Abstract
The 2009 H1N1 influenza pandemic posed challenges for governments worldwide. Strategies designed to limit community transmission, such as antiviral deployment, were largely ineffective due to both feasibility constraints and the generally mild nature of disease, resulting in incomplete case ascertainment. Reviews of national pandemic plans have identified pandemic impact, primarily linked to measures of transmissibility and severity, as a key concept to incorporate into the next generation of plans. While an assessment of impact provides the rationale under which interventions may be warranted, it does not directly provide an assessment on whether particular interventions may be effective. Such considerations motivate our introduction of the concept of pandemic controllability. For case-targeted interventions, such as antiviral treatment and post-exposure prophylaxis, we identify the visibility and transmissibility of a pandemic as the key drivers of controllability. Taking a case-study approach, we suggest that high-impact pandemics, for which control is most desirable, are likely uncontrollable with case-targeted interventions. Strategies that do not rely on the identification of cases may prove relatively more effective. By introducing a pragmatic framework for relating the assessment of impact to the ability to mitigate an epidemic (controllability), we hope to address a present omission identified in pandemic response plans.
Keywords: communicable diseases, pandemic influenza, management and policy, research
Introduction
Following the emergence of the influenza A(H1N1)pdm in 2009, national governments have been evaluating the strengths and weaknesses of their pandemic plans.1–5 In Australia, as in most other jurisdictions, pre-2009 plans focused primarily on responding to a severe pandemic with a 1918-like signature of high severity and high clinical attack rate.6–9 The generally mild nature of the 2009 pandemic posed challenges for targeted case-based intervention strategies designed to limit transmission such as antiviral deployment, reactive quarantine and selective school closures.1,5,10,11
Public health agencies including the US Centers for Disease Control and Prevention (CDC), European CDC, World Health Organization and the Australian Government's Office of Health Protection have identified the concept of pandemic impact as a key issue for further consideration in developing the next generation of pandemic plans.12–16 The impact of a pandemic derives from a number of factors, including the transmissibility17,18 and pathogenicity19 of the virus, the age-specific incidence of disease,20,21 health and socio-economic status of the population22–27 and timing of virus introduction relative to the annual seasonal cycle of influenza.22,28–30
Expanding on concepts articulated during the 2009 pandemic,31 here we identify an additional epidemiological measure, controllability, which may be influential in determining response strategies. We argue that the concept of controllability, complementary to impact, adds pragmatic value to current revisions of pandemic plans. Its inclusion will aid decision-makers considering whether and how to implement alternative responses to an emergent pandemic.
Pandemic impact
Influenza pandemics, sporadic in their occurrence over the course of human history, have varied in their impact on the human population.32–34 The 1918 influenza pandemic was characterized by high clinical infection counts and high mortality. In contrast, the 2009 H1N1 pandemic had an overall mortality rate similar to that seen in yearly seasonal epidemics, while the distinct age profile provided a clear indication of its non-seasonal nature.35,36 While it is recognized that pandemics exact substantial societal and economic costs,37 for the purposes of this work we will confine the definition of impact to a consideration of transmissibilityAQ and severity, respectively, capturing the burden of morbidity and mortality. Our focus aligns with the recently introduced framework for the assessment of impact by Reed et al.,16 who mapped past pandemics onto transmissibility-severity axes and evaluated the data requirements for real-time estimation of impact.
Virus transmissibility, captured by the basic reproduction number (R0) (assuming the population is fully susceptible), is the prime determinant of the total number of individuals infected over the course of a pandemic. The time course of infections depends further upon the average time between onset of symptoms in sequential generations of infection.
Clinical severity may be considered as the risk of severe outcome [e.g. hospitalization, intensive care unit (ICU) admission or death] per infected individual. The case-fatality ratio (CFR), which measures the risk of death given clinical infection, has been widely utilized as the primary measure of clinical severity,35 but measures such as the infection-fatality ratio (IFR)36 may also be appropriate, particularly from a transmission modeling point of view where the number of infections (clinical or otherwise) is imputed. However, the CFR (and IFR) has been seen to vary by orders of magnitude, both in its global average value across pandemics and by geographic location within pandemics.33
Real-time modeling to assess impact
The 2009 pandemic was the first to occur in the era of ubiquitous computational resources, highly developed national pandemic preparedness plans and with the availability of detailed virological surveillance data. Although algorithms to infer the reproduction number from real-time epidemiological surveillance data were available,38,39 limitations to their use were rapidly identified,20,40 with extensions to account for age- and mixing effects developed during or following the pandemic.11,20,41 Whether robust inferences on transmissibility can be made during the earliest stages of a pandemic remain an open challenge.35
Estimation of the CFR in real time is also fraught with challenges,35,42 based on uncertainties in the estimation of deaths and cases, inaccurate ascertainment of cause of death, delays to death from the time of infection and reporting bias.43 Early estimates of the 2009 pandemic CFR from Mexico were alarmingly high.44 As methodological flaws were addressed,45 estimates did indeed decrease.44,46–48
Pandemic intervention strategies
Knowledge of the likely impact of a pandemic will clearly motivate public health officials to implement responses that may themselves be socially and/or economically costly. These costs must be balanced against the burden of a high severity pandemic on medical services, business continuity and social stability.
Intervention measures designed to save lives and maintain core health-care facilities and staff—such as the use of antiviral agents for treatment—would likely be recommended for use wherever available. However, it is less clear under what circumstances, if any, pandemic intervention strategies designed to modify the course of the outbreak itself may be effective (and warranted). Lipsitch et al.35 have developed a decision-making framework that maps transmissibility and severity to the ‘overall scale of response’. Their proposed framework identifies the characteristics of a pandemic that would conceivably warrant intervention. The estimated effectiveness of control measures is considered as an additional element within this framework, but is not directly tied to the drivers of impact.35 A working group convened during the 2009 pandemic suggested that decisions on attempted containment should be based on worst-case estimates for the CFR and best-case estimates for R0, noting that the latter is a key driver of controllability.31
We suggest that the factors that contribute to impact may also critically determine the controllability of an epidemic and therefore controllability should be considered as an additional element in the decision-making process.
Visibility and controllability versus severity and impact
Identification of the predicted impact of a nascent pandemic may help guide a decision on whether or not intervention is warranted, but it cannot directly inform on whether or not an intervention is likely to succeed. For example, case-targeted interventions rely on the timely and sustained identification of a reasonable proportion of all infectious cases10 and so a primary driver of controllability for this intervention is the visibility of the pandemic.
Visibility encompasses both sociological and biological phenomena. The proportion of cases (of a given clinical severity) that choose to present to health authorities and thus become ‘visible’ and amenable to intervention will vary based on many factors, including cultural and social factors, the current (time-dependent) level of public perception of risk (itself likely positively correlated with the CFR), accessibility of health-care services and any effect of government information campaigns. A successful transmission reducing intervention also requires timely identification of cases, and so factors such as the proportion of transmission that pre- and post- dates onset of symptoms will also contribute to controllability.49
Impact and controllability are distinct but intertwined concepts. Combined with an assessment of transmissibility, severity helps to determine impact, while visibility (for case-targeted interventions) helps to determine controllability. To further explore this relationship between controllability and impact, we now take a case-study approach, using a previously published model of antiviral distribution and school-based intervention strategies.10,50
Case studies
Background
The Australian Government's Health Management Plan for Pandemic Influenza 2008 identified the distribution of antiviral agents for treatment and post-exposure prophylaxis as one of a number of strategies designed to reduce the community spread of influenza.6 Selective school closure was also envisaged to play a role in community mitigation. These recommendations were informed, in part, by earlier modeling studies conducted by the authors.9,51,52 In light of the 2009 experience, the model framework was updated to consider how varying levels of severity, and crucially, visibility, along with ‘real-world’ constraints in the health sector's capacity for diagnosis and drug delivery, may modify the likely utility of interventions and the circumstances under which they may be effective.10
Here we employ that modeling framework to consider the ability of different interventions to mitigate key characteristics of epidemics including the total and clinical attack rate and time to peak incidence. We run the model repeatedly, sampling disease and intervention parameters from appropriate distributions to simulate a range of possible future pandemic scenarios of widely varying impact. Model outputs under application of interventions are related to baseline epidemic scenarios to allow the assessment of controllability and how it varies with different assumptions for transmissibility and visibility. The sensitivity of model outputs to uncertain epidemic and intervention parameters is examined to provide guidance to public health authorities on key requirements for real-time decision support and current limitations in response capability.
Interventions
We consider two contrasting frontline interventions designed to modify the transmission of an influenza pandemic: community-based antiviral distribution and ‘school-based measures’, the latter intended to capture a range of possible interventions designed to reduce transmission among the child and adolescent populations. The antiviral strategy is targeted towards identified cases and their contacts, while school-based measures can be deployed to a segment of the population without need for identification of particular infected individuals.
We assume that treatment with antiviral drugs results in a modest reduction in transmissibility and provision of post-exposure prophylaxis to suspected contacts of cases results in a reduced susceptibility.10 Logistical constraints on case identification and drug delivery are imposed and have important implications for the overall ability of the response to modify the course of the epidemic, particularly for higher values of R0.
School-based measures are employed for a fixed period (weeks to months) following a delay to their introduction, reducing child–child mixing while active. We consider two alternative age-mixing models in which to deploy school-based measures: homogenous mixing and enhanced child–child mixing.
For both interventions we assume the availability of a strain-specific vaccine after 18 weeks of transmission, representing a definitive control measure. Full details of the mathematical model are presented in the Supplementary data.
Findings and analysis
Transmissibility, characterized through the basic reproduction number R0, is a key driver of impact and controllability. Figure 1 plots the association between R0 (sampled from [1.2, 2.0]) and impact, as measured by the overall attack rate (total infections) with all other parameters (biological, epidemiological and intervention) co-varied over plausible ranges. An antiviral intervention (Fig. 1a) is only able to meaningfully reduce the attack rate if R0 < 1.5. Furthermore, if R0 < 1.25, the intervention may be expected to definitively control the epidemic. In contrast, a school-based measure (Fig. 1b) implemented for a period of 12 weeks is never expected to control the epidemic, but its modest effect is sustained across a wider range of values for R0.
Fig. 1.
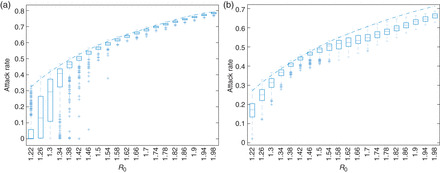
The distribution of attack rates (all cumulative infections, clinical or otherwise) as a function of transmissibility (R0). Each box plot shows the range of outcomes from the set of simulations, with the bar indicating the median, boxes covering the inter-quartile range, whiskers extending to the adjacent values and crosses indicating outliers. The dash-dotted line indicates the median attack rate over all simulations in the absence of intervention. Note that a strain-specific vaccine is rolled out to the population at Week 18 of the simulation, providing definitive control. (a) A targeted antiviral intervention, providing treatment to identified cases and post-exposure prophylaxis to contacts of identified cases. For high R0 (>1.5), the box plots are tightly constrained (i.e. all simulations give roughly the same result) and overlap with the baseline (no intervention) median result. For low R0 (<1.25), the intervention is expected to significantly reduce the attack rate. For intermediate values of R0, between 1.25 and 1.5 the intervention may reduce the attack rate—the broad inter-quartile range, and significant tail of outliers extending towards very low attack rates indicates that the utility of the intervention is highly dependent upon other model parameters that are sampled in the scenarios. (b) A school-based measure (reduced child–child mixing) implemented for 12 weeks from the initiation of transmission under the baseline assumption that child–child mixing is enhanced compared with adult–adult mixing. The intervention's effect is less substantial than in (a), and unable to completely control the epidemic. However, it is maintained over the broad range of R0 values considered. The relative reduction in intervention success for intermediate values of R0 is a complex result of the interplay between the timing of exponential growth, intervention withdrawal (at 12 weeks) and vaccine introduction (at 18 weeks), and is explored in detail in the Supplementary data.
Having established that transmissibility is a key driver of both impact and intervention success, we introduce a second axis, the visibility of the epidemic, measured as the proportion of all non-hospitalized infectious cases that present to medical authorities (αm). As detailed in the Supplementary data, visibility in our model is assumed to be correlated with the underlying severity of the epidemic, in principle allowing for controllability to be linked to outcomes such as ICU admissions or death.
Figure 2 demonstrates how impact and controllability may differ. We consider an antiviral intervention including both case treatment and post-exposure prophylaxis of contacts and take the median clinical attack rate as a measure of impact. In the absence of intervention (Fig. 2a), impact increases with increasing transmissibility (R0) and visibility (αm). Figure 2b shows the median clinical attack rate in the presence of the antiviral intervention. If we consider the controllability, a material change in the outcome is only evident if R0 is low and visibility is high (large negative values in upper left corner of Fig. 2c). That is, high-impact epidemics are uncontrollable, whereas lower impact pandemics of high visibility may be controllable. We present equivalent figures with severity in pace of visibility in the Supplementary data.
Fig. 2.
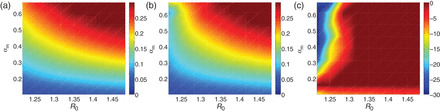
Impact and controllability, assessed by the median clinical attack rate over the LHS sample as a function of R0 (transmissibility) and αm (visibility). (a) Baseline (no intervention) median clinical attack rate. (b) The median clinical attack rate with the antiviral intervention. (c) The mean percentage change in the median clinical attack rate (calculated from the difference between plots (a) and (b)). The median value for the outcome measure (clinical attack rate) over the LHS samples increases with increasing R0 and αm, indicating higher impact of simulated pandemics in the upper right region of the R0–αm plane (equivalent figures with severity in place of visibility are provided in the Supplementary data). Only for low transmissibility and high visibility scenarios (upper left) is the antiviral intervention able to modify the course of the pandemic (the larger negative values in plot (c)). Note that the narrow horizontal strip at the bottom of plot (c) is simply a boundary effect due to the plotting routine, and not an indication of control in this region.
While case-targeted interventions may only be effective at modifying population transmission in high visibility scenarios, interventions that do not require the identification of individual infectious cases may have different controllability profiles. Figure 3 shows how controllability for a school-based measure maps to the axes of transmissibility (R0) and visibility (αm). Using the time-to-peak incidence as a measure of pandemic impact, we find a uniform median percentage delay may be expected, in particular independent of the visibility (αm). Note however that this finding is critically dependent upon the assumed mixing pattern in the population, and only holds if child–child mixing is naturally enhanced compared with adult–adult and child–adult mixing (Fig. 3a). Where child–child mixing is not enhanced, school-based measures are seen to be ineffective (Fig. 3b).
Fig. 3.
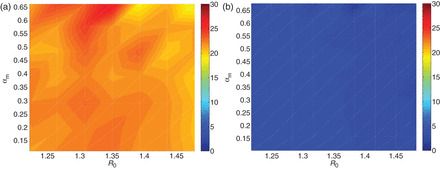
The average percentage change in the time-of-peak of the epidemic with school-based measures under two assumptions on mixing. The start time and duration of the intervention are randomly sampled over plausible ranges as described in the Supplementary data. (a) Enhanced child–child mixing assumption. The underlying age-dependent mixing between children is assumed to be enhanced compared with between adults. Measures to reduce child–child mixing result in an appreciable delay to epidemic peak, constant in percentage terms at around 20%. Note that for increasing R0, the time to peak shortens and thus so does the absolute delay achieved by the intervention. (b) Homogeneous mixing assumption. The underlying age-dependent mixing is assumed to be uniform. A reduction in child–child mixing has (on average) a negligible effect on the epidemic regardless of assumed transmissibility or severity.
Figures 2 and 3 display median results over many simulations. Parameters other than R0 and αm also contribute to variation in the outcome (Fig. 1). We may explore this variation in detail using partial rank correlation coefficient analysis, as discussed in the Supplementary data.
Discussion
Main findings of this study
We have introduced the concept of pandemic controllability to address a present omission in pandemic response plans identified following reviews of the 2009 H1N1 influenza pandemic,1–5 arguing that pandemic impact, while an appropriate measure to assess the likely burden on health-care systems and the population, is but one part of the equation. Our framework is designed to support planners with their attempts to characterize the circumstances under which interventions—designed to minimize societal disruption and reduce the impact of the pandemic—are both warranted and achievable.
Given that interventions come at a (potentially great) societal and economic cost, only pandemics of sufficient impact and for which interventions are anticipated to be both achievable and effective warrant deployment of extensive mitigation strategies.35,37,53–58 The concept of controllability should play a central role in considering how to intervene. Extending the concept as introduced in ref. 31 we have argued that different interventions have different controllability profiles and so the relationship between impact and controllability is dependent upon the intervention under consideration.
Furthermore, as the decision to intervene must be made early, almost certainly before strong evidence on likely future unmitigated impact is available, our results highlight that the development of decision algorithms for refocusing of efforts to provide direct protection to those most at risk is critical for improving public health response strategies. Such algorithms should consider multiple measures of severity—e.g. deaths, ICU admissions, the CFR or IFR—to ensure a precautionary approach to refocusing interventions.
Taking a case-study approach, we have demonstrated how two particular intervention strategies—targeted population distribution of antiviral agents and school-based measures—may differ in their ability to control epidemics of varying characteristics. We demonstrated that targeted case-based intervention strategies, such as wide-scale community provision of antiviral agents, are only likely to be effective in limiting transmission if an epidemic is of low transmissibility and high visibility. As such, epidemics of greatest anticipated impact are likely not controllable (with antivirals alone). This finding must be considered in relation to the high anticipated demand from the public for such interventions in high impact scenarios, and of course, the direct protective effect of antivirals for limiting severe outcomes or death.59 In contrast, strategies designed to reduce transmission in the general population, or specific sub-populations such as schools, may be effective whether or not a pandemic is ‘visible’ as the decision to deploy an intervention and the efficacy of that intervention is less dependent on the identification of individual cases. Accordingly, such interventions remain effective at materially reducing transmission over a broad range of assumed impacts. Any recommendation to deploy such interventions, targeted or otherwise, must still be weighed against their societal and economic cost.
What is already known on this topic
The concept of pandemic impact has recently been explored by multiple independent authorities, including the US CDC,15,16 European CDC,14 World Health Organization12 and the authors in conjunction with the Australian Government.13 All of these assessments have suggested that the two key elements of impact are transmissibility (e.g. R0) and severity (e.g. CFR). Gathering the appropriate information in a timely manner to assess these two key drivers of impact has been identified as a priority for national surveillance and real-time data analysis endeavors.
How an assessment of impact relates to the ability to mitigate an epidemic has received less attention.31
What this study adds
Our study, by introducing controllability as a context and intervention-dependent concept, addresses this gap. We have explored the relationship between impact and controllability using a case-study approach and suggested a framework under which to use scenario analysis techniques and mathematical modeling to develop flexible and proportionate response strategies for incorporation into pandemic management plans.
Our results indicate that rapid assessment of epidemic growth rates and case ‘visibility’ (i.e. the proportion of all infectious cases that come to the prompt attention of health authorities), as well as case severity, is critical to inform the likely success of an intervention.
Limitations of this study
Our study has two primary limitations. From a policy and planning perspective, a formal cost–benefit analysis is still arguably necessary before a particular course of action could be recommended in a given pandemic situation, even where likely to be effective. Given that such an assessment is anticipated to be impossible to achieve in real time,11,35 development of a fully integrated pre-emptive modeling and scenario analysis strategy remains a worthy goal.
The second limitation of our study is that we have only explored a limited set of mitigation strategies using one particular modeling framework based on knowledge of the Australian context.10 All models are necessary simplifications of ‘real-world’ complexities, and should be considered as useful frameworks within which to explore alternative scenarios, to gain key insights into epidemic drivers and potential mechanisms of disease control. As such, each will differ when determining scenarios which are controllable or otherwise. For robust, country-specific policy development, findings from a suite of modeling analyses drawn from different research groups and building on independent evaluations of the epidemiological literature should be considered.
Funding
This work was funded by a contract with the Office of Health Protection, Australian Government Department of Health and Ageing. James McCaw is supported by an Australian Research Council Future Fellowship with additional support provided by the Defense Science Institute.
Supplementary Material
Acknowledgements
The authors thank Jenny Firman and colleagues from the Australian Government Department of Health and Ageing Office of Health Protection for input into the conceptualization of impact and controllability and Nicholas Geard (University of Melbourne) for suggestions on the manuscript.
References
- 1.Bishop JF, Murnane MP, Owen R. Australia's winter with the 2009 pandemic influenza A (H1N1) virus. N Engl J Med. 2009;361(27):2591–4. doi: 10.1056/NEJMp0910445. [DOI] [PubMed] [Google Scholar]
- 2.Heine D. London: Pandemic Flu Response Review Team, Cabinet Office; 2010. An independent review of the UK response to the 2009 influenza pandemic. [Google Scholar]
- 3.Stockholm, Sweden: European Centre for Disease Prevention and Control; 2011. Review of ECDC's response to the influenza pandemic 2009–2010. [Google Scholar]
- 4.Cammarth, Tunisia: World Health Organization,; 2010. Public health measures during the influenza A (H1N1)2009 pandemic. Meeting report. WHO Technical Consultation. [Google Scholar]
- 5.Canberra, Australia: Australian Government Department of Health and Ageing; 2011. Review of Australia's health sector response to pandemic (H1N1) 2009. Lessons identified. [Google Scholar]
- 6.Canberra, Australia: Australian Government Department of Health and Ageing; 2008. The Australian health management plan for pandemic influenza 2008. [Google Scholar]
- 7.Ferguson NM, Cummings DAT, Fraser C, et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longini I, Halloran M, Nizam A, et al. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 9.McVernon J, McCaw JM, Nolan TM. Modelling strategic use of the national antiviral stockpile during the CONTAIN and SUSTAIN phases of an Australian pandemic influenza response. Aust NZ J Publ Health. 2010;34(2):113–9. doi: 10.1111/j.1753-6405.2010.00493.x. [DOI] [PubMed] [Google Scholar]
- 10.Moss R, McCaw JM, McVernon J. Diagnosis and Intervention strategies for mitigating an influenza epidemic. PLoS ONE. 2011;6(2):e14505. doi: 10.1371/journal.pone.0014505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkhove MDV, Ferguson NM. Epidemic and intervention modelling—a scientific rationale for policy decisions? Lessons from the 2009 influenza pandemic. Bull World Health Organ. 2012;90(4):306–10. doi: 10.2471/BLT.11.097949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briand S. Munich, Germany: ISIRV; 2012. Assessing the severity of influenza, WHO perspective. ISIRV Incidence, Severity and Impact. [Google Scholar]
- 13.McCaw JM, McVernon J. Munich, Germany: ISIRV; 2012. Defining pandemic impact levels to guide a proportionate and flexible operational response to the next influenza pandemic—modelling studies to guide Australia's pandemic policy development. ISIRV. [Google Scholar]
- 14.Nicoll A. Munich, Germany: ISIRV; 2012. Assessing the severity of influenza, ECDC perspective. ISIRV Incidence, Severity and Impact. [Google Scholar]
- 15.Reed C. Munich, Germany: ISIRV; 2012. Assessing the severity of influenza, CDC perspective. ISIRV Incidence, Severity and Impact. [Google Scholar]
- 16.Reed C, Biggerstaff M, Finelli L, et al. Novel framework for assessing epidemiologic effects of influenza epidemics and pandemics. Emerg Infect Dis. 2013;19(1):85–91. doi: 10.3201/eid1901.120124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiura H, Castillo-Chavez C, Safan M, et al. Transmission potential of the new influenza A(H1N1) virus and its age-specificity in Japan. Euro Surveill. 2009;14(22) doi: 10.2807/ese.14.22.19227-en. pii=19227. [DOI] [PubMed] [Google Scholar]
- 18.Jackson C, Vynnycky E, Mangtani P. Estimates of the transmissibility of the 1968 (Hong Kong) influenza pandemic: evidence of increased transmissibility between successive waves. Am J Epidemiol. 2010;171(4):465–78. doi: 10.1093/aje/kwp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed R, Oldstone MBA, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8(11):1188–93. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McBryde ES, Bergeri I, van Gemert C, et al. Early transmission characteristics of influenza A(H1N1)v in Australia: Victorian state, 16 May–3 June 2009. Euro Surveill. 2009;14(42) doi: 10.2807/ese.14.42.19363-en. pii=19363. [DOI] [PubMed] [Google Scholar]
- 21.Fielding J, Higgins N, Gregory J, et al. Pandemic H1N1 influenza surveillance in Victoria, Australia, April–September, 2009. Euro Surveill. 2009;14(42) doi: 10.2807/ese.14.42.19368-en. pii=19368. [DOI] [PubMed] [Google Scholar]
- 22.Hu W, Williams G, Phung H, et al. Did socio-ecological factors drive the spatiotemporal patterns of pandemic influenza A (H1N1)? Environ Int. 2012;45:39–43. doi: 10.1016/j.envint.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Kerkhove MDV, Vandemaele KAH, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8(7):e1001053. doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boggild AK, Yuan L, Low DE, et al. The impact of influenza on the Canadian First Nations. Can J Public Health. 2011;102(5):345–8. doi: 10.1007/BF03404174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly H, Mercer G, Cheng A. Quantifying the risk of pandemic influenza in pregnancy and indigenous people in Australia in 2009. Euro Surveill. 2009;14(50) pii=19441. [PubMed] [Google Scholar]
- 26.Goggin LS, Carcione D, Mak DB, et al. Chronic disease and hospitalisation for pandemic (H1N1) 2009 influenza in Indigenous and non-Indigenous Western Australians. Commun Dis Intell. 2011;35(2):172–6. [PubMed] [Google Scholar]
- 27.Balasegaram S, Ogilvie F, Glasswell A, et al. Patterns of early transmission of pandemic influenza in London—link with deprivation. Influenza Other Respi Viruses. 2012;6(3):e35–41. doi: 10.1111/j.1750-2659.2011.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merler S, Ajelli M, Pugliese A, et al. Determinants of the spatiotemporal dynamics of the 2009 H1N1 pandemic in Europe: implications for real-time modelling. PLoS Comput Biol. 2011;7(9):e1002205. doi: 10.1371/journal.pcbi.1002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mak GC, Wong AH, Ho WYY, et al. The impact of pandemic influenza A (H1N1) 2009 on the circulation of respiratory viruses 2009–2011. Influenza Other Respi Viruses. 2012;6(3):e6–10. doi: 10.1111/j.1750-2659.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowling BJ, Ng S, Ma ESK, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis. 2010;51(12):1370–9. doi: 10.1086/657311. [DOI] [PubMed] [Google Scholar]
- 31.Mathematical modeling of the pandemic H1N1 2009. Wkly Epidemiol Rec. 2009;84(34):341–8. [PubMed] [Google Scholar]
- 32.MacKellar L. Pandemic influenza: a review. Popul Dev Rev. 2007;33(3):429–51. doi: 10.1111/j.1728-4457.2007.00179.x. [DOI] [Google Scholar]
- 33.Mathews JD, Chesson JM, McCaw JM, et al. Understanding influenza transmission, immunity and pandemic threats. Influenza Other Respi Viruses. 2009;3(4):143–9. doi: 10.1111/j.1750-2659.2009.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxford JS. Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol. 2000;10(2):119–33. doi: 10.1002/(SICI)1099-1654(200003/04)10:2&lt;119::AID-RMV272&gt;3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.Lipsitch M, Finelli L, Heffernan RT, et al. Improving the evidence base for decision making during a pandemic: the example of 2009 influenza A/H1N1. Biosecur Bioterror. 2011;9(2):89–115. doi: 10.1089/bsp.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley S, Kwok KO, Wu KM, et al. Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a Longitudinal Community Cohort Study. PLoS Med. 2011;8(6):e1000442. doi: 10.1371/journal.pmed.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verikios G, McCaw JM, McVernon J, et al. H1N1 influenza and the Australian macroeconomy. J Asia Pacific Econ. 2012;17(1):22–51. doi: 10.1080/13547860.2012.639999. [DOI] [Google Scholar]
- 38.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. Am J Epidemiol. 2006;164(10):936–44. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- 39.White LF, Pagano M. Transmissibility of the influenza virus in the 1918 pandemic. PLoS ONE. 2008;3(1):e1498. doi: 10.1371/journal.pone.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer GN, Glass K, Becker NG. Effective reproduction numbers are commonly overestimated early in a disease outbreak. Stat Med. 2011;30(9):984–94. doi: 10.1002/sim.4174. [DOI] [PubMed] [Google Scholar]
- 41.Glass K, Mercer GN, Nishiura H, et al. Estimating reproduction numbers for adults and children from case data. J R Soc Interface. 2011;8(62):1248–59. doi: 10.1098/rsif.2010.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong JY, Cowling BJ, Kelly H, et al. Munich, Germany: ISIRV; 2012. The case fatality risk of 2009 pandemic influenza A(H1N1): a systematic review. ISIRV Incidence, Severity and Impact. [Google Scholar]
- 43.Ejima K, Omori R, Cowling BJ, et al. The time required to estimate the case fatality ratio of influenza using only the tip of an iceberg: joint estimation of the virulence and the transmission potential. Comput Math Methods Med. 2012;2012:978901. doi: 10.1155/2012/978901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipsitch M, Riley S, Cauchemez S, et al. Managing and reducing uncertainty in an emerging influenza pandemic. N Engl J Med. 2009;361:112–5. doi: 10.1056/NEJMp0904380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Presanis AM, Angelis DD, Team NYCSFI, et al. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6(12):e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Presanis AM, Pebody RG, Paterson BJ, et al. Changes in severity of 2009 pandemic A/H1N1 influenza in England: a Bayesian evidence synthesis. BMJ. 2011;343:d5408. doi: 10.1136/bmj.d5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donaldson LJ, Rutter PD, Ellis BM, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraser C, Riley S, Anderson R, et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci USA. 2004;101(16):6146–51. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolton KJ, McCaw JM, Moss R, et al. Likely effectiveness of pharmaceutical and non-pharmaceutical interventions for mitigating influenza virus transmission in Mongolia. Bull World Health Organ. 2012;90(4):264–71. doi: 10.2471/BLT.11.093419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCaw JM, McVernon J. Prophylaxis or treatment? Optimal use of an antiviral stockpile during an influenza pandemic. Math Biosci. 2007;209(2):336–60. doi: 10.1016/j.mbs.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 52.McCaw JM, Wood JG, McBryde ES, et al. Understanding Australia's influenza pandemic policy on the strategic use of the antiviral drug stockpile. Med J Aust. 2009;191(3):136–7. doi: 10.5694/j.1326-5377.2009.tb02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McVernon J, Mason K, Petrony S, et al. Recommendations for and compliance with social restrictions during implementation of school closures in the early phase of the influenza A (H1N1) 2009 outbreak in Melbourne, Australia. BMC Infect Dis. 2011;11(1):257. doi: 10.1186/1471-2334-11-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borse RH, Behravesh CB, Dumanovsky T, et al. Closing schools in response to the 2009 pandemic influenza A H1N1 virus in New York City: economic impact on households. Clin Infect Dis. 2011;52(Suppl. 1):S168–72. doi: 10.1093/cid/ciq033. [DOI] [PubMed] [Google Scholar]
- 55.Chen W-C, Huang AS, Chuang J-H, et al. Social and economic impact of school closure resulting from pandemic influenza A/H1N1. J Infect. 2011;62(3):200–3. doi: 10.1016/j.jinf.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Postma MJ, Milne G, Nelson EAS, et al. Pharmaceutical interventions for mitigating an influenza pandemic: modeling the risks and health-economic impacts. Expert Rev Anti Infect Ther. 2010;8(12):1431–9. doi: 10.1586/eri.10.136. [DOI] [PubMed] [Google Scholar]
- 57.Smith RD, Keogh-Brown MR, Barnett T. Estimating the economic impact of pandemic influenza: an application of the computable general equilibrium model to the U.K. Soc Sci Med. 2011;73(2):235–44. doi: 10.1016/j.socscimed.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uribe-Sanchez A, Savachkin A. Two resource distribution strategies for dynamic mitigation of influenza pandemics. J Multidiscip Healthc. 2010;3:65–77. doi: 10.2147/jmdh.s11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muthuri SG, Myles PR, Venkatesan S, et al. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009-2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207(4):553–63. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


