic influenza.
Table 1.
Input parameters used to estimate the number of outcomes averted by neuraminidase inhibitors (NAI) treatment and the cost per averted hospitalization
| Parameter | Value | Range (probability distribution) | Source |
|---|---|---|---|
| Total population | 62 280 000 | Fixed | 35 |
| Clinical attack rate (CAR) | |||
| 2009 pandemic | 7% | Fixed | Box A1, page 31 of Ref.46 |
| Transmissibility scenario 1 | 20% | Fixed | 28 |
| Transmissibility scenario 2 | 30% | Fixed | 28 |
| % Seeking outpatient care (non-high-risk)a | |||
| 2009 pandemic | 40% | 32–43% (Uniform) | 47 |
| Severity 1 | 60% | Fixed | 28 |
| Severity 2 | 70% | Fixed | 28 |
| % Seeking outpatient care (high-risk) | |||
| 2009 pandemic | 51.2% | 43.2–54.2% (Uniform) | Assumed from Ref.48 |
| Severity 1 | 71.2% | Fixed | Assumed in line with Ref.48,28 |
| Severity 2 | 81.2% | Fixed | Assumed in line with Ref.48,28 |
| % Of high-risk individuals | 30% | 27–33% (Uniform) | 49 |
| Case hospitalization risk (non-high-risk) | |||
| 2009 Pandemic | 0.30% | 0.27–0.33% (Uniform) | From Annex G, page 171 of Ref.50 |
| Severity 1 | 1.05% | Fixed | 28 |
| Severity 2 | 4.00% | Fixed | 28 |
| Case hospitalization risk (high-risk) | |||
| 2009 pandemic | 1.50% | 1.35–1.65% (Uniform) | Assumed from page 10 of Ref.29 that at-risk groups would have an increased risk of hospital admission by five times |
| Severity 1 | 5.25% | Fixed | Assumed in line with Refs.28,29 |
| Severity 2 | 20.00% | Fixed | Assumed in line with Refs.28,29 |
| % Of care-seeking patients prescribed NAI | 73% | 60–85% (Triangular) | 32 |
| Prescription of NAIs for non-influenza ILI as a % of those receiving NAIs for influenza | 40% | 30–50% (Uniform) | Assumed from Ref.31 |
| NAI (any time) compliance (as a %) | 64% | 55–70% (Uniform) | 32 |
| Effectiveness of NAI treatment (<48 h from symptom onset) on hospitalization (risk reduction) (Intention-to-treat-infected) |
63% | 83–19% (Triangular) | Assumed for pandemic influenza from Ref.19 |
| Mortality risk (in-hospital) | |||
| 2009 Pandemic | 5.3% | Fixed | 51 |
| Severity 1 | 5.3% | Fixed | Assumed to be fixed between scenarios |
| Severity 2 | 5.3% | Fixed | Assumed to be fixed between scenarios |
| Costs | |||
| Before being input into the model, all costs listed below were inflated to the 2017 UK Pound Sterling (£) (The hospital & community health services (HCHS) index) | |||
| Cost of GP consultation | £ 37 | 52,55 | |
| Cost of telephone consultation | £ 22 | 52,55 | |
| Average (weighted) outpatient consultation cost | £24.2 | £22–£37 (Truncated log normal) | |
| Cost of NAI (+delivery) | £ 16 | Fixed | 53 |
| High-risk patients: Cost of Hospitalization due to influenza (per patient) | £1727 | £1263–£2075 (Truncated log normal) | 54, 55 |
| Low-risk patients: Cost of Hospitalization due to influenza (per patient) | £436 | £307–£504 (Truncated log normal) | 54,55 |
aThis includes consultations made through the National Pandemic Flu Service (NPFS) telephone line.
We did not differentiate between oseltamivir and zanamivir in the definition of NAIs in our model; however, we based our cost and treatment effectiveness estimates on data specific for oseltamivir. We focus on community-based treatment and do not consider NAI prophylaxis. We used NAI effectiveness estimates from an individual participant data (IPD) meta-analysis of clinical trials data on otherwise healthy patients with seasonal influenza19 based on ITTI analysis (relative risk: 0.37, 95% confidence interval: 0.17–0.81) since NAIs are not active against non-influenza respiratory infections.30 To account for NAI prescriptions to patients with non-influenza ILI, we assumed a ‘wastage factor’ of 40%, i.e. patients with non-influenza ILI would be prescribed 40% of the number of regimens that are prescribed to patients with influenza.31 We assumed that all patients would start NAI treatment ≤48 h of symptom onset in our main model and then performed a sensitivity analysis varying the promptness of care-seeking within 48 h of symptom onset from 25 to 75% (percentage of all care-seeking patients who do so ≤48 h of symptom onset). Based on estimates from 2009, we also assumed that 64% of patients would be compliant with the prescribed regimen.32
Unit cost data for our model were obtained from secondary sources including the British National Formulary and UK-based reports on the cost of health and social care (Table 1). Briefly, we used a weighted average cost of physician-based consultation of £24.20. This cost was calculated as a weighted average cost of either a conventional primary care consultation or a phone-based consultation with the 2009 National Pandemic Flu Service (NPFS).33 The weighting of the costs was done using the proportion of assessments routed through each consultation service in 2009. We used a cost of £16 for an NAI prescription, which included the cost of delivery. Costs of hospitalizations ranged from £436 for non-high-risk patients to £1727 for high-risk patients (Table 1). All costs were inflated to the 2017 British Pound Sterling (£) using the hospital and community health services (HCHS) index.34
The overall population of 62.28 million was based on the 2009 UK population.35 We performed the analyses from the perspective of the healthcare payer, the UK National Health Service (NHS). Given that we did not undertake a full cost-utility analysis, we chose to measure our outcomes in natural units (deaths and hospitalizations) rather than in standardized units (QALYs).36 We considered a time horizon of less than one year (one pandemic event), therefore a discounting rate would not apply.
In each pandemic scenario, we compared the number of outcomes averted (hospitalizations and deaths) and total costs associated with NAI treatment compared to no NAI treatment. We assessed cost-effectiveness of community-based NAI treatment by estimating the cost per averted hospitalization. Our primary analysis was performed using the middle values of our input parameters using formulas provided in Appendix 1. To account for uncertainty in parameter estimates, we performed sensitivity analyses by probabilistically varying input parameters along pre-defined probability distributions (Table 1) and using Monte Carlo simulations (5000 iterations using Latin hypercube sampling) to calculate mean output values and 95% confidence intervals for different combinations of input parameters. The sensitivity analyses were performed using the software @Risk version 7.3 (Palisade Corporation). Further, we also performed two-way sensitivity analysis to assess the impact of varying NAI effectiveness and patient compliance on the outcome (hospitalizations averted).
Results
In a 2009-like pandemic scenario, we estimated that in our base-case model (no NAI treatment) there would be 28 773 hospitalizations in the overall population. We estimated that 1.9 million regimens of NAIs would be dispensed for outpatient treatment. NAI treatment would have averted 4034 (14%) hospitalizations in a population of 62.28 million (65 hospitalizations averted/million population) at a cost of £7110 per hospitalization averted (Table 2). The cost to avert one hospitalization was £2238 in high-risk populations and £20 473 in the non-high-risk population (Table 2).
Table 2.
Outpatient NAI treatment for averting outcomes and the cost per averted hospitalization
| NAI regimens dispensed to pandemic influenza patients | NAI regimens dispensed to non-influenza ILI patients | Total NAI regimens dispensed | Total Hospitalizations | NAI costs (£) | Total costs (£) | Hospitalizations averted (%) | Incremental cost per averted hospitalization (£) | Deaths averted, No. | |
|---|---|---|---|---|---|---|---|---|---|
| 2009 A(H1N1) pandemic | |||||||||
| High-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 19 618 | NA | 56 713 354 | NA | NA | NA |
| NAI treatment | 488 833 | 195 533 | 684 367 | 16 662 | 12 397 546 | 63 330 048 | 2956 (15.1) | 2238 | 157 |
| Non-high-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 9155 | NA | 37 976 039 | NA | NA | NA |
| NAI treatment | 891 102 | 356 441 | 1 247 543 | 8077 | 22 599 693 | 60 043 645 | 1078 (11.8) | 20 473 | 57 |
| Total population | |||||||||
| No NAI treatment | NA | NA | NA | 28 773 | NA | 94 689 393 | NA | NA | NA |
| NAI treatment | 1 379 935 | 551 974 | 1 931 910 | 24 739 | 34 997 239 | 123 373 693 | 4034 (14.0) | 7110 | 214 |
| 20% CAR-Severity 1 | |||||||||
| High-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 196 182 | NA | 456 499 003 | NA | NA | NA |
| NAI treatment | 1 942 239 | 776 896 | 2 719 135 | 155 069 | 49 258 106 | 425 367 141 | 41 113 (21.0) | CS | 2179 |
| Non-high-risk patients | – | ||||||||
| No NAI treatment | NA | NA | NA | 91 552 | NA | 188 534 796 | NA | NA | NA |
| NAI treatment | 3 819 010 | 1 527 604 | 5 346 613 | 75 383 | 96 855 827 | 277 409 315 | 16 168 (17.7) | 5497 | 857 |
| Total population | – | ||||||||
| No NAI treatment | NA | NA | NA | 287 734 | NA | 645 033 798 | NA | NA | NA |
| NAI treatment | 5 761 249 | 2 304 500 | 8 065 748 | 230 452 | 146 113 933 | 702 776 456 | 57 281 (19.9) | 1008 | 3036 |
| 20% CAR-Severity 2 | |||||||||
| High-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 747 360 | NA | 1 544 470 684 | NA | NA | NA |
| NAI treatment | 2 215 026 | 886 010 | 3 101 036 | 568 740 | 56 176 380 | 1 251 387 276 | 178 620 (23.9) | CS | 9467 |
| Non-high-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 348 768 | NA | 339 398 172 | NA | NA | NA |
| NAI treatment | 4 455 511 | 1 782 204 | 6 237 716 | 276 910 | 112 998 465 | 416 924 159 | 71 858 (20.6) | 1079 | 3808 |
| Total population | |||||||||
| No NAI treatment | NA | NA | NA | 1 096 128 | NA | 1 883 868 856 | NA | NA | NA |
| NAI treatment | 6 670 537 | 2 668 215 | 9 338 751 | 845 650 | 169 174 845 | 1 668 311 434 | 250 478 (22.9) | CS | 13 275 |
| 30% CAR-Severity 1 | |||||||||
| High-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 294 273 | NA | 684 748 504 | NA | NA | NA |
| NAI treatment | 2 913 359 | 1 165 344 | 4 078 702 | 232 603 | 73 887 160 | 638 050 710 | 61 670 (21.0) | CS | 3269 |
| Non-high-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 137 327 | NA | 282 802 193 | NA | NA | NA |
| NAI treatment | 5 728 514 | 2 291 406 | 8 019 920 | 113 075 | 145 283 741 | 416 113 973 | 24 252 (17.7) | 5497 | 1285 |
| Total population | |||||||||
| No NAI treatment | NA | NA | NA | 431 600 | NA | 967 550 697 | NA | NA | NA |
| NAI treatment | 8 641 873 | 3 456 749 | 12 098 622 | 345 678 | 219 170 901 | 1 054 164 684 | 85 922 (19.9) | 1008 | 4554 |
| 30% CAR-Severity 2 | |||||||||
| High-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 1 121 040 | NA | 2 316 706 026 | NA | NA | NA |
| NAI treatment | 3 322 538 | 1 329 015 | 4 651 554 | 853 111 | 84 264 570 | 1 877 080 913 | 267 929 (23.9) | CS | 14 200 |
| Non-high-risk patients | |||||||||
| No NAI treatment | NA | NA | NA | 523 152 | NA | 509 097 257 | NA | NA | NA |
| NAI treatment | 6 683 267 | 2 673 307 | 9 356 574 | 415 364 | 169 497 697 | 625 386 237 | 107 788 (20.6) | 1079 | 5713 |
| Total population | |||||||||
| No NAI treatment | NA | NA | NA | 1 644 192 | NA | 2 825 803 283 | NA | NA | NA |
| NAI treatment | 10 005 805 | 4 002 322 | 14 008 127 | 1 268 475 | 253 762 267 | 2 502 467 151 | 375 717 (22.9) | CS | 19 913 |
CAR, clinical attack rate; CS, cost saving; NA, not applicable.
In the 20% CAR-Severity 1 scenario (CHR: non-high-risk = 1.05%; high-risk = 5.25%), we estimated that 287 734 hospitalizations would occur. The 8.07 million regimens of NAIs would be dispensed, averting 57 281 (19.9%) hospitalizations at a cost per averted hospitalization of £1008 in the overall population and £5497 in the non-high-risk population. NAI treatment was seen to be cost-saving in the high-risk population.
In the 20% CAR-Severity 2 scenario (CHR: non-high-risk = 4%; high-risk = 20%), we estimated that over 1.09 million hospitalizations would occur. The 9.34 million NAI regimens would be dispensed, averting 250 478 (22.9%) hospitalizations in the total population at a cost per averted hospitalization of £1079 in the non-high-risk population. NAI treatment was seen to be cost-saving in the overall population and in the high-risk population.
In the 30% CAR–Severity 1 scenario, (CHR: non-high-risk = 1.05%; high-risk = 5.25%), we estimated that over 430 000 hospitalizations would occur. The 12.1 million NAI regimens would be dispensed, averting 85 922 (19.9%) hospitalizations at a cost per averted hospitalization of £1 008 in the overall population and £5497 in the non-high-risk population. NAI treatment was seen to be cost-saving in the high-risk population.
In the fourth pandemic scenario, (CHR: non-high-risk = 4%; high-risk = 20%), we estimated that over 1.6 million hospitalizations would occur. The 14.01 million NAI regimens would be dispensed, averting 375 717 (22.9%) hospitalizations in the overall population at a cost per averted hospitalization of £1079 in the non-high-risk population. NAI treatment was seen to be cost-saving in the overall population and in the high-risk population.
We found that varying the proportion of care-seeking patients who do so within 48 h of symptom onset, while keeping all other variables constant, lowered the percentage of averted hospitalizations in the overall population from 14.0% (assuming 100%) to 3.5% (assuming 25%) in the 2009-like pandemic scenario (Table 2, Supplemental Table S1).
Our sensitivity analyses revealed that using just the middle values of input parameters in a simple multiplicative model without probability distributions was likely to overestimate the number of hospitalizations averted and underestimate the cost per averted hospitalization. For the 2009-like pandemic scenario, multiplying the middle values of input parameters (Table 2) overestimated the overall number of averted hospitalizations by 28% and underestimated the overall cost per-averted hospitalization by 34% when compared to the mean estimated from the Monte Carlo simulation (Supplemental Table S2). Similar differences in estimates were observed in the other scenarios as well.
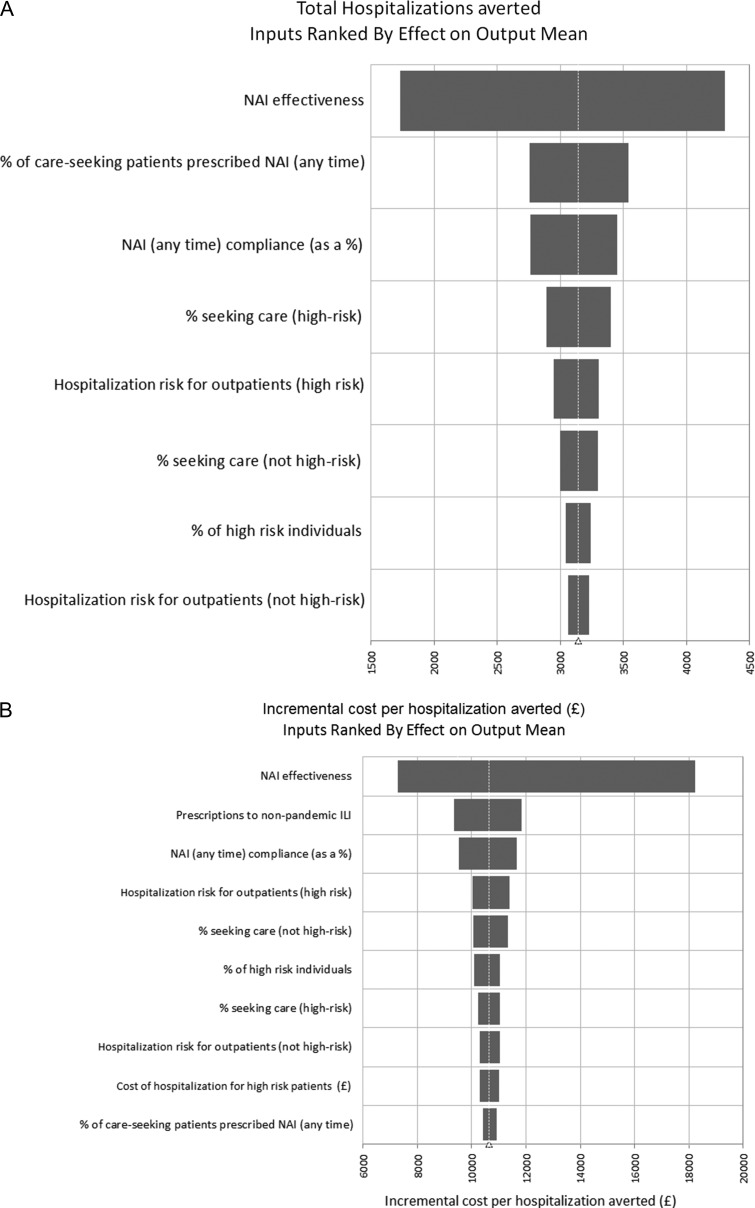
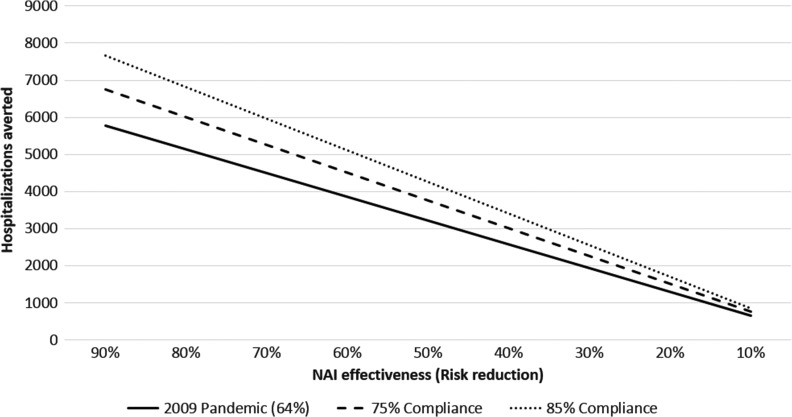
The sensitivity analyses, based on a 2009-like pandemic scenario, indicated that NAI effectiveness had the greatest impact on both the total number of hospitalizations averted, as well as on the cost per hospitalization averted (see Fig. 2 for 2009 scenario). When the NAI effectiveness was varied from 19 to 83%, the resulting overall proportion of averted hospitalizations ranged between 6 and 15%, at a cost per averted hospitalization of £6936–£19 338. The percentage of care-seeking patients who were prescribed NAI, the proportion of NAI prescriptions to non-influenza patients, and NAI treatment compliance were in the top three influential parameters for one or both outcomes (Fig. 2). In our two-way sensitivity analysis we varied the treatment compliance level along with NAI effectiveness beyond the 95% confidence intervals of our input parameter (from 90% effectiveness to 10% effectiveness). Increased compliance levels were consistently associated with an increased number of averted hospitalizations across NAI effectiveness estimates (Fig. 3). The impact of prescribing NAIs to non-influenza ILI patients had a considerable effect on the cost per averted hospitalization. For the 2009-like pandemic scenario, this ranged from £7983 per averted hospitalization (wastage factor = 30%) to £11 032 per averted hospitalization (wastage factor = 70%).
Fig. 2.
Probabilistic sensitivity analysis. (A) The impact of various parameters on total hospitalizations averted and (B) the impact of various parameters on cost-effectiveness (2009-like pandemic scenario). The width of the bars indicate the change in the output from several replications when each parameter is varied over its range. NAI, neuraminidase inhibitors; ILI, influenza-like illness.
Fig. 3.
Impact of varying treatment compliance on hospitalizations averted at different NAI effectiveness estimates. This plot is based on a 2009-like influenza pandemic where the number of hospitalizations in the base-case scenario was estimated to be 24 739. NAI, neuraminidase inhibitor.
Discussion
Main finding of this study
We found that community-based NAI treatment would avert a significant proportion of hospitalizations and deaths, particularly in high-risk patients, across the pandemic scenarios we explored in this analysis. However, a substantial number of hospitalizations and deaths would continue to occur even with community-based NAI treatment. The proportion of hospitalizations averted by NAIs could be an important consideration while planning for conditions when hospital capacity could be exceeded. Community-based NAI treatment was seen to be cost-saving for the overall population in a pandemic with a high CAR and high severity, and cost-saving for patients at high risk of complications from influenza across all the pandemic influenza scenarios tested. The value of NAI treatment for population groups not at high risk and for milder pandemic scenarios will have to be determined by careful review under country-specific willingness-to-pay thresholds and the desire to reduce the number of hospitalizations and potential hospital capacity issues.
What is already known on this topic
NAI treatment for pandemic influenza has generally been shown to be cost-effective, when compared to no NAI treatment.23–25,37 Previous studies have found that NAI effectiveness is, by far, the most influential factor affecting the numbers of outcomes averted and the associated cost-effectiveness.23,31 Results from our sensitivity analysis support this finding. A study based in the United States that used a similar model31 showed slightly lower proportions of hospitalizations averted due to NAI treatment when compared to ours, but the difference could be because of the lower level of treatment effectiveness assumed in the US study. The US study further found that while NAI treatment averted many hospitalizations, large numbers of hospitalizations would remain,31 which is similar to what we have found.
What this study adds
We found that variations in NAI prescription rate, treatment compliance and healthcare-seeking behaviour (to include the choice to seek care and the promptness in care-seeking) impacted considerably on the outcomes, suggesting that even with a drug of fixed effectiveness, factors relating to healthcare-seeking and healthcare delivery could significantly influence the total number of hospitalizations and deaths averted. These data indicate that a successful pandemic stockpiling strategy must be linked to operational procedures which optimize timely access to antivirals, widespread treatment implementation, and high levels of compliance in targeted groups.
One recognized limitation of some previous economic analyses of NAI treatment has been that entire populations have been modelled homogenously without accounting for the increase in the likelihood of influenza-related care-seeking and complications in patients with underlying at-risk conditions.23,24 In our model, we vary the propensity to seek care and CHR by patients’ at-risk status. The significance of this is that countries with limited resources could consider obtaining smaller antiviral stockpiles to target at-risk population groups and avert a higher number of hospitalizations and deaths for each antiviral course dispensed than if they adopted a treat-all approach.
The CAR was an important factor in determining the number of NAI regimens that would be needed for community-based treatment. Our model showed that a highly transmissible, but low severity pandemic would require a larger NAI stockpile than a pandemic with lower transmissibility and higher severity. However, across all pandemic scenarios, the number of NAI regimens dispensed for outpatient treatment was well below the UK’s published national NAI stockpile size of almost 40 million courses of the drug.38
We have adopted a simple and transparent approach to model building in which we account for important epidemiological factors, population healthcare-seeking behaviour and service utilization rates in a range of pandemic scenarios. Our analyses are UK-focussed, but the spreadsheet tool is easily adaptable to represent other healthcare systems. While the epidemiological parameters are unlikely to change drastically by country, input parameters relating to healthcare utilization and costs will need to be replaced with country-specific ones. We provide the simple version of the spreadsheet tool (without the sensitivity analysis) in Appendix 2. We used updated NAI effectiveness estimates from seasonal influenza data, although observational data from the 2009 A(H1N1) pandemic in a high-severity (high risk of hospitalization) population suggest similar estimates of NAI effectiveness (≤48 h from symptom onset).39 We assumed NAI effectiveness is the same in patients with and without at-risk conditions. While there is some evidence to suggest that the level of effectiveness against hospitalization is similar for both groups,39 there is also evidence that suggests a reduction in NAI effectiveness in patients with at-risk conditions.40
Limitations of this study
This study is subject to limitations. We used a decision tree model (not a transmission dynamic model) and assumed no effect of NAI treatment on transmission. There is evidence to suggest that NAI treatment, at a population level, is likely to have minimal impact on influenza transmission.41 However, decision tree models are known to be limited, especially in their ability to describe the change in influenza attack rates in different risk groups over the course of a pandemic.37 A comparison of static and dynamic models of NAI treatment for pandemic influenza concluded NAI treatment was seen to be cost-effective with both modelling paradigms; although the associated cost-effectiveness ratios were seen to differ.37 Due to a lack of evidence specific to hospitalization, we did not consider benefits of NAI treatment >48 h of symptom onset. NAI treatment has, however, been shown be beneficial even when started beyond 48 h from symptom onset.12 The use of NAIs may be associated with additional costs to the healthcare system due to possible adverse effects of NAIs21 but we have not considered these costs in our model since most side effects are known to be minor.19 Finally, we have assumed that the multiplier for high-risk patients remains constant between severity scenarios resulting in a CHR as high as 20%. CHRs of 20%, even for high-risk patients, may be unlikely.
Conclusions
Our analyses show that NAI treatment in outpatients can be cost-saving, particularly for population groups at high risk of influenza-related complications. Model-based estimates like these of the potential hospitalizations, deaths and costs associated with different pandemic scenarios can help countries consider different treatment options and inform stockpiling decisions while developing pandemic preparedness plans. NAI stockpiling decisions are also influenced by other costs to the healthcare system related to storage and maintenance of the NAI stockpile. Currently, the shelf-life for the 75 mg hard capsules of oseltamivir phosphate that comprise most of the NAI stockpile is estimated to be 10 years if stored as per instructions.42 However, influenza pandemics cannot be predicted, and NAI stockpiles could remain unused at the end of their shelf-life, or they may be rendered ineffective or less relevant by the development of antiviral drug resistance or newer, more effective influenza antiviral therapies. Additionally, evidence suggests that in-hospital NAI treatment may also be associated with protective effects43,44 and NAI treatment has been shown to be cost-effective if the benefits of NAI usage are confined only to those treated in hospital.45 If a pandemic treatment policy was pursued which combined community use of NAIs to prevent hospital admission and NAI treatment of hospitalized patients to reduce mortality, then cost-effectiveness and stockpile strategies across both scenarios would need to be considered. Future research in optimizing NAI distribution to risk groups during a pandemic will further inform the cost-effectiveness of stockpiling.
Supplementary Material
Acknowledgements
We would like to thank Anita Patel from CDC, Atlanta, for reviewing this manuscript and offering helpful comments.
Conflict of interest
SV, JSN-V-T and PRM are currently working on outputs from the PRIDE study which is supported by an unrestricted educational grant from F. Hoffman La Roche. JSN-V-T reports grants from F. Hoffmann-La Roche, personal fees from Shionogi Ltd. (in 2016), outside the submitted work and is currently on secondment from the University of Nottingham to the Department of Health and Social Care (England). All other authors report no conflicts of interest. The work and conclusions presented here are those of the authors and do not necessarily represent the official position of the Department of Health and Social Care (England). The work and conclusions presented here are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention (CDC) or the US Department of Health and Human Services.
References
- 1. DH Pandemic Influenza Preparedness Team UK Influenza Pandemic Preparedness Strategy 2011. 2012.
- 2. Programme WHOGI Pandemic Influenza Preparedness and Response: A WHO Guidance Document. Geneva, Switzerland: World Health Organization, 2009. [PubMed] [Google Scholar]
- 3. U.S. Department of Health and Human Services Pandemic Influenza Plan: 2017 Update. 2017.
- 4. Centers for Disease Control and Prevention Selecting Viruses for the Seasonal Influenza Vaccine 2016. [cited]. https://www.cdc.gov/flu/about/season/vaccine-selection.htm (14 June 2017, date last accessed).
- 5. Monto AS, Fleming D, Henry D et al. . Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis 1999;180(2):254–61. [DOI] [PubMed] [Google Scholar]
- 6. Monto AS, Webster A, Keene O. Randomized, placebo-controlled studies of inhaled zanamivir in the treatment of influenza A and B: pooled efficacy analysis. J Antimicrob Chemother 1999;44(suppl_2):23–9. [DOI] [PubMed] [Google Scholar]
- 7. Nicholson K, Aoki F, Osterhaus A et al. . Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 2000;355(9218):1845–50. [DOI] [PubMed] [Google Scholar]
- 8. Treanor JJ, Hayden FG, Vrooman PS et al. . Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. J Am Med Assoc 2000;283(8):1016–24. [DOI] [PubMed] [Google Scholar]
- 9. Hedrick JA, Barzilai A, Behre U et al. . Zanamivir for treatment of symptomatic influenza A and B infection in children five to twelve years of age: a randomized controlled trial. Pediatr Infect Dis J 2000;19(5):410–7. [DOI] [PubMed] [Google Scholar]
- 10. Whitley RJ, Hayden FG, Reisinger KS et al. . Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001;20(2):127–33. [DOI] [PubMed] [Google Scholar]
- 11. Heinonen S, Silvennoinen H, Lehtinen P et al. . Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin Infect Dis 2010;51(8):887–94. [DOI] [PubMed] [Google Scholar]
- 12. Fry AM, Goswami D, Nahar K et al. . Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis 2014;14(2):109–18. [DOI] [PubMed] [Google Scholar]
- 13. Kohno S, Kida H, Mizuguchi M et al. . Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother 2011;55(6):2803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitley R, Laughlin A, Carson S et al. . Single dose peramivir for the treatment of acute seasonal influenza: integrated analysis of efficacy and safety from two placebo-controlled trials. Antivir Ther 2015;20(7):709–19. [DOI] [PubMed] [Google Scholar]
- 15. Kelly H, Cowling B. Evidence and policy for influenza control. Euro Surveill 2014;19(27):2–4. [PubMed] [Google Scholar]
- 16. Nguyen-Van-Tam JS, Openshaw PJM, Nicholson KG. Antivirals for influenza: where now for clinical practice and pandemic preparedness? Lancet 2014;384(9941):386–7. [DOI] [PubMed] [Google Scholar]
- 17. Jefferson T, Jones MA, Doshi P et al. . Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2012;1(1). 10.1002/14651858.CD008965.pub3. [DOI] [PubMed] [Google Scholar]
- 18. Kaiser L, Wat C, Mills T et al. . Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med 2003;163(14):1667–72. [DOI] [PubMed] [Google Scholar]
- 19. Dobson J, Whitley RJ, Pocock S et al. . Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015;385(9979):1729–37. [DOI] [PubMed] [Google Scholar]
- 20. Ebell MH, Call M, Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract 2012;30(2):125–33. [DOI] [PubMed] [Google Scholar]
- 21. Jefferson T, Jones M, Doshi P et al. . Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. Br Med J 2014;348:g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Department of Health Pandemic Influenza: Guidance for Primary Care Trusts and Primary Care Professionals on the Provision of Healthcare in a Community Setting in England. 2007.
- 23. Siddiqui MR, Edmunds WJ. Cost-effectiveness of antiviral stockpiling and near-patient testing for potential influenza pandemic. Emerg Infect Dis 2008;14(2):267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carrasco LR, Lee VJ, Chen MI et al. . Strategies for antiviral stockpiling for future influenza pandemics: a global epidemic-economic perspective. J R Soc Interface 2011;8(62):1307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balicer RD, Huerta M, Davidovitch N et al. . Cost-benefit of stockpiling drugs for influenza pandemic. Emerg Infect Dis 2005;11(8):1280–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drake TL, Chalabi Z, Coker R. Cost-effectiveness analysis of pandemic influenza preparedness: what’s missing? Bull World Health Organ 2012;90:940–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reed C, Biggerstaff M, Finelli L et al. . Novel framework for assessing epidemiologic effects of influenza epidemics and pandemics. Emerg Infect Dis 2013;19(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meltzer MI, Gambhir M, Atkins CY et al. . Standardizing scenarios to assess the need to respond to an influenza pandemic. Clin Infect Dis 2015;60(suppl_1):S1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Health Protection Agency Pandemic (H1N1) 2009 in England; An Overview of Initial Epidemiological Findings and Implications for the Second Wave. London: HPA, 2009. [Google Scholar]
- 30. Academy of Medical Sciences, Wellcome Trust Use of Neuraminidase Inhibitors in Influenza 2015. [cited]. http://www.acmedsci.ac.uk/policy/policy-projects/treating-influenza/ (14 June 2017, date last accessed).
- 31. O’Hagan JJ, Wong KK, Campbell AP et al. . Estimating the United States demand for influenza antivirals and the effect on severe influenza disease during a potential pandemic. Clin Infect Dis 2015;60(suppl_1):S30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rutter P, Mytton O, Ellis B et al. . Access to the NHS by telephone and Internet during an influenza pandemic: an observational study. BMJ Open 2014;4(2):e004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Department of Health The National Pandemic Flu Service: An Evaluation. 2011.
- 34. Curtis LA. Unit Costs of Health and Social Care 2017. 2017.
- 35. World Bank UK Population Total 2017. [cited 12/05/2017]. https://data.worldbank.org/indicator/SP.POP.TOTL?locations=GB (21 February 2017, date last accessed).
- 36. Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull 2010;96(1):5–21. [DOI] [PubMed] [Google Scholar]
- 37. Lugnér AK, Mylius SD, Wallinga J. Dynamic versus static models in cost‐effectiveness analyses of anti‐viral drug therapy to mitigate an influenza pandemic. Health Econ 2010;19(5):518–31. [DOI] [PubMed] [Google Scholar]
- 38. Public Accounts Committee 2 Stockpiling Tamiflu and the Management of the Stockpile In: Parliament U, editor.; 2013.
- 39. Venkatesan S, Myles PR, Leonardi-Bee J et al. . Impact of outpatient neuraminidase inhibitor treatment in patients infected with influenza A(H1N1)pdm09 at high risk of hospitalization: an individual participant data metaanalysis. Clin Infect Dis 2017;64(10):1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marra F, Chong M, Henry B et al. . Effectiveness of neuraminidase inhibitors in preventing hospitalization during the H1N1 influenza pandemic in British Columbia, Canada. J Antimicrob Chemother 2013;69(5):1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghani A, Baguelin M, Griffin J et al. . The early transmission dynamics of H1N1pdm influenza in the United Kingdom. PLoS Curr 2010;1:RRN1130. 06/1310/26/created11/20/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Electronic Medicines Compendium (eMC) Tamiflu 30, 45 and 75 mg Hard Capsules 2017. [cited]. http://www.medicines.org.uk/emc/medicine/20294 (14 June 2017, date last accessed).
- 43. Muthuri SG, Venkatesan S, Myles PR et al. . Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014;2(5):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muthuri SG, Venkatesan S, Myles PR et al. . Impact of neuraminidase inhibitors on influenza A(H1N1)pdm09-related pneumonia: an individual participant data meta-analysis. Influenza Other Respir Viruses 2016;10(3):192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watson S, Chen Y, Nguyen-Van-Tam J et al. Evidence Synthesis and Decision Modelling to Support Complex Decisions: Stockpiling Neuraminidase Inhibitors for Pandemic Influenza Usage [Version 2; Referees: 2 Approved]. 2017. [DOI] [PMC free article] [PubMed]
- 46. Department of Health Scientific Summary of Pandemic Influenza & its Mitigation: Scientific Evidence Base Review. 2011.
- 47. Brooks-Pollock E, Tilston N, Edmunds WJ et al. . Using an online survey of healthcare-seeking behaviour to estimate the magnitude and severity of the 2009 H1N1v influenza epidemic in England. BMC Infect Dis 2011;11(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biggerstaff M, Jhung MA, Reed C et al. . Influenza-like illness, the time to seek healthcare, and influenza antiviral receipt during the 2010–2011 influenza season—United States. J Infect Dis 2014;210(4):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Public Health England Seasonal Influenza Vaccine Uptake Amongst GP Patient Groups in England: Provisional Monthly Data for 1 September 2016 to 30 November 2016. In: Department of Health, editor. 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/580075/Seasonal_flu_GP_patients_2016_2017_01_September_30_November_CCG.pdf (14 June 2017, date last accessed).
- 50. Hine D. The 2009 Influenza Pandemic: An Independent Review of the UK Response to the 2009 Influenza Pandemic. 2010.
- 51. Myles PR, Semple MG, Lim WS et al. . Predictors of clinical outcome in a national hospitalised cohort across both waves of the influenza A/H1N1 pandemic 2009–2010 in the UK. Thorax 2012;67(8):709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curtis L. Unit Costs of Health and Social Care 2008. Kent: Personal Social Services Research Unit, University of Kent at Canterbury, 2009. [Google Scholar]
- 53. British Medical Association and the Royal Pharmaceutical Society of Great Britain British National Formulary 58. September 2009 edition. 2009.
- 54. Department of Health NHS Reference Costs 2008. 2008.
- 55. Baguelin M, Van Hoek AJ, Jit M et al. . Vaccination against pandemic influenza A/H1N1v in England: a real-time economic evaluation. Vaccine 2010;28(12):2370–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.