The etiological agent of coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged in December 2019 in Wuhan, Hubei Province, China [1]. SARS-CoV-2 has spread globally and the World Health Organization has declared a global pandemic. As of May 2020, more than 5 million cases have been identified worldwide, with more than 1.6 million cases in the United States alone. Numerous case reports and features of SARS-CoV-2 infection in adults have been reported. Common symptoms include, fever, myalgia, and dry cough [1]. Progression to pneumonia is most often seen in patients with underlying conditions and the elderly [1]. In contrast, there is a paucity of data on clinical presentation and course in pediatric SARS-CoV-2 infections, particularly among infants and neonates. The Chinese Center for Disease Control and Prevention reported that children accounted for <1% of 72 314 COVID-19 cases and that pediatric disease was most often mild [1]. Additionally, the US Centers for Disease Control and Prevention (CDC) reported that 1.7% of 150 000 COVID-19 cases in the United States through 2 April 2020 occurred in patients aged <18 years, with few requiring hospitalization [2].
Here, we report a case of COVID-19 in a 10-day-old infant with no underlying health conditions who presented with acute respiratory failure. We discuss the clinical presentation, treatment course, clinical outcomes, and testing of alternative sample types for the presence of SARS-CoV-2.
CASE PRESENTATION
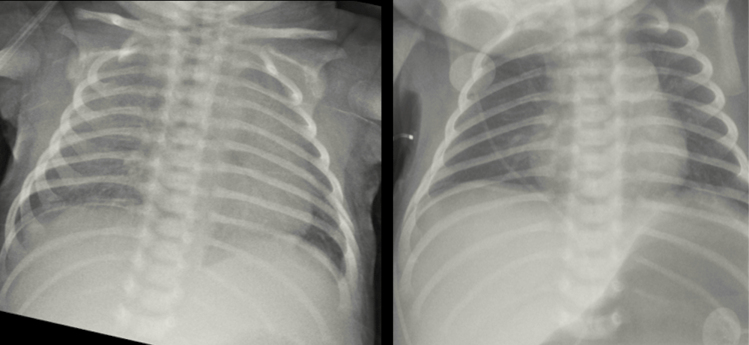
On 1 April 2020, a 10-day-old male born at 39 weeks’ gestation via normal spontaneous vaginal delivery presented to the emergency department (ED) of a California hospital with increased nasal secretion and labored breathing approximately 1 week after exposure to his grandmother and older sibling who experienced upper respiratory symptoms the week prior. No other family members reported being symptomatic. A capillary blood gas (CBG) analysis revealed a pH of 7.36 and partial pressure of carbon dioxide (PCO2) and oxygen (PO2) of 39 and 53 mm Hg, respectively; serum lactic acid was 4.1 mmol/L. The patient was treated with oxygen by nasal cannula (NC). Oxygen saturation (SpO2) on 2 L/min was 90%, and increased work of breathing (WOB) was noted; therefore, he was escalated to 5 L/min high flow (HF) NC. Taken together, these findings were consistent with hypoxic respiratory failure. Blood cultures were collected, and the patient was placed empirically on intravenous ampicillin and gentamicin. He was immediately transferred to the pediatric intensive care unit (PICU) at Children’s Hospital of Los Angeles (CHLA) for higher-level care. A chest radiograph from the outside hospital demonstrated bilateral ground-glass opacities with no focal consolidations suggestive of viral etiology (Figure 1). On admission to the PICU, increased nasal flaring and secretions, increased WOB, subcostal retractions, and lethargy were noted. He was afebrile with no cough. The patient was continued on HFNC, and SpO2 was monitored hourly and kept above 90%. His respiratory rate varied between 20 and 40 breaths/minute. Because he demonstrated clinical improvement with SpO2 maintained at 99% on 5 L/min HFNC, he was weaned to 0.5 L/min NC oxygen within 24 hours.
Figure 1.
Chest radiograph showing bilateral ground-glass opacities with no focal consolidations. The image was obtained and provided by the outside hospital on the same day the patient was admitted to Children’s Hospital of Los Angeles.
Overall, blood tests were unremarkable ,with slightly low mean corpuscular volume and mean corpuscular hemoglobin (Supplementary Table 1). A nasopharyngeal (NP) swab was collected, and SARS-CoV-2 RNA was detected by reverse transcriptase real-time polymerase chain reaction (RT-PCR) using the CDC Emergency Use Authorization SARS-CoV-2 RT-PCR protocol and reported within 12 hours. Cycle threshold (Ct) values of 21.9 and 21.7 for neuraminidase gene targets 1 and 2 (N1, N2) were detected by the assay, respectively. A FilmArray respiratory viral panel (FA-RVP) (BioFire Diagnostics, Salt Lake City, UT) performed on the same specimen was negative, ruling out other viral etiologies or viral coinfections (Supplementary Table 1). Additional SARS-CoV-2 RT-PCR testing was performed on blood and a nares swab; SARS-CoV-2 was detected from nares only. No antivirals were initiated. No other family members were tested for SARS-CoV-2 at that time.
The patient remained stable on 0.5 L/min NC oxygen and weaned to 0.25 L/min later the same day. Normal appetite and fluid intake were noted, along with no apparent respiratory distress. On day 3 of admission, the patient was successfully weaned off NC oxygen to room air. Blood culture from the outside hospital grew Staphylococcus epidermidis, which was deemed a contaminant. The patient was stable and afebrile on day 4 of admission and was discharged.
The patient returned to CHLA ED 5 days later with increased nasal congestion, subcostal retractions, and decreased feeding. He was afebrile with no other symptoms. SpO2 was 100% and CBG PCO2 and PO2 were slightly abnormal, measuring 46 and 43 mm Hg, respectively. The mother, who was previously asymptomatic, was noted to have nasal congestion at that time. NP swabs from the patient and the mother tested positive for SARS-CoV-2. No other family members were tested. The patient’s NP swab exhibited Ct values of 31.1 and 34.8 for N1 and N2, respectively, qualitatively indicating a lower viral load than the first NP specimen tested 5 days prior. At that time, the patient also tested positive for human metapneumovirus by FA-RVP. On the same day, SARS-CoV-2 was detected in stool, but a nares swab was negative. The patient was monitored overnight, no investigational therapies were initiated, and his respiratory symptoms resolved. He was stable on room air and discharged the next morning.
DISCUSSION
Here, we present a confirmed COVID-19 case in a 10-day-old full-term male with no known underlying medical conditions who was hospitalized with acute respiratory failure for approximately 3 days. The patient was managed with HFNC oxygen and did not require invasive mechanical ventilation. Previous pediatric COVID-19 case reports indicate that patients who required mechanical ventilation had preexisting conditions [3]. To date, few cases of COVID-19 in newborns have been published. A case report on a confirmed SARS-CoV-2–positive pregnant woman and her newborn showed that the child tested SARS-CoV-2–positive 36 hours after birth; the child was afebrile and stable [4]. No treatment was needed despite the presence of a high-density nodular shadow in the right lung [4]. Similarly, a 15-day-old infant admitted for fever, lethargy, cutaneous mottling, respiratory distress, and cough was diagnosed with COVID-19; chest X ray was normal, and supportive care was administered [5].
Several pediatric case series have been reported, with the majority from China. A retrospective analysis of clinical data and chest computed tomography (CT) images for 9 children diagnosed with COVID-19 showed that 5 were asymptomatic, 4 had fever, 2 had cough, and 1 had rhinorrhea. CT scans revealed ground-glass opacities with consolidation (n = 6), nodular lesions (n = 6), or patchy lesions (n = 7) [6]. Another report of 10 children indicated no patients sought medical care directly but were tested for SARS-CoV-2 due to exposure history and hospitalized only after testing positive [7]. Upon evaluation, most patients were febrile, but cough, sore throat, nasal congestion, rhinorrhea, and diarrhea were less common, additionally 2 were asymptomatic [7]. Taken together, infants and young children tend to range from being asymptomatic to having mild/moderate clinical symptoms. However, as described in our case, these patients can present with acute respiratory failure that requires noninvasive oxygen therapy. Additionally, the epidemiological history of COVID-19–positive children is often linked to family cluster and exposure to sick contacts [3,6]. This was also the case for our patient who lives with 7 relatives and presented following exposure to ill family members at home.
Detection of SARS-CoV-2 from alternate sources, including stool/rectal swabs, alternative respiratory samples other than NP swabs, urine, and blood, have been documented in a small subset of studies. Here, we report 2 separate NP swabs from the same patient collected approximately 8 days apart, both of which tested positive for SARS-CoV-2 via RT-PCR. Despite an initial positive nares swab, subsequent sampling upon readmission was negative, indicating lower clinical sensitivity compared with the NP swab. However, SARS-CoV-2 was detected from fresh stool collected approximately 8 days after initial presentation. Interestingly, fecal viral shedding up to 27 days after admission was reported in children, while paired NP specimens from the same patients tested negative [7]. Intermittent shedding and prolonged SARS-CoV-2 shedding were reported in a child with 2 consecutive negative tests for both NP and rectal swabs, but the child was positive by rectal swab 6 days later [7]. Current data suggest respiratory samples and stool/rectal swabs are appropriate specimen types for SARS-CoV-2 testing in pediatric patients, with stool/rectal swabs having higher potential utility for monitoring viral shedding. Overall, this case represents a unique presentation of respiratory failure due to SARS-CoV-2 in a neonatal patient and expands the clinical spectrum of pediatric COVID-19.
CONCLUSIONS
More extensive studies are needed to better understand the pediatric disease spectrum and clinical outcomes of COVID-19, particularly in newborns and children aged <1 year. It is important for pediatricians to know that COVID-19 could potentially present as respiratory failure in young children, given that current clinical data indicate that children most often do not require oxygen therapy, intensive care support, and/or invasive mechanical ventilation. Pleural effusion, enlarged lymph nodes, or progression to pneumonia that occurs in critically ill adults was not seen in our patient, nor are these common in children based on other reports. Last, while respiratory and, potentially, stool specimens have high yield of SARS-CoV-2 detection in children, the prevalence of viremia appears to be low.
Supplementary Material
Note
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2. Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus disease 2019 in children— United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team SARS-CoV-2 infection in children. N Engl J Med 2020; 382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Guo L, Chen L, et al. . A case report of neonatal COVID-19 infection in China. Clin Infect Dis 2020; ciaa225, 10.1093/cid/ciaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 2020; 52:427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Y, Yang GD, Feng K, et al. . Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Chinese J Contemp Pediatr 2020; 22:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Y, Li X, Zhu B, et al. . Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020; 26:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.