Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected from at least 1 buccal specimen in 9 of 11 coronavirus disease 2019 (COVID-19)–infected children (81.8%). Viral loads in buccal specimens were substantially lower than those in nasopharyngeal specimens. Buccal swabs are not good as COVID-19 screening specimens in children.
Keywords: COVID-19, buccal, saliva, SARS-CoV-2, viral load
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative novel coronavirus for coronavirus disease 2019 (COVID-19), has been found in various clinical specimens of infected individuals [1]. Nasopharyngeal and oropharyngeal swabs are the recommended upper respiratory tract specimens for detection of SARS-CoV-2 [2]; however, the method of collection comes with risks and challenges. Nasopharyngeal and oropharyngeal specimen collection requires healthcare workers to be in close contact with individuals who may be infected with COVID-19, posing a risk of nosocomial viral transmission to the healthcare workers [3]. Nasopharyngeal specimen collection is uncomfortable, and young children are often uncooperative during the procedure, thus increasing the risk of nasal trauma. Collection of nasopharyngeal samples is potentially aerosol-generating, with an inherent requirement for negative-pressure isolation facilities.
Recent studies showed evidence of consistent detection of SARS-CoV-2 in saliva specimens of infected adults, raising the possibility of using saliva as a form of screening for SARS-CoV-2 [4–6]. The use of saliva specimens to detect the presence of Zika virus in children has been reported [7]. Although children are often unable to produce saliva specimens spontaneously, buccal swabs can be performed to obtain saliva for testing. Buccal swabs are less invasive and cause less discomfort for children. Buccal swabs are also unlikely to trigger a sneezing or coughing response, in contrast to nasopharyngeal specimen collection. The concern regarding sneezing and coughing in response to the procedure of nasopharyngeal specimen collection is negated when buccal swabs are done instead. Hence, the collection of buccal swabs does not require negative-pressure isolation facilities as it is not aerosol-generating.
In addition, if the virus could be detected in saliva, this suggests a potential route of viral transmission in children, especially in infants who tend to drool and place objects in their mouths. It is important to understand the viral shedding pattern in buccal specimens of children in order to predict the routes of viral transmission. We conducted a study to evaluate the presence of SARS-CoV-2 in buccal specimens in COVID-19–infected children.
METHODS
Study Design and Participants
KK Women’s and Children’s Hospital is an 830-bed hospital that provides care for approximately 500 children’s emergency daily attendances and 12 000 deliveries per year. It is the primary hospital for evaluation and isolation of COVID-19 in the pediatric population in Singapore.
From 23 March 2020 to 3 April 2020, all inpatient pediatric confirmed COVID-19 cases diagnosed via positive SARS-CoV-2 polymerase chain reaction (PCR) from nasopharyngeal swabs using the real-time reverse transcription (rRT)-PCR assay for the E gene were included in this study. In addition to daily nasopharyngeal swabs for SARS-CoV-2 PCR taken for these children, daily buccal swabs were also taken on bilateral buccal mucosa to assess for viral shedding in saliva. Buccal specimen collection was stopped if there were negative buccal SARS-CoV-2 specimens on 2 consecutive days. The cycle threshold (Ct) values of all nasopharyngeal and buccal swabs for SARS-CoV-2 for each child were obtained. The Ct values were reported in relation to the day of illness or day of diagnosis for symptomatic and asymptomatic patients, respectively.
The study was approved by the institutional ethics review board. Written informed consent was waived in light of the need to inform public health outbreak control policies.
rRT-PCR E Gene Assays
Nasopharyngeal and buccal swabs were collected using Mini UTM Kits (Copan, Brescia, Italy) with flocked swabs and 1 mL of universal transport medium. From this medium, 200 µL was used for extraction of viral nucleic acids using the EZ1 Virus Mini Kit v2.0 (Qiagen, Hilden, Germany) into 60 µL of eluate. rRT-PCR targeting the SARS-CoV-2 E gene was performed according to the method described by Corman et al [8]. All reactions were run on a QuantStudio 5 instrument (ThermoFisher Applied Biosystems, Foster City, CA). A volume of 5 µL was used in PCR assays; with conversion factors, this represented 1/60 of the swab contents per reaction. The positive control consisted of a plasmid with a SARS-CoV-2 E gene insert, adjusted to 1000 copies per reaction. During the study period, the mean Ct value of all positive control PCR assays was 29.86 and the standard deviation was ±0.68. Thus, with the conversion factor, a Ct value of 29.86 corresponded to approximately 6 × 104 virus genome copies per swab.
Statistical Analyses
Continuous variables were expressed as median (range), and the Mann-Whitney U test or T test was used for analysis. Categorical variables were expressed as percentage proportions. The Spearman rank correlation was used to analyze the association between 2 continuous variables. Statistical significance was set at P < .05. All analyses were done using the SPSS software, version 23 (IBM, Armonk, NY).
RESULTS
Eleven COVID-19–infected children were included in this study. Six (54.5%) children were asymptomatic, and 5 symptomatic children (45.5%) had a mild course of illness. The asymptomatic children remained well throughout the admission, with no subsequent development of symptoms. The median ages of asymptomatic and symptomatic children were 8.4 years (range, 2.1–12.5) and 3.8 years (range, 0.3–11.8), respectively. However, the difference was not statistically significant (P = .24).
SARS-CoV-2 was detected from at least 1 buccal specimen in 9 of 11 children (81.8%). One asymptomatic child with nasopharyngeal Ct values of 33.0 and 30.0 on days 1 and 2 of diagnosis, respectively, had undetectable buccal SARS-CoV-2. Another symptomatic child with nasopharyngeal Ct values of 26.9 and 32.6 on days 2 and 3 of illness, respectively, had undetectable buccal SARS-CoV-2.
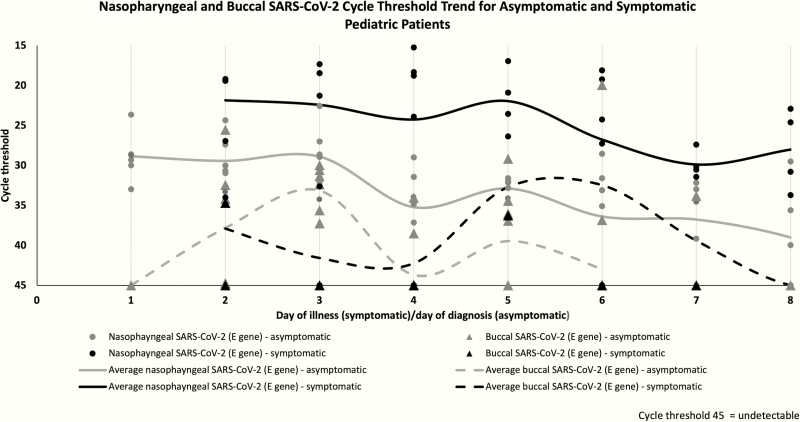
In the 9 infected children with detectable SARS-CoV-2 in buccal specimens, the mean difference of Ct values between buccal and nasopharyngeal specimens for all infected patients was 10.7 (range, 6.1–16.1), and this was statistically significant (P < .001). There was a general trend for buccal specimens to contain lower SARS-CoV-2 viral loads (higher Ct values) compared with nasopharyngeal specimens, as shown in Figure 1. The sensitivity of buccal swabs compared with nasopharyngeal swabs ranged from 25% to 71.4% on different days of collection during the first week of illness/diagnosis. Buccal SARS-CoV-2 was undetectable by day 8 of admission/diagnosis, although the nasopharyngeal SARS-CoV-2 was still detectable. There was no statistically significant association between buccal or nasopharyngeal Ct values with age (P > .05).
Figure 1.
Nasopharyngeal and buccal SARS-CoV-2 polymerase chain reaction cycle threshold trends for asymptomatic and symptomatic pediatric patients. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
Our findings confirm that SARS-CoV-2 can be detected in buccal specimens of infected children and that the viral load is the highest in the first week of illness or diagnosis. The detection of virus in buccal specimens from children suggests a high possibility that, as with adults [4–6], SARS-CoV-2 is present and potentially transmissible via the saliva of children. In our study, the average viral loads of buccal SARS-CoV-2 were consistently lower than the respective nasopharyngeal specimens, with substantial differences between the average Ct values. Some studies have shown that no growth of the virus in culture was obtained in specimens that yielded positive SARS-CoV-2 PCR results with higher Ct values [9, 10]. Thus, saliva may not be a major route of viral transmission for SARS-CoV-2.
Two COVID-19–infected children had negative buccal specimens despite detectable nasopharyngeal viral load. Although there are advantages with buccal specimen collection compared with nasopharyngeal swabs, buccal specimen collection does not appear to be a good screening modality for COVID-19 due to the reduced sensitivity and higher Ct values. In our cohort, buccal SARS-CoV-2 was undetectable by day 8 of illness/diagnosis, despite continued detection of the virus in the nasopharynx of all patients. Hence, buccal swabs similarly do not appear to be a good alternative to document viral clearance in infected children. However, in resource-limited places where isolation facilities are unavailable for nasopharyngeal specimen collection from children, buccal swabs would appear to be an alternative, albeit less sensitive, screening procedure.
Our study is limited by a small sample size of 11 children with COVID-19. No additional buccal specimens were collected after 3 April 2020; hence, we could not extend our observations beyond that time. Although our PCR assay was not set up to be quantitative, viral loads can be estimated from Ct values using the laboratory’s dilution factors and the fact that positive controls contained 1000 copies of target per reaction.
In conclusion, we confirm the detection of SARS-CoV-2 from buccal specimens of children with COVID-19. However, buccal specimens yielded substantially lower viral loads and had poor sensitivity compared with nasopharyngeal specimens. Buccal swabs for SARS-CoV-2 are not good as screening specimens for COVID-19 in children.
Notes
Acknowledgments. The authors thank the staff of the Microbiology Section, Department of Pathology and Laboratory Medicine, KK Women’s and Children’s Hospital, for their dedication and commitment in the challenging working conditions during the coronavirus 2019 pandemic.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wang W, Xu Y, Gao R, et al. . Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020. Mar 11. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (COVID-19) Updated on 14 April 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed 29 April 2020.
- 3. Qian Y, Zeng T, Wang H, et al. . Safety management of nasopharyngeal specimen collection from suspected cases of coronavirus disease 2019. Int J Nurs Sci 2020. Apr 4. doi: 10.1016/j.ijnss.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. To KK, Tsang OT, Chik-Yan Yip C, et al. . Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020. Feb 12. pii: ciaa149. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. To KK, Tsang OT, Leung WS, et al. . Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020. Mar 23. pii: S1473-3099(20)30196-1. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azzi L, Carcano G, Gianfagna F, et al. . Saliva is a reliable tool to detect SARS-CoV-2. J Infect 2020. Apr 14. pii: S0163-4453(20)30213–9. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musso D, Roche C, Nhan TX, et al. . Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–5. [DOI] [PubMed] [Google Scholar]
- 8. Corman VM, Landt O, Kaiser M, et al. . Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020. Jan; 25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020. Apr 1. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 10. Arons MM, Hatfield KM, Reddy SC, et al. . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020. Apr 24. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]