Abstract
Background
This is an ecological study that examines the relationship between antiviral drug collection during the 2009/2010 A/H1N1 influenza pandemic, and area-level ethnicity, socioeconomic deprivation and distance from an antiviral collection point (ACP).
Methods
Age-standardized antiviral collection rates (ACR) were calculated for each super output area (geographic areas representing a population of ∼1500) in Sandwell, UK for all residents who received an antiviral drug for influenza-like illness between 23 July 2009 and 7 February 2010. Multivariable regression was used to examine the relationship between ACR and ethnicity (percentage population non-white), socioeconomic deprivation (index of multiple deprivation, IMD) and distance from an ACP.
Results
Socioeconomic deprivation, ethnicity and distance from an ACP were independently associated with a reduction in ACR. Each one-point increase in the IMD score was associated with a drop in the ACR of 15.7 prescriptions per 100 000 population (P= 0.013).
Conclusions
Socioeconomic deprivation, ethnicity and distance from an ACP may have influenced health-seeking behaviour during the 2009/2010 influenza pandemic. This suggests possible inequalities in access to antivirals during the most recent influenza pandemic. Qualitative research is needed to examine the reasons for this. Individual-level data on ethnicity should be routinely collected in the event of a future pandemic.
Keywords: A/H1N1, antivirals, deprivation, ethnicity, health-seeking behaviour, pandemic influenza
Introduction
In April 2009 a novel strain of influenza A/H1N1 was isolated in Mexico and the USA. On 11th June the World Health Organization declared a global pandemic. The National Pandemic Flu Service (NPFS) was activated in England on 23rd July as part of the UK's response to the pandemic. This was a telephone and web-based advice line which could be contacted by people with influenza-like illness (ILI) as a pathway to seeking the antiviral drugs oseltamivir or zanamivir from an antiviral collection point (ACP). Neuraminidase inhibitors such as oseltamivir and zanamivir have been shown to prevent the complications of influenza.1 ‘Flu friends’ were asked to collect the antiviral drug on behalf of the person suffering from ILI.
A raft of observational studies, particularly from the USA, indicate that people from ethnic/racial minorities and socioeconomically deprived populations are less likely than the general population to access preventive health services such as seasonal influenza vaccine2–4 and this lack of parity contributes to significant excess mortality.5 This has been attributed to a range of factors including poor health literacy,6 education,7,8 health beliefs,7,9,10 attitudes,11 access to healthcare services,9,12,13 language barriers,14 socioeconomic deprivation4,15 and provider recommendations.10 There is also evidence that belonging to an ethnic/racial minority is an independent risk factor for low influenza vaccination uptake.16
Actual acceptance of the offer of influenza vaccination may be similar between ethnic/racial minority groups and whites17 suggesting that poor access to services and awareness may be significant barriers. The negative association between ethnic/racial minorities and uptake of influenza vaccination, however, is not universal and in some settings the uptake amongst ethnic minority groups may actually be higher than in the white population.18 Few studies have evaluated the impact of influenza vaccination programs by ethnicity and socioeconomic status19 and we are unaware of any published studies that examine the equality of access to antiviral drugs during an influenza pandemic by socioeconomic and ethnic status.
This is an ecological study that aims to determine whether socioeconomic deprivation, ethnicity and distance from an ACP influenced access to antiviral drugs during the 2009/2010 A/H1N1 influenza pandemic in the West Midlands, UK. The aim is to identify if further work is needed to ensure equality of access to influenza-related services during future pandemics.
Methods
The NPFS provided data on the age, sex and residential postcode of the 10 655 residents in Sandwell, West Midlands, UK who sought and collected an antiviral drug (oseltamivir or zanamivir) for ILI from an ACP between 23 July 2009 and 7 February 2010. Sandwell is a metropolitan borough in the West Midlands with a population of 280 790. It has a high proportion of ethnic minorities (predominantly Indian, Black Caribbean, Pakistani, Bangladeshi and mixed ethnic group) and high levels of socioeconomic deprivation. It consists of six towns made up of 187 super output areas (geographic areas representing a population of ∼1500 people). Across the six towns the proportion of the population belonging to an ethnic minority ranges from 8 to 44%. It is the 14th most socioeconomically deprived local authority area in England.20
Postcodes from the NPFS data were mapped to their corresponding super output area and were used with population denominators from the 2001 census21 and the European standard population to calculate direct age-standardized antiviral collection rates (ACR). These rates were then tabulated and mapped against area-level ethnicity, socioeconomic deprivation and distance from an ACP for each super output area in the borough.
The NPFS did not collect data on ethnicity. Area-level ethnicity was therefore measured as the population percentage non-white as recorded in the 2001 census.21 This was based on the Office for National Statistics 2001 level 1 classification of ethnic groups in which white ethnic group includes ‘white British’, ‘Irish’ and ‘other white’. Socioeconomic status was measured as the index of multiple deprivation (IMD) for the super output area.20 IMD is based on domains of deprivation including income, employment, education and health.20 A higher score indicates a higher level of socioeconomic deprivation.
There were two ACPs in Sandwell. One was open from 23 July 2009 to 15 January 2010 and the other from 1st October to 31st January. The standard opening hours were 7 a.m. to 8 p.m. Monday to Friday and opening times were more variable on the weekends depending on staff capacity. Although it was possible to receive antivirals from other health bodies such as general practitioners, ACPs were the predominant source of mass distribution. The direct geometric distance between the population-weighted centroid and the nearest ACP was measured for each super output area using easting and northing coordinates from the Office for National Statistics.22
Population characteristics were summarized and scatter plots were produced for the ACR and each of the independent variables. Density maps were produced using MapInfo version 10.0 to demonstrate the spatial relationship between the ACR and socioeconomic deprivation, ethnicity, and the location of the ACPs (Fig. 1). Univariable and multivariable linear regression were performed with the ACR as the dependent variable and socioeconomic deprivation, ethnicity and distance from an ACP as the independent variables. The scatter plots and statistical analysis were performed using Stata version 11.
Fig. 1.
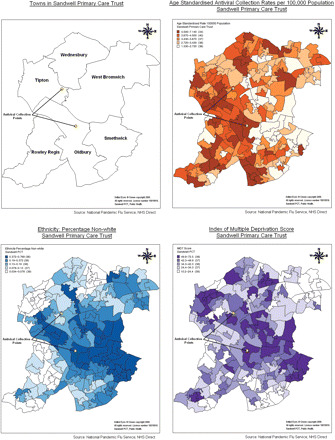
Maps showing the six towns of Sandwell, the location of the ACPs, age-standardized ACR, ethnicity (percentage population non-white) and IMD.
Results
The population characteristics are summarized in Table 1. Antiviral recipients were predominantly young adults and children and there were more females than males. The mean IMD score of 37.2 indicates a high level of socioeconomic deprivation amongst the general population. The mean ACR was 3681 prescriptions per 100 000 population (SD 1120, range: 1134–7133). Around 1.1million prescriptions of antivirals were distributed by the NPFS in England23 which had an estimated population in 2009 of 51.8million.21 This equates to a national (non-age standardized) ACR of ∼2100 prescriptions per 100 000 population.
Table 1.
Summary of population characteristics (ACR, age-standardized antiviral collection per 100 000 population; distance, distance from an ACP; ethnicity, percentage population non-white; IMD, index of multiple deprivation; IQR, interquartile range; SD, standard deviation)
| Number | % | Mean | SD | Median | IQR |
Range
|
||
|---|---|---|---|---|---|---|---|---|
| Upper | Lower | |||||||
| Population | 280 790 | |||||||
| ACR | 3681 | 1120 | 1334 | 7133 | ||||
| IMD | 37.2 | 13.6 | 10.2 | 72.5 | ||||
| Ethnicity (%) | 15.3 | 8.8, 27.9 | 3.4 | 76.8 | ||||
| Distance (m) | 3104 | 1424 | 157 | 7104 | ||||
| Antiviral recipients | ||||||||
| Male | 4728 | 43.5 | 26.6* | 17.5* | 1* | 84* | ||
| Female | 6128 | 56.4 | 28.9* | 17.5* | 1* | 95* | ||
| Overall | 10856 | 100 | 27.9* | 17.5* | 1* | 95* | ||
*Age in years.
The density maps in Fig. 1 demonstrate an inverse relationship between the ACR and socioeconomic deprivation and the percentage population non-white. Relatively fewer antivirals were collected in the town of Smethwick which has the highest proportion of ethnic minorities (44.2%) and some of the highest levels of socioeconomic deprivation in Sandwell.
Figure 2 illustrates that although the data in the scatter plots are widely dispersed, there is a general trend for the ACR to decrease with an increase in socioeconomic deprivation, ethnicity and distance from an ACP.
Fig. 2.
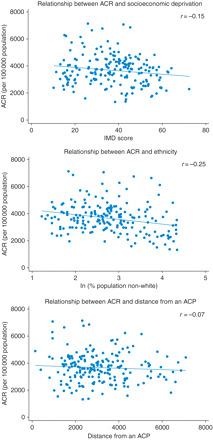
Scatter plots showing the relationship between ACR and socioeconomic deprivation (IMD), ethnicity (percentage population non-white) and distance from an ACP.
ACR, socioeconomic deprivation and the distance from an ACP were normally distributed. Ethnicity had a skewed distribution and was therefore log transformed for the regression analysis.
In the univariable analysis socioeconomic deprivation and ethnicity were both associated with a reduction in ACR (P < 0.05; Table 2). In the multivariable model ethnicity, socioeconomic deprivation and distance from an ACP were found to be independently associated with a statistically significant reduction in ACR. The effect was largest for socioeconomic deprivation—every increased point in the IMD score was associated with a drop in ACR of 15.7 prescriptions per 100 000 population. Similarly an increase in population non-white of 1% was associated with a reduction in ACR of 3.49 per 100 000 population. While there was also a statistically significant reduction in ACR with an increase in the distance from an ACP, this effect was very small after adjusting for socioeconomic deprivation and ethnicity.
Table 2.
Results of the uni- and multi-variable linear regression analysis for the relationship between age-standardized ACR and socioeconomic deprivation, ethnicity and distance from an ACP
| Variable |
Univariable (unadjusted)
|
Multivariable (adjusted)
|
||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | |||
| IMD | −12.6 | −24.4 | −0.85 | 0.036 | −15.7 | −28.0 | −3.35 | 0.013 |
| Ln(ethnicity) | −353 | −551 | −155 | 0.001 | −351 | −548 | −154 | 0.001 |
| ethnicity | −3.51 | −5.48 | −1.54 | 0.001 | −3.49 | −5.45 | −1.53 | 0.001 |
| distance | −0.055 | −0.169 | 0.059 | 0.344 | −0.135 | −0.253 | −0.017 | 0.025 |
β-change in number of antivirals prescribed per 100 000 population per unit change in explanatory variable. R2= 0.10. Results highlighted in bold are statistically significant at the P = 0.05 level.
The residuals of the multivariable regression model followed a normal distribution. There was little correlation between socioeconomic status and ethnicity (Spearman's rank correlation = 0.28). The model however only accounted for 10% (R2= 0.10) of the overall variation in the ACR.
Discussion
Main findings of this study
The ACR was negatively associated with area-level socioeconomic deprivation, proportion of the population non-white and distance from an ACP, suggesting that these factors adversely influenced health-seeking behaviour during the influenza pandemic in Sandwell.
What is already known on this topic
It is becoming increasingly clear that people from ethnic minorities were disproportionately affected by A/H1N1. Data from the Flu Clinical Information Network (FLU-CIN) project24 showed that 62% of patients hospitalized in England with A/H1N1 during the pandemic were from ethnic minorities despite non-white ethnic minorities representing only 7.1% of the UK population.25 Cleary and McKerr from the Health Protection Agency (unpublished, personal communication) identified that 28.5% of all confirmed cases of A/H1N1 from the beginning of the pandemic to mid-October 2009 were of South Asian ethnicity. South Asian cases were also younger and more likely to live in deprived areas and were strongly clustered in London and the West Midlands. Black and minority ethnic groups, particularly those with high levels of deprivation, have been previously shown in the West Midlands, UK to have higher asthma-related hospital admission rates than the white population26 and people with asthma are known to be at higher risk of complications from influenza.27 This may partly explain the increased morbidity from A/H1N1 in ethnic minorities.
This trend was also seen in ethnic minority and indigenous groups globally. In one hospital in New Zealand 38% of patients hospitalized with A/H1N1 were Maori and 25% were of Pacific races.28 The majority of these patients had comorbidities including asthma, obesity and diabetes. In Manitoba Canada, the proportion of First Nations residents confirmed with A/H1N1 increased with disease severity from 28% of community cases to 54% of cases admitted to hospital and 60% of those admitted to ICU.29 First Nations ethnicity, interval between onset of symptoms to initiation of antiviral therapy, and the presence of an underlying comorbidity were associated with a significantly increased odds of being admitted to an intensive care unit [odds ratio 6.52 (95% CI 2.04–20.8), 8.24 (95% CI 2.82–24.1) and 3.19 (95% CI 1.07–9.52), respectively].29 In 12 states of the US two indigenous ethnic groups with some of the greatest health disparities30—American Indians and Alaskan natives, were found to have a mortality rate from A/H1N1 fourfold higher than patients in all other ethnic populations combined.31 The reasons for this were postulated to include a high prevalence of chronic comorbidities such as diabetes and asthma, social deprivation, poor living conditions, and delayed access to care. This was also thought to have been true during the influenza pandemic of 1918–19.32
What this study adds
Poor antiviral uptake by ethnic minority and socioeconomically deprived groups may have contributed to their disproportionately high morbidity from A/H1N1. There is some evidence that use of antivirals reduced household transmission during the pandemic.33 The effectiveness of oseltamivir is time dependent29 and under-access of the NPFS could have delayed the administration of oseltamivir and consequently led to poorer outcomes, although the impact of this has not been established. The relative significance of this with respect to other potentially contributing factors, such as comorbid asthma, overcrowded housing34 and generally poorer access to health services is unknown.
The reasons for the relatively low ACR in areas with higher levels of deprivation and non-white ethnic minorities remain unclear but may be related to health literacy, access to health services, and a lack of a tailored communication strategy. These findings are compatible with evidence from the UK of health inequalities and the inverse care law in the provision of seasonal influenza immunization.35,36 This may contribute to and compound the disproportionate morbidity and hospitalization people from socioeconomically deprived backgrounds experience from respiratory infections including influenza.37
The negative correlation between ethnicity and ACR seems to run counter to the findings of Rubin et al.38 whose cross-sectional survey suggests that ethnicity is associated with an increased likelihood of an intention to adopt protective behaviours for A/H1N1 influenza. This suggests that although people from ethnic minorities are ready to make behavioural changes in response to recommendations from healthcare professionals other barriers prevent this from translating into health-seeking action. Disparities in beliefs about influenza across demographic subgroups suggest a need for a more targeted communication strategy.7 Qualitative research is needed to explore this issue further and data on ethnicity should be routinely collected and reported in morbidity and mortality surveillance systems31 in future pandemics.
The finding in this study of a higher ACR amongst females and young adults and children is consistent with the demography of health-seeking behaviour and the epidemiology of A/H1N1. Females are known to be more likely to adopt protective behaviours during a pandemic39 and the younger population are known to be more susceptible to novel strains of influenza.40
Limitations of this study
The results of this study align with the findings of the broader literature but there are several limitations. First there is some inaccuracy in the age-standardization of the ACR as the population size has increased since the 2001 census from which the population denominators were obtained. Synthetic estimates of population size at the super output area level, however, were not considered accurate enough to justify using them in this analysis.
Ethnicity was measured at an area level rather than at an individual level due to the unavailability of individual-level data. It is therefore unknown at an individual level if the people collecting antivirals were representative of the ethnic makeup of their resident area so an ecological fallacy cannot be excluded. Furthermore this index of ethnicity does not include white Eastern Europeans who in this locality fall predominantly in a lower socioeconomic group. Measuring ethnicity is further complicated by the undercount of ethnic minorities in the 2001 census. The relationship between ACR and ethnicity, however, was highly statistically significant (P< 0.001) indicating a very low probability of this result being due to chance.
The population of Sandwell is almost uniformly deprived. This lack of variation in socioeconomic status may to some extent have hidden the true magnitude of the relationship between deprivation and a fall in ACR.
The regression model only accounted for 10% of the variation in ACR across Sandwell. The purpose of this study, however, was not to produce a predictive model but to generate hypotheses on potential factors that may have influenced antiviral collection during the pandemic. It does highlight though that there are many other potential factors interacting with health-seeking behaviour that might explain the variation in antiviral collection.
Finally the geometric measurement of the distance between a super output area population-weighted centroid and an ACP should be interpreted with caution since antivirals could have been collected by either ‘flu friends’ (who may not necessarily have resided in the same locality as the antiviral recipients) or the antiviral recipients themselves. In addition they may not necessarily reflect the ease of access to ACPs as it does not take into account transport times or available modes of transport. An alternative would be to use isochrone maps that calculate travel times for different modes of transport. Use of isochrones maps would be helpful for planning the location of future ACPs.
Acknowledgements
We would like to thank Nicola Grayson (Strategic Operations Centre Co-ordinator, NHS West Midlands Strategic Health Authority) for providing the data from the National Pandemic Flu Service, Ralph Smith (deputy head of information and intelligence, Sandwell Primary Care Trust) for advice on geographic mapping and indices of socioeconomic deprivation and Gillian Smith (regional epidemiologist, Health Protection Agency, West Midlands) for her comments on the final draft.
References
- 1.Matheson N, Harnden A, Perera R, et al. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database of Syst Rev. 2007;(1):1–47. doi: 10.1002/14651858.CD002744.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Racial/ethnic disparities in influenza and pneumococcal vaccination levels among persons aged > or =65 years–United States, 1989–2001. MMWR Morb Mortal Wkly Rep. 2003;52(40):958–62. [PubMed] [Google Scholar]
- 3.Lu P, Bridges CB, Euler GL, et al. Influenza vaccination of recommended adult populations, U.S., 1989–2005. Vaccine. 2008;26(14):1786–93. doi: 10.1016/j.vaccine.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Fiscella K, Holt K. Impact of primary care patient visits on racial and ethnic disparities in preventive care in the United States. J Am Board Fam Med. 2007;20(6):587–97. doi: 10.3122/jabfm.2007.06.070053. [DOI] [PubMed] [Google Scholar]
- 5.Fiscella K, Dressler R, Meldrum S, et al. Impact of influenza vaccination disparities on elderly mortality in the United States. Prev Med. 2007;45(1):83–7. doi: 10.1016/j.ypmed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Bennett IM, Chen J, Soroui JS, et al. The contribution of health literacy to disparities in self-rated health status and preventive health behaviors in older adults. Ann Fam Med. 2009;7(3):204–11. doi: 10.1370/afm.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santibanez TA, Mootrey GT, Euler GL, et al. Behavior and beliefs about influenza vaccine among adults aged 50–64 years. Am J Health Behav. 2010;34(1):77–89. doi: 10.5993/ajhb.34.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Mannino DM, Williams SG. Asthma and influenza vaccination: findings from the 1999–2001 National Health Interview Surveys. Chest. 2003;124(3):783–9. doi: 10.1378/chest.124.3.783. [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Fox SA, Cantrell CH, et al. Health disparities and prevention: racial/ethnic barriers to flu vaccinations. J Community Health. 2007;32(1):5–20. doi: 10.1007/s10900-006-9031-7. [DOI] [PubMed] [Google Scholar]
- 10.Winston CA, Wortley PM, Lees KA. Factors associated with vaccination of medicare beneficiaries in five U.S. communities: Results from the racial and ethnic adult disparities in immunization initiative survey, 2003. J Am Geriatr Soc. 2006;54(2):303–10. doi: 10.1111/j.1532-5415.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 11.Lindley MC, Wortley PM, Winston CA, et al. The role of attitudes in understanding disparities in adult influenza vaccination. Am J Prev Med. 2006;31(4):281–5. doi: 10.1016/j.amepre.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Chen JY, Diamant A, Pourat N, et al. Racial/ethnic disparities in the use of preventive services among the elderly. Am J Prev Med. 2005;29(5):388–95. doi: 10.1016/j.amepre.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Rangel MC, Shoenbach VJ, Weigle KA, et al. Racial and ethnic disparities in influenza vaccination among elderly adults. J Gen Intern Med. 2005;20(5):426–31. doi: 10.1111/j.1525-1497.2005.0097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiscella K, Franks P, Doescher MP, et al. Disparities in health care by race, ethnicity, and language among the insured: findings from a national sample. Med Care. 2002;40(1):52–9. doi: 10.1097/00005650-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Coupland C, Harcourt S, Vinogradova Y, et al. Inequalities in uptake of influenza vaccine by deprivation and risk group: time trends analysis. Vaccine. 2007;25(42):7363–71. doi: 10.1016/j.vaccine.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Egede LE, Zheng D. Racial/ethnic differences in influenza vaccination coverage in high-risk adults. Am J Public Health. 2003;93(12):2074–8. doi: 10.2105/ajph.93.12.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz KL, Neale AV, Northrup J, et al. Racial similarities in response to standardized offer of influenza vaccination. A MetroNet study. J Gen Intern Med. 2006;21(4):346–51. doi: 10.1111/j.1525-1497.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appel A, Everhart R, Mehler PS, et al. Lack of ethnic disparities in adult immunization rates among underserved older patients in an urban public health system. Med Care. 2006;44(11):1054–8. doi: 10.1097/01.mlr.0000228017.83672.c3. [DOI] [PubMed] [Google Scholar]
- 19.Ompad DC, Galea S, Vlahov D. Distribution of influenza vaccine to high-risk groups. Epidemiol Rev. 2006;28:54–70. doi: 10.1093/epirev/mxj004. [DOI] [PubMed] [Google Scholar]
- 20.Noble M, McLennan D, Wilkinson K, et al. The English Indices of Deprivation. London: Communities and Local Government; 2007. [Google Scholar]
- 21.2001 Census. London: Office for National Statistics; 2001. [Google Scholar]
- 22.ONS. Super Output Area Look-up Files. London: Office for National Statistics; 2009. [Google Scholar]
- 23.Hine D. The 2009 Influenza Pandemic: An Independent Review of the UK Response to the 2009 Influenza Pandemic. London: Cabinet Office; 2010. [Google Scholar]
- 24.Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009) Thorax. 2010;65(7):645–51. doi: 10.1136/thx.2010.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Office for National Statistics. Focus on Ethnicity and Identity. London: 2005. [Google Scholar]
- 26.Gilthorpe MS, Lay-Yee R, Wilson RC, et al. Variations in hospitalization rates for asthma among black and minority ethnic communities. Respir Med. 1998;92(4):642–8. doi: 10.1016/s0954-6111(98)90511-x. [DOI] [PubMed] [Google Scholar]
- 27.CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb Mortal Wkly Rep. 2007;56(RR06):1–54. [Google Scholar]
- 28.Dee S, Jayathissa S. Clinical and epidemiological characteristics of the hospitalised patients due to pandemic H1N1 2009 viral infection: experience at Hutt Hospital, New Zealand. N Z Med J. 2010;123(1312):45–53. [PubMed] [Google Scholar]
- 29.Zarychanski R, Stuart TL, Kumar A, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182(3):257–64. doi: 10.1503/cmaj.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drewette-Card RJ, Landen MG. The disparity change score: a new methodology to examine health disparities in New Mexico. J Public Health Manag Pract. 2005;11(6):484–92. doi: 10.1097/00124784-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Deaths related to 2009 pandemic influenza A (H1N1) among American Indian/Alaska Natives—12 states, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(48):1341–4. [PubMed] [Google Scholar]
- 32.Groom AV, Jim C, Laroque M, et al. Pandemic influenza preparedness and vulnerable populations in tribal communities. Am J Public Health. 2009;99(Suppl. 2):S271–8. doi: 10.2105/AJPH.2008.157453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carcione D, Giele C, Goggin L, et al. Secondary attack rate of pandemic influenza A(H1N1) 2009 in Western Australian households, 29 May- 7 August 2009. Eurosurveillance. 2011;16(3):1. [PubMed] [Google Scholar]
- 34.Alwash R, McCarthy M. Accidents in the home among children under 5: ethnic differences or social disadvantage? Br Med J Clin Res Ed. 1988;296(6634):1450–3. doi: 10.1136/bmj.296.6634.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLean G, Sutton M, Guthrie B. Deprivation and quality of primary care services: evidence for persistence of the inverse care law from the UK Quality and Outcomes Framework. J Epidemiol Community Health. 2006;60(11):917–22. doi: 10.1136/jech.2005.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangtani P, Breeze E, Kovats S, et al. Inequalities in influenza vaccine uptake among people aged over 74 years in Britain. Prev Med. 2005;41(2):545–53. doi: 10.1016/j.ypmed.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Hawker JI, Olowokure B, Sufi F, et al. Social deprivation and hospital admission for respiratory infection: an ecological study. Respir Med. 2003;97(11):1219–24. doi: 10.1016/s0954-6111(03)00252-x. [DOI] [PubMed] [Google Scholar]
- 38.Rubin GJ, Amlot R, Page L, et al. Public perceptions, anxiety, and behaviour change in relation to the swine flu outbreak: cross sectional telephone survey. BMJ. 2009;339:b2651. doi: 10.1136/bmj.b2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal S, Pourbohloul B, Hupert N, et al. The shifting demographic landscape of pandemic influenza. PLoS ONE [Electronic Resource] 2010;5(2):1–8. doi: 10.1371/journal.pone.0009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bish A, Michie S. Demographic and attitudinal determinants of protective behaviours during a pandemic: a review. Br J Health Psychol. 2010;15(Pt 4):797–824. doi: 10.1348/135910710X485826. [DOI] [PMC free article] [PubMed] [Google Scholar]


