It has recently been shown that SARS-CoV-2 enters cells via receptor-mediated endocytosis, as expected from previous studies of other coronaviruses.1 ACE2 is the receptor for SARS-CoV-2, which unlocks access to the endocytic uptake of the virus.2 It has also been demonstrated that a two-pore channel (TPC2) is critically important for SARS-CoV-2 entry into cells.1 From earlier studies, we know that TPCs are channels in the endo-lysosomal system through which Ca2+ is released when activated by the intracellular messenger nicotinic acid adenine dinucleotide phosphate (NAADP).3 The new findings on SARS-CoV-2 entry1 link two hitherto completely separate research fields, namely physiological Ca2+ signaling and virology, opening up intriguing and important opportunities for future work on the cellular pathophysiology of COVID-19.
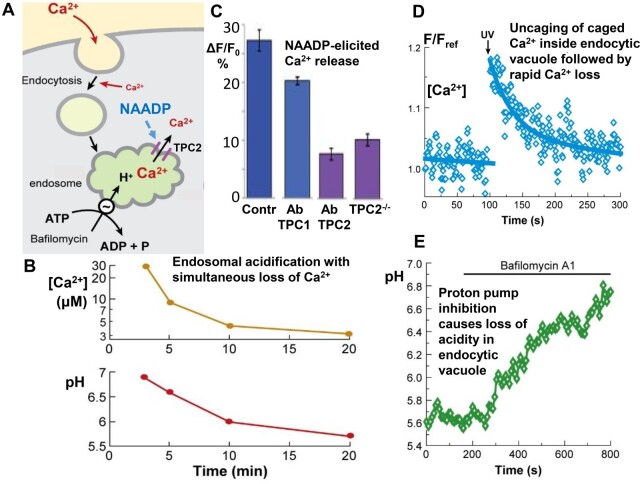
Endocytosis is a process whereby substances in the extracellular fluid can be taken into cells without endangering the integrity of the plasma membrane (Figure 1). Endosomes have an acid interior due to the operation of a proton pump that can be very specifically inhibited by bafilomycin (Figure 1). Ou et al.1 have shown that SARS-CoV-2 entry is completely blocked when the proton pump is inhibited by 100 nM bafilomycin A, a concentration of the inhibitor that has previously been shown to prevent endosomal acidification in different cell types (Figure 1).4,6
Figure 1.
The link between endosomal acidification and loss of Ca2+ from endosomal organelles. (A) Simplified sketch of endosomal Ca2+ uptake, acidification of the endosome by the bafilomycin-sensitive proton pump and leak of Ca2+ from the endosome, via NAADP-sensitive TPCs. (B) Time course of endosomal acidification and Ca2+ loss in fibroblasts. (C) Effects of antibodies against TPC1 and TPC2 as well as deletion of TPC2 on NAADP-elicited Ca2+ release from permeabilized pancreatic acinar cells. (D) Rapid loss of Ca2+ from pancreatic acinar endocytic vacuole following sudden elevation of [Ca2+] inside vacuole due to Ca2+ uncaging. (E) Rapid increase in pH of pancreatic acinar endocytic vacuole after blockage of proton pump by 100 nM bafilomycin A1. Data in (B) are from Gerasimenko et al. 1998,4 in (C) from Gerasimenko et al. 20155 and in (D) and (E) from Sherwood et al. (2007).6
It is particularly intriguing that pharmacological inhibition of TPC2, with tetrandrine, markedly reduced cellular entry of SARS-CoV-2.1 This finding is similar to what has been shown in much more detail with regard to the entry of the Ebola virus.7 Deletion of TPC1 or TPC2 resulted in marked inhibition of virus entry.7 Furthermore, several known inhibitors of NAADP-elicited Ca2+ release from acid stores, for example, Ned-19 and tetrandrine, also severely reduced entry of this virus.7 From earlier Ca2+ signaling studies, it is known that deletion of TPC2 markedly reduced NAADP-elicited Ca2+ release in permeabilized cells, as did an antibody to TPC2 (Figure 1). Ca2+ release mediated by NAADP in intact cells was also markedly inhibited by Ned-19.5
Endocytic acidification occurs at the same time as Ca2+ is lost from endosomes (Figure 1). The fluid taken into cells by endocytosis has, of course, the normal concentration of free Ca2+ found in the extracellular fluid, namely ∼1mM. However, as seen in Figure 1, at the earliest time points allowing assessment of endosomal [Ca2+], the concentration had already dropped to ∼30 μM, which is still markedly higher than the level in the cytosol (∼0.1 μM). The endosomal [Ca2+] then declined further to ∼4 μM in late endosomes (Figure 1). Endosomal acidification and loss of Ca2+ are interlinked. Blockade of the proton pump by bafilomycin prevented release of Ca2+ from the endosomes, with endosomal [Ca2+] remaining high.4 If [Ca2+] in the early endosomes was kept low, by reducing the extracellular [Ca2+] to less than 300 μM, acidification did not occur.4 The mechanism underlying the linkage between Ca2+ and H+ movements is not fully understood, but the fact that the uptake of H+ into endosomes occurs simultaneously with release of Ca2+, and that the two processes are interdependent, means that it is difficult to determine whether inhibition of virus entry by blockade of the proton pump or by TPC inhibition1,7 is primarily due to the high pH or to the relatively high [Ca2+] in the endosomes, or to a mixture of both.
Since TPCs are NAADP-sensitive channels, it is important to understand the mechanism by which NAADP is formed. The CD38 enzyme responsible for NAADP generation is an ecto-enzyme that is internalized by endocytosis,8 and requires an acid environment to perform its task.8 In pancreatic acinar cells, the process of NAADP formation and the mechanism of action of this messenger have been investigated in some detail.5,8 In this cell type, there is evidence showing that it is in the acidic endosomes that NAADP generation takes place.8 Furthermore, the initial Ca2+ release triggered by NAADP may depend on actions of this messenger on both endosomes and lysosomes.5 At least part of the effect of proton pump inhibition on virus entry1,7 could therefore be explained by lack of NAADP formation8 and action, thereby preventing TPC activation.5
SARS-CoV-2 is not only entering cells in the respiratory tract,2 but is also taken up in the central nervous system, where it can cause serious damage,9 as well as in the gastro-intestinal tract, including the pancreas.10 From a mechanistic perspective, it may be of particular interest to study virus uptake in pancreatic acinar cells, since in these cells there is clear evidence for a physiological role of NAADP.5 In this context, it is interesting that COVID-19 can be associated with acute pancreatitis and that ACE2 is present in the pancreas.10 In acute pancreatitis, a human disease in which the pancreas digests itself, there is a secretion defect and large endocytic vacuoles, which are different from endosomes and post-exocytic in nature, appear in the acinar cells.6 Nevertheless, these vacuoles have functional features in common with endosomes. When NAADP is generated by hormonal stimulation, the vacuole membrane is very permeable to Ca2+, as demonstrated by the rapid loss of Ca2+ from vacuoles following a sudden increase in [Ca2+] elicited by uncaging of caged Ca2+ by UV light (Figure 1D). The endocytic vacuoles are also acidic, with a pH similar to that found in endosomes, and bafilomycin A1—the very specific proton pump blocker—rapidly increased pH inside the vacuoles (Figure 1E). The endocytic vacuoles may provide an additional and important entry pathway for viruses, including SARS-CoV-2. Furthermore, the post-exocytic vacuoles contain large amounts of many different proteases,6 which could also play a role in SARS-CoV-2 infection.
Exocrine pancreatic secretion is principally controlled by the neurotransmitter acetylcholine (ACh) and the hormone cholecystokinin (CCK). Both agents elicit secretion by mobilizing intracellular Ca2+, which subsequently triggers Ca2+ entry from the extracellular solution. CCK primarily evokes Ca2+ release mediated by NAADP acting not only on TPCs—but also on ryanodine receptors—in a complex arrangement involving endosomes and lysosomes.5 In contrast, ACh primarily causes intracellular Ca2+ release mediated by inositol trisphosphate (IP3), acting on IP3 receptors principally located in the endoplasmic reticulum.5 Given that endosomal acidification depends on endosomal Ca2+ release,4 and that the entry of SARS-CoV-2 and Ebola virus is abolished or severely reduced when the proton pump is blocked or when TPCs are inhibited,1,7 it would be of considerable interest to know if and how NAADP-dependent Ca2+ signaling influences SARS-CoV-2 entry. Taking advantage of the co-existence in pancreatic acinar cells of two different Ca2+ signaling mechanisms, activated by separate receptor pathways, it would seem possible to provide well-defined test and control conditions in such studies.
We conclude that there is evidence for the involvement of acidic endosomes and TPC-mediated Ca2+ release from the endo-lysosomal system in both SARS-CoV-2 entry and NAADP-mediated Ca2+ signaling. The pancreatic acinar cells may prove to be a particularly useful system for exploring the relationship between these phenomena.
Conflicts of interest statement
None declared.
Funding
Experimental work in the authors’ laboratory was supported by grants from the UK’s Medical Research Council (MR/J002771/1 and G19/22/2).
References
- 1. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calcraft PJ, Ruas M, Pan Z, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009;459:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV.. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 1998;8:1335–1338. [DOI] [PubMed] [Google Scholar]
- 5. Gerasimenko JV, Charlesworth RM, Sherwood MW, et al. Both RyRs and TPCs are required for NAADP-induced intracellular Ca2+ release. Cell Calcium 2015;58:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sherwood MW, Prior I, Voronina SG, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci (PNAS) 2007;104:5674–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakurai Y, Kolokoltsov AA, Chen C-C, et al. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015;347:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosker F, Cheviron N, Yamasaki M, et al. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J Biol Chem 2010;285:38251–38259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steardo L, Steardo L Jr, Verkhratsky Zorec R,. A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol 2020; 229:e13473, doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hadi A, Werge M, Kristiansen KT, et al. Coronavirus disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology, 2020; 20:665–667, doi: 10.1016/j.pan.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]