Abstract
Background
The effect of neuraminidase inhibitor (NAI) treatment on length of stay (LoS) in patients hospitalized with influenza is unclear.
Methods
We conducted a one-stage individual participant data (IPD) meta-analysis exploring the association between NAI treatment and LoS in patients hospitalized with 2009 influenza A(H1N1) virus (A[H1N1]pdm09) infection. Using mixed-effects negative binomial regression and adjusting for the propensity to receive NAI, antibiotic, and corticosteroid treatment, we calculated incidence rate ratios (IRRs) and 95% confidence intervals (CIs). Patients with a LoS of <1 day and those who died while hospitalized were excluded.
Results
We analyzed data on 18 309 patients from 70 clinical centers. After adjustment, NAI treatment initiated at hospitalization was associated with a 19% reduction in the LoS among patients with clinically suspected or laboratory-confirmed influenza A(H1N1)pdm09 infection (IRR, 0.81; 95% CI, .78–.85), compared with later or no initiation of NAI treatment. Similar statistically significant associations were seen in all clinical subgroups. NAI treatment (at any time), compared with no NAI treatment, and NAI treatment initiated <2 days after symptom onset, compared with later or no initiation of NAI treatment, showed mixed patterns of association with the LoS.
Conclusions
When patients hospitalized with influenza are treated with NAIs, treatment initiated on admission, regardless of time since symptom onset, is associated with a reduced LoS, compared with later or no initiation of treatment.
Keywords: Neuraminidase inhibitors, pandemic influenza, IPD meta-analysis, length of stay, antivirals
We found that neuraminidase inhibitor (NAI) treatment initiated on hospital admission to patients with clinically diagnosed or laboratory-confirmed A(H1N1)pdm09 virus infection was associated with a reduction in hospital length of stay when compared to later or no NAI treatment.
(See the Editorial commentary by Monto, on pages 340–2.)
Seasonal influenza epidemics and pandemics increase pressure on hospital bed capacity. Early initiation of monotherapy with neuraminidase inhibitors (NAIs) reduces illness duration in patients with uncomplicated influenza [1–3]; associated reductions in complications, hospitalizations, and mortality are supported by systematic reviews of observational data [4–8]. The evidence is less clear that NAI treatment reduces length of stay (LoS) in hospitalized patients with influenza, compared with supportive care without antiviral treatment [9–15]. Minimizing the LoS is important in managing hospital surge and limiting healthcare costs due to seasonal influenza epidemics and pandemics. We undertook a one-stage individual participant data (IPD) [16] meta-analysis to explore the association between NAI treatment of patients hospitalized with 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) infection and the LoS during the 2009–2010 influenza pandemic.
METHODS
Details regarding identification of study centers and inclusion of patients have been published previously [6]. Briefly, we requested data from multiple clinical centers worldwide on patients admitted to hospital with laboratory-confirmed or clinically diagnosed A(H1N1)pdm09 infection for whom a minimum data set was available. Of the IPD that we received, we excluded patients who had laboratory-confirmed absence of A(H1N1)pdm09 infection, retaining only patients who had laboratory-confirmed A(H1N1)pdm09 infection and patients with clinically diagnosed pandemic influenza (ie, those for whom the clinical suspicion and working diagnosis was one of pandemic influenza but laboratory confirmation was not performed) [6]. The PRIDE study protocol was registered with the PROSPERO register of systematic reviews (CRD42011001273) prior to data collection [17]. This states that the study will investigate the impact of NAI treatment on multiple outcomes of public health interest in A(H1N1)pdm09-infected patients, using mixed-effects models. After collection and standardization of the data, sufficient data existed to assess 2 indicators of “severe hospital outcomes”—requirement for ventilatory support (ie, intensive care unit [ICU] admission) and LoS. In this article, we present the findings relating to the LoS.
Data Standardization, Exposure, and Outcome
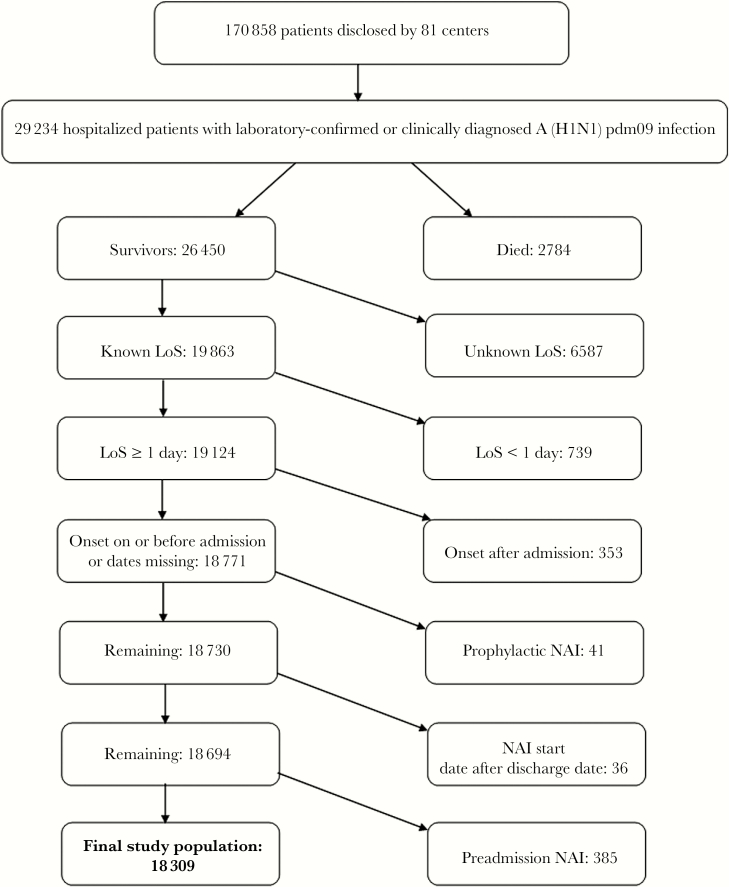
We standardized data from individual data sets before we pooled the data (Supplementary Table 1). The primary outcome was the LoS (in whole days). We excluded patients with known receipt of NAI treatment before admission, to ensure uniform potential for treatment to influence the LoS. We excluded patients with continuing postdischarge NAI treatment; patients with a LoS <1 day, on the grounds that they would have received a maximum of 2 doses of NAI inpatient treatment and that their admission may have been precautionary; and patients with nosocomial influenza (defined as influenza with symptom onset after the hospital admission date; Figure 1). Finally, since rapid deterioration and early death during hospitalization would be an adverse outcome associated with a paradoxically short LoS, those who died in the hospital were excluded from analysis.
Figure 1.
Identification of the study population. A(H1N1)pdm09, 2009 pandemic influenza A(H1N1) virus; LoS, length of stay; NAI, neuraminidase inhibitor.
The primary exposure variable was in-hospital NAI treatment received on the day of hospital admission, compared with later or no NAI treatment. Additionally, where data were available, we defined 3 further exposure variables: NAI treatment (at any time) versus no NAI treatment, early NAI treatment (initiated within ≤2 days after symptom onset) versus no NAI treatment, and early NAI treatment versus later treatment (initiated >2 days after symptom onset).
Propensity Scores
We derived propensity scores via multivariable logistic regression for each exposure variable, as described by Hirano and Imbens [18], separately for individual study centers, based on patient characteristics recorded on admission. Propensity score derivation models included, a priori, the following variables: age, sex, comorbidity (yes/no), and an indicator of disease severity, plus any additional covariates (ie, obesity, smoking, pregnancy, asthma, chronic obstructive pulmonary disease, lung disease, heart disease, immunosuppression, neurological disease, renal disease, and/or diabetes) that remained statistically significant in a regression model. Variables for which data from >25% of participants were missing were excluded from propensity score derivation.
Statistical Analysis
To investigate the impact of NAI treatment on the LoS, we performed a one-stage IPD meta-analysis using a mixed-effects negative binomial regression model, including study center as a random intercept to account for clustering. A negative binomial model was chosen to account for overdispersion in the LoS data (as represented in Supplementary Figure 1). We tested a zero-inflated negative binomial regression model on a subgroup of the data and found that the model fit was inferior to that of a negative binomial regression model.
In our primary analysis, we aimed to quantify the potential benefits of a pragmatic treat-on-admission policy (irrespective of the time elapsed since symptom onset), compared with patients who received no NAI treatment and those whose treatment was delayed until after the day of admission. By way of sensitivity analysis, we restricted the comparator group to patients who did not receive NAI treatment at any point. For both analyses, we adjusted for propensity score quintile, in-hospital antibiotic treatment, in-hospital corticosteroid treatment, and the delay between symptom onset and hospital admission. In addition, we performed secondary analyses for the following exposures: NAI treatment (at any time) versus no NAI treatment, early NAI treatment (≤48 hours after symptom onset) versus later NAI treatment (>48 hours after symptom onset), and early NAI treatment versus no NAI treatment, adjusting for propensity score, in-hospital antibiotic treatment, and corticosteroid treatment.
We performed a priori–specified analyses for the following subgroups: patients with laboratory-confirmed A(H1N1)pdm09 infection, children (age, <16 years), elderly patients (age, ≥65 years), patients with chest radiography–confirmed influenza-related pneumonia (IRP), and patients with confirmed absence of IRP. We looked at pregnant women and obese patients as post hoc subgroups. Furthermore, we investigated, by stratification, the impact of NAI treatment on the total LoS in patients admitted to critical care facilities (ie, ICUs) at any point and patients treated exclusively by using standard ward-based care.
Both unadjusted and adjusted models were run, and results are presented as unadjusted incidence rate ratios (IRRs) or adjusted IRRs (aIRRs) with 95% confidence intervals (CIs). Missing data in the covariates were included in the analysis as dummy variable categories. Using aIRR point estimates, we determined the difference in the LoS (in days) between a treated patient and an untreated patient with similar characteristics by scaling the model prediction for LoS without treatment by (aIRR-1). Repeating this for all patients in our data set gave us a distribution of expected changes in the LoS due to treatment (with timing as defined for each regression analysis). This does not account for error in the estimates of model covariates, which would require a Bayesian approach; however, it offers a clinically relevant interpretation of aIRRs. The statistical analyses were performed using Stata (version 14.2; StataCorp, College Station, TX).
RESULTS
We identified 29 234 patients admitted to the hospital between 2 January 2009 and 14 March 2011 with laboratory-confirmed or clinically diagnosed A(H1N1)pdm09 infection [4]. The analysis population included 18 309 patients (62.6%; Figure 1).
The included patients came from 70 clinical centers in 36 countries across all 6 World Health Organization regions. The Americas contributed the most data (46.2% of patients), followed by Europe (for 33.3%). The country that contributed the most to the pooled data set was Mexico (28.8% of patients), followed by Spain (8.6%), the United States (7.6%), and the United Kingdom (7.5%). Among patients in the final study population, 67.4% were adults, and 81.1% had laboratory-confirmed A(H1N1)pdm09 infection; general characteristics of the included population are further described in Table 1.
Table 1.
General Characteristics of the Study Population
| Characteristic | Overall | No NAI Treatment | In-Hospital NAI Treatment |
|---|---|---|---|
| Patients | 18 309/18 309 (100) | 6075/18 309 (33.2) | 12 234/18 309 (66.8) |
| Male sex | 9114/18 306 (49.8) | 2852 (47) | 6262 (51.2) |
| Age | |||
| Overall (n = 18 238) | 26 (10–44) | 24 (6–41) | 27 (12–46) |
| ≥16 y (adults) | 12 331/18 238 (67.4) | 3686 (60.8) | 8645 (70.7) |
| <16 y (children) | 5907/18 238 (32.3) | 2344 (38.6) | 3563 (29.1) |
| ≥65 y (elderly individuals) | 1035/18 238 (5.7) | 304 (5) | 731 (6) |
| Obese | 1677/13 695 (12.3) | 475 (8.9) | 1202 (14.4) |
| Smoker | 1728/12 851 (13.5) | 429 (8.2) | 1299 (17.1) |
| Pregnanta | 1197/5318 (22.5) | 380 (21.1) | 817 (23.2) |
| WHO region | |||
| African Region | 23/18 309 (0.1) | 0/6075 (0) | 23/12 234 (0.19) |
| Region of the Americas | 8466/18 309 (46.2) | 4606/6075 (75.8) | 3860/12 234 (31.6) |
| Eastern Mediterranean Region | 1649/18 309 (9) | 41/6075 (0.7) | 1608/12 234 (13.1) |
| European Region | 6090/18 309 (33.3) | 918/6075 (15.1) | 5172/12 234 (42.3) |
| South-East Asia Region | 180/18 309 (1) | 107/6075 (1.8) | 73/12 234 (0.6) |
| Western Pacific Region | 1901/18 309 (10.3) | 403/6075 (6.6) | 1498/12 234 (12.2) |
| A(H1N1)pdm09 infection diagnosis | |||
| Laboratory confirmed | 14 844/18 309 (81.1) | 3588/6075 (59.1) | 11 256/12 234 (92) |
| Clinically diagnosed | 3465/18 309 (18.9) | 2487/6075 (40.9) | 978/12 234 (8) |
| Comorbidity | |||
| Any | 7017/18 282 (38.4) | 1749 (28.8) | 5268 (43.2) |
| Asthma | 2461/16 625 (14.8) | 607 (10.2) | 1854 (17.4) |
| COPD | 792/13 812 (5.7) | 187 (3.6) | 605 (7.1) |
| Other chronic lung disease | 1393/9800 (14.2) | 190 (12.9) | 1203 (14.5) |
| Heart disease | 1030/12 146 (8.5) | 140 (8.2) | 890 (8.5) |
| Renal disease | 401/11 373 (3.5) | 44 (3.1) | 357 (3.6) |
| Liver disease | 187/9564 (2) | 24 (1.7) | 163 (2) |
| Cerebrovascular disease | 239/7751 (3.1) | 32 (3.2) | 207 (3.1) |
| Neurological disease | 743/8929 (8.3) | 105 (7) | 638 (8.6) |
| Diabetes | 1375/17 377 (7.9) | 418 (7.3) | 957 (8.2) |
| Immunosuppression | 1051/17 180 (6.1) | 245 (4.3) | 806 (7) |
| Chest radiography for influenza-related pneumonia | |||
| Confirmed presence | 4591/7611 (60.3) | 426 (46.1) | 4165 (62.3) |
| Confirmed absence | 3020/7611 (39.7) | 498 (53.9) | 2522 (37.7) |
| A(H1N1)pdm09 vaccination | 292/5371 (5.4) | 33 (4.7) | 259 (5.5) |
| Time from symptom onset to hospital admission, d (n = 16 736) | 2 (1–5) | 2 (1–5) | 2 (1–5) |
| NAI used | |||
| None | 6075/18 309 (33.2) | 6075/6075 (100) | … |
| Any | 12 234/18 309 (66.8) | … | 12 234/12 234 (100) |
| Treated with oral oseltamivir | 11 082/12 234 (90.6) | … | 11 082 (98.8) |
| Treated with intravenous/inhaled zanamivir | 295/12 234 (2.4) | … | 295 (4.3) |
| Treated with intravenous peramivir | 13/12 234 (0.1) | … | 13 (0.2) |
| Early NAI initiation (≤2 d after symptom onset) | 3678/8621 (42.7) | … | 3678/8621 (42.7) |
| Later NAI initiation (>2 d after symptom onset) | 4943/8621 (57.3) | … | 4943/8621 (57.3) |
| Time from symptom onset to antiviral treatment, d (n = 7433) | 3 (2–5) | … | 3 (2–5) |
| Treated with any NAI on day of hospital admission | 4816/12 234 (39.4) | … | 4816/12 234 (39.4) |
| Treated with antibiotics | 9153/14 599 (62.7) | 2981 (52.2) | 6172 (69.5) |
| Treated with corticosteroids | 2024/8075 (25.1) | 165 (15.3) | 1859 (26.6) |
| Hospital LoS, db (n = 18 309) | 5 (3–9) | 4 (2–6) | 6 (3–10) |
| Admitted to critical care unit | 4243/17 348 (24.5) | 411 (6.9) | 3832 (33.7) |
Data are proportion (%) of patients or median value (interquartile range).
Abbreviations: A(H1N1)pdm09, 2009 pandemic influenza A(H1N1) virus; COPD, chronic obstructive pulmonary disease; LoS, length of stay; NAI, neuraminidase inhibitor; WHO, World Health Organization.
aProportions were calculated as a percentage of pregnant patients among female patients of reproductive age (13–54 years). The broader age range was selected in preference to the WHO definition (age, 15–44 years) after consultation with data contributors, to reflect the actual fertility experience of the sample. This also includes data from a hospital obstetrics unit (n = 72)
cThe LoS in the NAI-treated group is the overall LoS in this group. Precise NAI administration dates were not uniformly available to work out the LoS after NAI administration in the NAI-treated group.
Among the 8621 patients (47.1%) for whom data on the timing of NAI treatment were available, 3678 (42.7%) received early NAI treatment, and 4816 (55.9%) initiated treatment on the day of admission. The median delay from illness onset to hospital admission was 2 days (interquartile range [IQR], 1–5 days), and among patients with data on the timing of treatment, 42.7% presented ≤48 hours after symptom onset; the median LoS was 5 days (IQR, 3–9 days; Supplementary Figure 1). In patients whose NAI treatment was initiated on the day of hospital admission, the median interval between symptom onset and admission was 2 days (IQR, 1–4 days).
Impact of NAI Treatment on LoS
In our primary analysis, we observed that NAI treatment started on the day of admission was associated with a 19% overall reduction in the LoS (aIRR, 0.81 [95% CI, .78–.85]; median decrease, 1.19 days [IQR, 0.85–1.55 days]), compared with no or later initiation of NAI treatment. This association was of similar magnitude and remained significant in all subgroups (Table 2 and Supplementary Table 3).
Table 2.
Results From Mixed-Effects Negative Binomial Regression Analyses
| Variable | Unadjusted, IRR (95% CI) | Adjusted,a IRR (95% CI) |
|---|---|---|
| Primary analysis: NAI treatment on day of hospital admission vs later/no NAI treatmentb | ||
| Overall | 0.83 (.79–.87)c | 0.81 (.78–.85)c |
| Laboratory-confirmed A(H1N1)pdm09 infection | 0.83 (.79–.86)c | 0.81 (.77–.85)c |
| Children (age <16 y) | 0.90 (.83–.97)c | 0.85 (.78–.92)c |
| Elderly (age ≥65 y) | 0.78 (.67–.91)c | 0.78 (.67–.91)c |
| Patients requiring standard ward-based care only | 0.81 (.77–.85)c | 0.81 (.78–.86)c |
| ICU-admitted patients onlyd | 0.80 (.73–.88)c | 0.79 (.72–.87)c |
| Confirmed absence of influenza-related pneumonia | 0.71 (.66–.77)c | 0.73 (.68–.79)c |
| Confirmed presence of influenza-related pneumonia | 0.91 (.84–.98)c | 0.85 (.79–.93)c |
| Sensitivity analysis: NAI treatment on day of hospital admission vs no NAI treatmentb | ||
| Overall | 1.14 (1.07–1.22)c | 1.06 (.99–1.13) |
| Laboratory-confirmed A(H1N1)pdm09 infection | 1.15 (1.07–1.22)c | 1.04 (.97–1.12) |
| Children (age <16 y) | 1.09 (.98–1.20) | 0.98 (.88–1.09) |
| Elderly (age ≥65 y) | 0.84 (.67–1.06) | 0.83 (.65–1.07) |
| Patients requiring standard ward-based care only | 0.93 (.87–.99)c | 0.92 (.85–.98)c |
| ICU-admitted patients onlyd | 1.14 (.96–1.36) | 1.08 (.90–1.31) |
| Confirmed absence of influenza-related pneumonia | 0.83 (.75–.92)c | 0.81 (.73–.90)c |
| Confirmed presence of influenza-related pneumonia | 1.28 (1.12–1.47)c | 1.28 (1.11–1.48)c |
| Secondary analyses | ||
| NAI anytime vs no NAI treatment | ||
| Overall | 1.21 (1.17–1.26)c | 1.11 (1.07–1.16)c |
| Laboratory-confirmed A(H1N1)pdm09 infection | 1.31 (1.25–1.37)c | 1.17 (1.12–1.23)c |
| Children (age <16 y) | 1.18 (1.11–1.25)c | 1.11 (1.04–1.18)c |
| Elderly patients (age ≥65 y) | 1.00 (.86–1.17) | 0.98 (.83–1.14) |
| Patients requiring standard ward-based care only | 1.06 (1.02–1.10)c | 1.02 (.98–1.05) |
| ICU-admitted patients onlyd | 1.33 (1.19–1.49)c | 1.26 (1.13–1.41)c |
| Confirmed absence of influenza-related pneumonia | 0.98 (.90–1.07) | 0.97 (.89–1.06) |
| Confirmed presence of influenza-related pneumonia | 1.36 (1.24–1.49)c | 1.28 (1.16–1.40)c |
| Early NAI treatment vs later NAI treatment | ||
| Overall | 0.70 (.68–.73)c | 0.77 (.74–.80)c |
| Laboratory-confirmed A(H1N1)pdm09 infection | 0.70 (.68–.73)c | 0.77 (.74–.80)c |
| Children (age <16 y) | 0.80 (.74–.86)c | 0.87 (.81–.93)c |
| Elderly patients (age ≥65 y) | 0.71 (.62–.81)c | 0.71 (.62–.82)c |
| Patients requiring standard ward-based care only | 0.78 (.75–.81)c | 0.83 (.79–.86)c |
| ICU-admitted patients onlyd | 0.69 (.64–.74)c | 0.74 (.69–.80)c |
| Confirmed absence of influenza-related pneumonia | 0.80 (.75–.86)c | 0.84 (.78–.90)c |
| Confirmed presence of influenza-related pneumonia | 0.84 (.78–.90)c | 0.82 (.77–.88)c |
| Early NAI treatment vs no NAI treatment | ||
| Overall | 1.04 (.98–1.11) | 0.93 (.87–.99)c |
| Laboratory-confirmed A(H1N1)pdm09 infection | 1.05 (.98–1.11) | 0.93 (.87–.99)c |
| Children (age <16 y) | 1.00 (.91–1.10) | 0.92 (.83–1.01) |
| Elderly patients (age ≥65 y) | 0.82 (.67–1.01) | 0.79 (.63–.997)c |
| Patients requiring standard ward-based care only | 0.93 (.87–.99)c | 0.88 (.82–.94)c |
| ICU-admitted patients onlyd | 1.01 (.86–1.20) | 0.93 (.79–1.10) |
| Confirmed absence of influenza-related pneumonia | 0.79 (.71–.89)c | 0.76 (.68–.85)c |
| Confirmed presence of influenza-related pneumonia | 1.09 (.95–1.24) | 1.01 (.88–1.16) |
Abbreviations: A(H1N1)pdm09, 2009 pandemic influenza A(H1N1) virus; CI, confidence interval; IRR, incidence rate ratio; NAI, neuraminidase inhibitor.
aAdjusted for propensity scores (quintiles) for receiving treatment, antibiotic treatment received in the hospital, and steroid treatment received in the hospital.
bThe IRR was further adjusted for time from onset to admission.
cStatistically significant (P < .05).
dData are for patients admitted to the ICU at any point. The IRR was calculated for the total length of hospital stay, not time in the ICU. Our sensitivity analyses and secondary analyses must be interpreted with caution because they may be affected by various time-dependent biases
In the sensitivity analysis, we observed that NAI treatment on the day of hospital admission was associated with an 8% reduction in the LoS among patients not admitted to the ICU (aIRR, 0.92 [95% CI, .85–.98; median decrease, 0.50 days [IQR, 0.43–0.57 days]), a 19% reduction among patients with confirmed absence of IRP (aIRR, 0.81 [95% CI, .73–.90]; median decrease, 1.24 days [IQR, .93–1.38 days]), but a 28% increase among patients with confirmed presence of IRP (aIRR, 1.28 [95% CI, 1.11–1.48]; median increase, 1.73 days [IQR, 1.29–2.07 days]), compared with no NAI treatment.
Secondary Analyses
After adjustment, NAI treatment at any time was associated with an 11% overall increase in the LoS (aIRR, 1.11 [95% CI, 1.07–1.16]; median increase, 0.74 days [IQR, 0.60–1.05 days]), compared with no NAI treatment. By exploring subgroups, we identified corresponding statistically significant findings in patients with laboratory-confirmed A(H1N1)pdm09 infection, children, patients admitted to the ICU, and patients with confirmed IRP but not in the elderly, patients requiring non-ICU care, or patients with confirmed absence of IRP (Table 2). We did not find any evidence of effect modification by pandemic influenza vaccination (P = .68) or by in-hospital antibiotic treatment (P = .20); however, a borderline significant effect modification was observed for in-hospital corticosteroid treatment (P = .05), with NAI treatment plus corticosteroid treatment associated with a marginally increased LoS (aIRR, 1.17 days; 95% CI, 1.00–1.36).
In contrast, early NAI treatment was associated with a 7% overall reduction in the LoS (aIRR, 0.93 [95% CI, .87–.99]; median decrease, 0.40 days [IQR, 0.36–0.45 days]), compared with no NAI treatment. Similar or larger reductions were observed in most subgroups; however, this association was not statistically significant in children, patients admitted to the ICU, and patients with confirmed IRP (Table 2). Early NAI treatment was associated with an 23% overall reduction in the LoS (aIRR, 0.77 [95% CI, .74–.80]); median decrease, 1.78 days [IQR, 1.34–2.49 days]), compared with later NAI treatment; the reduction varied across all a priori–specified subgroups but remained statistically significant (Table 2).
In subgroups of pregnant women and obese patients, early NAI treatment was associated with statistically significant reductions of 39% (aIRR, 0.61 [95% CI, .52–.70]; median decrease, 3.10 days [IQR, 2.34–4.56 days]) and 27% (aIRR, 0.73 [95% CI, .65–.83]; median decrease, 2.11 days [IQR, 1.62–3.10 days]) in the LoS, respectively, compared with later NAI treatment. NAI treatment at any time and early NAI treatment were not statistically significantly associated with LoS, compared with no NAI treatment (Supplementary Table 3).
DISCUSSION
Our study extends the existing literature by offering data on the association between NAI treatment and the LoS in >18 000 adult and pediatric patients, of whom >80% had a laboratory-confirmed diagnosis of A(H1N1)pdm09 infection. We found a mixed pattern of association between NAI treatment and LoS, depending on the delay to initiation of treatment, age, and case severity.
The most pragmatic and important question is whether NAI treatment, started on admission, irrespective of delay since symptom onset, reduces the LoS in hospitalized patients with influenza. Clinically, this is important because there can be significant uncertainty in ascertaining symptom onset, even by the attending physician. The uncertainty in ascertaining symptom onset could mean prescribing NAI treatment outside the recommended (licensed) window of ≤48 hours after symptom onset. However, there is evidence pointing to the effectiveness of NAI therapy, albeit reduced, even when given >48 hours after symptom onset [6]. Statistically, by defining our exposure variable on the basis of treatment decisions made on admission, we avoided introducing correlations between exposure and LoS that can lead to survivorship bias in linear regression models of time-to-event data [19, 20]. Additionally, this approach ensures that the propensity scores, modeled on symptom severity at admission, should appropriately correct for treatment bias [21]. However, this choice of exposure variable also reflects the clinical reality that patients present to the hospital at varying intervals after symptom onset (ranging from 0–20 days in our study) and that clinicians and policy makers want to know whether a so-called treat-at-the-door policy applied to patients admitted to the hospital with clinically recognized influenza will be beneficial, compared with no NAI treatment or a watch-and-see approach. This was addressed by our primary analysis, which revealed that initiation of NAI treatment on the day of admission was associated with a 19% reduction in the LoS (median decrease, 1.19 days), compared with later or no treatment, with similar statistically significant findings across all patient subgroups including children, pregnant women, and obese patients. These findings emphasize the importance of presumptive NAI treatment in patients admitted to the hospital with suspected influenza, coupled with early diagnosis using standard laboratory or rapid diagnostic tests.
In our sensitivity analysis, we found a significant reduction of 19% in the LoS (median decrease, 1.24 days) among patients with confirmed absence of IRP and a reduction of 8% (median decrease, 0.5 days) among patients who required supportive ward-based care. In contrast, NAI treatment (compared with no treatment) was associated with a 28% increase in the LoS (median increase, 1.73 days) among patients with IRP. These data suggest that NAIs may be more effective in reducing the LOS when patients do not have IRP and are consistent with the fact that NAIs have no known antibacterial properties.
In secondary analyses, we observed an 11% overall increase in the LoS associated with NAI treatment, equivalent to a median increase of about 0.74 days and irrespective of the time between symptom onset and initiation of therapy. Compared with no treatment, NAI treatment initiated within 48 hours after symptom onset was associated with a 7% overall reduction in the LoS, equivalent to a median decrease of 0.40 days; this effect was not observed in children and patients requiring ICU care. This finding is clinically important because it suggests that rapid access to antiviral treatment after symptom onset may influence the LoS in adults and elderly individuals; nevertheless, we did not observe the same result among patients requiring ICU care. Our results in children may be influenced by a higher A(H1N1)pdm09 viral load in children [22] than in adults, leading to prolonged hospital stay, suboptimal dosing in very young children [23], increased likelihood of antiviral resistance emergence in children [24], secondary bacterial infections, confounding by indication related to baseline illness severity [25], or a combination of these factors. Although we attempted to adjust for influenza severity by using propensity scores, we found ICU care to be very strongly associated with a prolonged LoS (IRR, 2.96; 95% CI, 2.84–3.09) and NAI treatment to be associated with a higher likelihood of requiring ICU care (adjusted odds ratio, 3.11; 95% CI, 2.42–3.98). Furthermore, we found that patients who presented to the hospital >2 days after symptom onset were 73% more likely to eventually require ICU care than patients who presented earlier (odds ratio, 1.73; 95% CI, 1.53–1.95). In addition, patients requiring ICU care have frequently developed extrapulmonary manifestations of influenza and multiorgan decompensation; therefore, inhibition of virus replication may not correspond with rapid clinical recovery.
We noted no association between NAI treatment and LoS among hospitalized children with influenza when considering early treatment versus no treatment. The study may have been underpowered in children, but other factors might have contributed to our findings. The LoS is typically shorter among children, compared with adults; mortality and serious outcomes are less common among hospitalized children with influenza, compared with adults; and different discharge policies and thresholds for children could also influence the findings. In addition, vomiting is a recognized side effect of oseltamivir in children [3], and this may have prevented discharge in some cases.
Previous studies examining whether use of NAIs in patients hospitalized with influenza affects the LoS have generally been of smaller size (<1300 individuals) as compared to our study and reached variable conclusions. Of note, 8 studies [11–15, 26–28] (of which one [12] was a randomized trial) assessed NAI treatment of hospitalized children, but only 2 (both with an observational design) concluded that the total number of hospital days in the NAI-treated hospital cohort was reduced (by 18% [8.3 days]) [11, 28], with the other 6 reporting no differences [12–15, 26, 27]. Only 4 studies have addressed the same question in adults. In Hong Kong, a study of 356 adult patients hospitalized with laboratory-confirmed seasonal influenza showed that early oseltamivir treatment was associated with a reduced LoS in both unadjusted and multivariable analyses [9], compared with no or later treatment, with the median LoS decreasing from 6 to 4 days; this accords with our primary analysis. A Canadian study of adult patients with seasonal influenza found that oseltamivir treatment was not associated with the LoS among surviving patients [29]. A further study in 13 Spanish hospitals involving 538 patients with laboratory-confirmed A(H1N1)pdm09 infection noted that the LoS increased by 7% (odds ratio, 1.07), after adjustment for confounders, if NAI treatment was instigated <48 hours after symptom onset; however, this was of borderline statistical significance [10]. A recent American study analyzed data on 201 adult patients with laboratory-confirmed seasonal influenza, reporting that NAI treatment was not associated with the LoS overall but was associated with a reduced LoS among vaccinated individuals (hazard ratio of discharge, 1.6; 95% CI, 1.0–2.4; P = .04) [30]. Finally, 2 studies included patients of all age groups. One of them, performed in 813 hospitalized patients with A(H1N1)pdm09 infection in Spain, found that early NAI treatment reduced the LoS by 1.9 days (P = <.001) [31]. The other, an American study using insurance claims data from patients with seasonal influenza, reported that patients treated with NAIs spent fewer days in the hospital (P = <.0001) [32].
This study has a number of strengths and weaknesses. We combined data from geographically diverse centers, offering broad generalizability of our findings. We used propensity scores to adjust for major confounders. By excluding patients who died (10%), we removed the paradoxical possibility that a short LoS (a positive outcome in our analyses) was associated with an extremely unfavorable clinical outcome. However, a limitation of this approach is that it does not explain the impact of NAI treatment on the relationship between LoS and in-hospital mortality. In our primary analysis, we adjusted for the delay between illness onset and admission, to address length bias [20], and chose our exposure variable to avoid time-dependent/survivorship bias [19, 21]. However, our secondary analyses, which used time since onset to define the exposure variable, are subject to time-dependent biases and must therefore be interpreted with caution. Indeed, the benefit of early versus late treatment (Table 2) will be partially driven by this bias [19]. All of our analyses may be subject to residual competing risk bias, which was not removed through adjustment; for example, we found a significant difference between propensity scores to receive NAIs in the hospital for surviving and nonsurviving patients in the data set (P < .05, by the Kruskal-Wallis test), signaling that our removal of nonsurviving patients altered the aggregate presenting patient characteristics for which our results hold.
Our data, generated during the 2009–2010 influenza pandemic, contained relatively few elderly patients and children, consistent with patterns of A(H1N1)pdm09 infection [33], and differs in profile from seasonal influenza A(H3N2) virus infection, for which patients admitted to the hospital tend to be much older and to have a median LoS higher than the LoS of 5 days we observed [34, 35]. In addition, the prevalence proportions of clinically recorded obesity (12%) and pregnancy (23%) were both comparatively high.
Optimally, clinicians wish to treat patients with influenza within 48 hours after symptom onset, yet in many cases patients with influenza do not seek medical care during this therapeutic window. Our data show that 57.3% of included patients were hospitalized >48 hours after symptom onset. What then matters is whether initiation of treatment upon hospitalization (on the day of admission), irrespective of the time elapsed since symptom onset, is effective and whether this is preferable to nontreatment or further delays in treatment. We revealed a 19% reduction in the LoS (median decrease, 1.19 days) among patients who were treated with an NAI upon admission, compared with those who received no or later treatment; the trend was observed across all subgroups, including children. This treatment approach would avoid the uncertainties associated with ascertaining the symptom onset date.
Our data support current recommendations to treat adults hospitalized with clinically suspected influenza with NAIs as soon as possible upon admission; furthermore, this approach appears to be superior to no treatment or delayed treatment in terms of a reduced LoS. If used consistently, this strategy would contribute to the management of surge pressures and healthcare costs during seasonal influenza epidemics and pandemics.
STUDY GROUP MEMBERS
The PRIDE consortium investigators are as follows (affiliations are listed in Supplementary Table 4): Nisreen Amayiri, Robed Amin, Clarissa Baez, Carlos Bantar, Jing Bao, Mazen Mahmoud Barhoush, Ariful Basher, Julie Bettinger, Emilio Bouza, Ilkay Bozkurt, Elvira Čeljuska-Tošev, Kenny KC Chan, Yusheng Chen, Rebecca Cox, Maria R Cuezzo, Wei Cui, Simin Dashti-Khavidaki, Bin Du, Hicham El Rhaffouli, Hernan Escobar, Agnieszka Florek-Michalska, John Gerrard, Stuart Gormley, Sandra Götberg, Matthias Hoffmann, Behnam Honarvar, Edgar Bautista, Amr Kandeel, Jianmin Hu, Christoph Kemen, Gulam Khandaker, Marian Knight, Evelyn S C Koay, Miroslav Kojic, Koichiro Kudo, Arthur Kwan, Idriss Lahlou Amine, Win Mar Kyaw, Leonard Leibovici, Hongru Li, Xiao-Li Li, Pei Liu, Tze Ping Loh, Deborough Macbeth, Magdalena Marczyńska, Fabiane Pinto Mastalir, Allison McGeer, Mohsen Moghadami, Lilian Moriconi, Pagbajabyn Nymadawa, Bulent Ozbay, Fernando P Polack, Philippe Guillaume Poliquin, Wolfgang Pöppl, Alberto Rascon Pacheco, Blaž Pečavar, Mahmudur Rahman, Elena B Sarrouf, Brunhilde Schweiger, Fang Gao Smith, Antoni Torres, Selda Hancerli Torun, C B Tripathi, Daiva Velyvyte, Diego F. Viasus, Qin Yu, Kwok-Yung Yuen, Wei Zhang, and Wei Zuo.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Jerome Tokars and Dr Alicia Fry (Centers for Disease Control and Prevention, Atlanta, GA) for their comments on the manuscript.
J. S. N.-V.-T., P. R. M., S. V., and S. G. M. conceived and designed the study. All authors, apart from S. V., K. J. B., and S. G. M., contributed to the acquisition and local preparation of constituent data sets. S. V., P. R. M., K. J. B., and S. G. M. contributed to data set amalgamation and standardization, design of statistical analyses, and data analysis. J. S. N.-V.-T., P. R. M., K. J. B., and S. V. interpreted the data and wrote the manuscript. All authors contributed to critical examination of the manuscript for important intellectual content and approval of the final report. Each author acted as the guarantor of data from their individual study center. S. V. had full access to the pooled data set in the study and takes responsibility for the accuracy of the data analysis. J. S. N.-V.-T. acts as overall guarantor of the manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the UK Government or the United States Centers for Disease Control and Prevention. The funder has had no role in protocol design, no opportunity to comment on it, and no opportunity to see it other than via the PROSPERO website; no access to any data (and no rights to future access); no role in analysis or interpretation; no opportunity to preview results/findings before entry into the public domain; and no opportunity to contribute to, preview, or comment on manuscripts and presentations arising from this work. The research contract between the University of Nottingham and the Funder is freely available for inspection (with commercial details redacted) at: http://www.nottingham.ac.uk/research/groups/healthprotection/projects/pride.aspx. No data were provided or funded for collection by pharmaceutical companies.
Financial support. This work was supported by F. Hoffmann-La Roche (unrestricted educational grant to the PRIDE study).
Potential conflicts of interest. C. C. is a recipient of a PERIS (Strategic Plan for Research and Innovation in Health 2016–2020) postdoctoral grant. G. M. reports grants and other funding from Pfizer, MSD, and Gilead outside the submitted work. K. G. I. M. reports personal fees from Sanofi-Aventis Norway outside the submitted work. D. T. reports grants from the Canadian Institutes of Health Research/SickKids Foundation (new investigator grant XG08-049R), the Canadian Institutes of Health Research (Catalyst Grant CAT86860), and the University of Toronto Dean’s Fund (Pilot Study Grant) during the conduct of the study. W. V. reports grants from Canadian Pediatric Society during the conduct of the study. K. J. B. reports funding from the University of Nottingham (Anne McLaren Fellowship). J. S. N.-V.-T. reports grants from F. Hoffmann–La Roche for the conduct of this study and personal fees from Shionogi (in 2016) outside the submitted work; he is currently on secondment to the Department of Health and Social Care, England. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Sixth European Scientific Working Group on Influenza Conference, Riga, Latvia, 10–13 September 2017.
Contributor Information
PRIDE Consortium Investigators:
Nisreen Amayiri, Robed Amin, Clarissa Baez, Carlos Bantar, Jing Bao, Mazen Mahmoud Barhoush, Ariful Basher, Julie Bettinger, Emilio Bouza, Ilkay Bozkurt, Elvira Čeljuska-Tošev, Kenny K C Chan, Yusheng Chen, Rebecca Cox, Maria R Cuezzo, Wei Cui, Simin Dashti-Khavidaki, Bin Du, Hicham El Rhaffouli, Hernan Escobar, Agnieszka Florek-Michalska, John Gerrard, Stuart Gormley, Sandra Götberg, Matthias Hoffmann, Behnam Honarvar, Edgar Bautista, Amr Kandeel, Jianmin Hu, Christoph Kemen, Gulam Khandaker, Marian Knight, Evelyn S C Koay, Miroslav Kojic, Koichiro Kudo, Arthur Kwan, Idriss Lahlou Amine, Win Mar Kyaw, Leonard Leibovici, Hongru Li, Xiao-Li Li, Pei Liu, Tze Ping Loh, Deborough Macbeth, Magdalena Marczyńska, Fabiane Pinto Mastalir, Allison McGeer, Mohsen Moghadami, Lilian Moriconi, Pagbajabyn Nymadawa, Bulent Ozbay, Fernando P Polack, Philippe Guillaume Poliquin, Wolfgang Pöppl, Alberto Rascon Pacheco, Blaž Pečavar, Mahmudur Rahman, Elena B Sarrouf, Brunhilde Schweiger, Fang Gao Smith, Antoni Torres, Selda Hancerli Torun, C B Tripathi, Daiva Velyvyte, Diego F Viasus, Qin Yu, Kwok-Yung Yuen, Wei Zhang, and Wei Zuo
References
- 1. Jefferson T, Jones MA, Doshi P, et al. . Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2012. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008965.pub3/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385:1729–37. [DOI] [PubMed] [Google Scholar]
- 3. Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66:1492–500. [DOI] [PubMed] [Google Scholar]
- 4. Hsu J, Santesso N, Mustafa R, et al. . Antivirals for Treatment of Influenza. Ann Intern Med 2012; 156:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muthuri SG, Myles PR, Venkatesan S, Leonardi-Bee J, Nguyen-Van-Tam JS. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 Influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis 2013; 207:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muthuri SG, Venkatesan S, Myles PR, et al. ; PRIDE Consortium Investigators Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venkatesan S, Myles PR, Leonardi-Bee J, et al. . Impact of Outpatient Neuraminidase Inhibitor Treatment in Patients Infected With Influenza A(H1N1)pdm09 at High Risk of Hospitalization: An Individual Participant Data Metaanalysis. Clin Infect Dis 2017; 64:1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipsitch M, Hernán MA. Oseltamivir effect on antibiotic-treated lower respiratory tract complications in virologically positive randomized trial participants. Clin Infect Dis 2013; 57:1368–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee N, Chan PK, Choi KW, et al. . Factors associated with early hospital discharge of adult influenza patients. Antivir Ther 2007; 12:501–8. [PubMed] [Google Scholar]
- 10. Viasus D, Paño-Pardo JR, Pachón J, et al. . Timing of oseltamivir administration and outcomes in hospitalized adults with pandemic 2009 influenza A (H1N1) virus infection. Chest 2011; 140:1025–32. [DOI] [PubMed] [Google Scholar]
- 11. Coffin SE, Leckerman K, Keren R, Hall M, Localio R, Zaoutis TE. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J 2011; 30:962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawood FS, Jara J, Gonzalez R, et al. . A randomized, double-blind, placebo-controlled trial evaluating the safety of early oseltamivir treatment among children 0-9 years of age hospitalized with influenza in El Salvador and Panama. Antiviral Res 2016; 133:85–94. [DOI] [PubMed] [Google Scholar]
- 13. Youn SE, Chun JH, Lee KS, Rha YH, Choi SH. Clinical characteristics of Influenza B virus in children and the efficacy of oseltamivir: data from two university hospitals. Korean J Pediatr Infect Dis 2014; 21:199–206. [Google Scholar]
- 14. Bueno M, Calvo C, Méndez-Echevarría A, et al. . Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr Infect Dis J 2013; 32:1066–9. [DOI] [PubMed] [Google Scholar]
- 15. Khandaker G, Zurynski Y, Lester-Smith D, et al. . Clinical features, oseltamivir treatment and outcome in infants aged< 12 months with laboratory-confirmed influenza A in 2009. Antivir Ther 2011; 16:1005. [DOI] [PubMed] [Google Scholar]
- 16. Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340:c221. [DOI] [PubMed] [Google Scholar]
- 17. Myles P, Leonardi-Bee J, Van-Tam J, Muthuri S, Venkatesan S. A systematic review of the impact of neuraminidase inhibitor antiviral use on outcomes of public health importance during the 2009/10 (swine) influenza A/H1N1v pandemic http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001273. Accessed 20 May 2017.
- 18. Hirano K, Imbens GW.. The propensity score with continuous treatments. applied bayesian modeling and causal inference from incomplete-data perspectives. West Sussex: John Wiley and Sons, 2005:73–84. [Google Scholar]
- 19. Beyersmann J, Gastmeier P, Wolkewitz M, Schumacher M. An easy mathematical proof showed that time-dependent bias inevitably leads to biased effect estimation. J Clin Epidemiol 2008; 61:1216–21. [DOI] [PubMed] [Google Scholar]
- 20. Wolkewitz M, Allignol A, Harbarth S, de Angelis G, Schumacher M, Beyersmann J. Time-dependent study entries and exposures in cohort studies can easily be sources of different and avoidable types of bias. J Clin Epidemiol 2012; 65:1171–80. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC, Platt RW. Survivor treatment bias, treatment selection bias, and propensity scores in observational research. J Clin Epidemiol 2010; 63:136–8. [DOI] [PubMed] [Google Scholar]
- 22. Li C-C, Wang L, Eng H-L, et al. . Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg Infect Dis 2010; 16:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimberlin DW, Acosta EP, Prichard MN, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group Oseltamivir pharmacokinetics, dosing, and resistance among children aged <2 years with influenza. J Infect Dis 2013; 207:709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lina B, Boucher C, Osterhaus A, et al. . Five years of monitoring for the emergence of oseltamivir resistance in patients with influenza A infections in the Influenza Resistance Information Study. Influenza Other Respir Viruses 2018; 12:267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Signorello LB, McLaughlin JK, Lipworth L, Friis S, Sørensen HT, Blot WJ. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther 2002; 9:199–205. [DOI] [PubMed] [Google Scholar]
- 26. Lim JK, Kim TH, Kilgore PE, et al. . The association between influenza treatment and hospitalization-associated outcomes among Korean children with laboratory-confirmed influenza. J Korean Med Sci 2014; 29:485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Launes C, García-García JJ, Martínez-Planas A, et al. ; CIBERESP Cases and Controls in Pandemic Influenza Working Group Clinical features of influenza disease in admitted children during the first postpandemic season and risk factors for hospitalization: a multicentre Spanish experience. Clin Microbiol Infect 2013; 19:E157–62. [DOI] [PubMed] [Google Scholar]
- 28. Fanella ST, Pinto MA, Bridger NA, et al. . Pandemic (H1N1) 2009 influenza in hospitalized children in Manitoba: nosocomial transmission and lessons learned from the first wave. Infect Control Hosp Epidemiol 2011; 32:435–43. [DOI] [PubMed] [Google Scholar]
- 29. McGeer A, Green KA, Plevneshi A, et al. ; Toronto Invasive Bacterial Diseases Network Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568–75. [DOI] [PubMed] [Google Scholar]
- 30. Segaloff H, Petrie J, Malosh R, et al. . Severe morbidity among hospitalised adults with acute influenza and other respiratory infections: 2014–2015 and 2015–2016. Epidemiol Infect 2018; 146:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delgado-Rodríguez M, Castilla J, Godoy P, et al. . Prognosis of hospitalized patients with 2009 H1N1 influenza in Spain: influence of neuraminidase inhibitors. J Antimicrob Chemother 2012; 67:1739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gums JG, Pelletier EM, Blumentals WA. Oseltamivir and influenza-related complications, hospitalization and healthcare expenditure in healthy adults and children. Expert Opin Pharmacother 2008; 9:151–61. [DOI] [PubMed] [Google Scholar]
- 33. Van Kerkhove MD, Vandemaele KAH, Shinde V, et al. . Risk factors for severe outcomes following 2009 Influenza A (H1N1) infection: a global pooled analysis. PLoS Med 2011; 8:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee N, Chan PKS, Lui GCY, et al. . Complications and outcomes of pandemic 2009 Influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? J Infect Dis 2011; 203:1739–47. [DOI] [PubMed] [Google Scholar]
- 35. Matias G, Taylor RJ, Haguinet F, Schuck-Paim C, Lustig RL, Fleming DM. Modelling estimates of age-specific influenza-related hospitalisation and mortality in the United Kingdom. BMC Public Health 2016; 16:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.