Abstract
The aim of this study was to analyse the contemporary policies regarding avian and human pandemic influenza control in three South-East Asia countries: Thailand, Indonesia and Vietnam. An analysis of poultry vaccination policy was used to explore the broader policy of influenza A H5N1 control in the region. The policy of antiviral stockpiling with oseltamivir, a scarce regional resource, was used to explore human pandemic influenza preparedness policy. Several policy analysis theories were applied to analyse the debate on the use of vaccination for poultry and stockpiling of antiviral drugs in each country case study. We conducted a comparative analysis across emergent themes.
The study found that whilst Indonesia and Vietnam introduced poultry vaccination programmes, Thailand rejected this policy approach. By contrast, all three countries adopted similar strategic policies for antiviral stockpiling in preparation. In relation to highly pathogenic avian influenza, economic imperatives are of critical importance. Whilst Thailand's poultry industry is large and principally an export economy, Vietnam's and Indonesia's are for domestic consumption. The introduction of a poultry vaccination policy in Thailand would have threatened its potential to trade and had a major impact on its economy. Powerful domestic stakeholders in Vietnam and Indonesia, by contrast, were concerned less about international trade and more about maintaining a healthy domestic poultry population. Evidence on vaccination was drawn upon differently depending upon strategic economic positioning either to support or oppose the policy.
With influenza A H5N1 endemic in some countries of the region, these policy differences raise questions around regional coherence of policies and the pursuit of an agreed overarching goal, be that eradication or mitigation. Moreover, whilst economic imperatives have been critically important in guiding policy formulation in the agriculture sector, questions arise regarding whether agriculture sectoral policy is coherent with public health sectoral policy across the region.
Keywords: Influenza, pandemic, avian influenza, policy, South-East Asia, Indonesia, Thailand, Vietnam
KEY MESSAGES.
Indonesia and Vietnam introduced poultry vaccination programmes for avian and human pandemic influenza (HPAI) control, whereas Thailand did not. All three countries adopted similar strategic policies for antiviral stockpiling.
Economic imperatives associated with poultry production, rather than public health imperatives, were key in poultry vaccine policy formulation for HPAI in Indonesia, Thailand and Vietnam.
These policy differences raise questions around regional coherence of policies and the pursuit of an agreed overarching goal, be that eradication or mitigation.
Introduction
Influenza virus A H5N1 has been causing significant outbreaks of highly pathogenic avian influenza (HPAI) in poultry since late 2003. The challenge of HPAI has been especially profound in South-East Asia where it has become endemic in poultry in several countries (WHO 2006a).
HPAI is important for several reasons including its zoonotic potential. There have been 442 confirmed human cases and 262 deaths globally reported to the World Health Organization (WHO) as of 24 September 2009. Of these, 286 cases and 215 deaths were reported from South-East Asia; Indonesia, Vietnam and Thailand have reported 141, 111 and 25 cases, respectively, and 115, 56 and 17 deaths, respectively (WHO 2009). Thus, South-East Asia has shouldered a substantial amount of the global human burden of HPAI.
Control of HPAI remains a global public health challenge because of concerns that viral change may result in a global pandemic. This concern was recognized by the international community. By 2007, Indonesia, Vietnam and Thailand had received commitments of substantial sums of aid, with US$132 million, US$115 million and US$11 million being committed, respectively (UN System Influenza Co-ordinator and World Bank 2008). Concerns regarding a potential pandemic arising as a result of the spread of H5N1 persist despite the emergence of the pandemic (H1N1) 2009. Indeed fears of re-assortment between H1N1 and H5N1 have the potential to further heighten concerns given the endemic nature of H5N1 in South-East Asia and the pandemicity of H1N1 (Belshe 2005).
As well as affecting human health, HPAI has had an impact on poultry with flock mortality often above 50%, and culling of flocks as a control measure resulting in an economic burden felt by families and communities as well as impacting upon domestic and international trade. Since 2003, at least 150 million poultry birds have died or been culled as a result of the H5N1 epidemic in Indonesia (Ministry of Agriculture 2006; Foster 2009). The economic loss has been estimated at US$470 million (KOMNAS Presentation 2008). In Thailand, more than 75 million poultry have been killed, and the economic cost has been estimated to be at least US$3 billion to the poultry industry alone (Department of Disease Control 2008). In Vietnam, since 2003, 52 million poultry have died or been culled, with 86.5% of poultry culled in 2003-04 (Pfeiffer et al. 2007). The economic impact in 2004 was estimated to be US$45 million due to loss of poultry, with additional costs of US$22 million for the poultry vaccine programme. Moreover, the government set aside a budget allocation of US$41 million for the entire year for support for birds culled during outbreaks.
Over the past 5 years, two interventions have received considerable attention from policy makers and received substantial policy attention and implementation funding: vaccination of poultry and antiviral stockpiling for humans. This paper is focuses on these two policies.
In March 2007, the World Organization for Animal Health (OIE), the Food and Agriculture Organization (FAO) and the Instituto Zooprofilatttico Sperimentale delle Venezie (IZSVe) issued recommendations to support the eradication of HPAI, following a joint OIE/FAO/IZSVe conference in Verona (OIE 2007). The 2007 Verona Recommendations acknowledged that the control of HPAI was a complex issue and that new strategies were needed that complemented traditional approaches to eradicating the disease. They suggested that control strategies could be based on a combination of culling, movement restrictions and emergency vaccination. The purpose of poultry vaccination is primarily to reduce replication and viral shedding through the induction of protective immunity in poultry flocks. Vaccination may also, it was suggested, prevent the introduction of avian influenza. The agencies recommended that countries consider introducing poultry vaccination given local epidemiological profile, a determination of costs and benefits to different stakeholders, and issues of operational feasibility amongst others.
In 2006, WHO issued guidelines on the management of a regional stockpile of the antiviral drug oseltamivir on the prediction that an influenza pandemic may result globally in more than 1 billion cases and 2–7 million deaths (WHO 2006b). The need for a regional stockpile of oseltamivir was predicated on its strategic use as an intervention to supplement national capacity to contain the emergence of a potential pandemic at its epicentre through the rapid deployment of the drug to halt transmission and contain disease. The guidance advocating national stockpiling of antiviral agents was aimed at securing capacity for mitigation purposes. In 2005, it was estimated that stockpiles that cover 20–25% of the population would be sufficient to treat most clinical cases and could lead to 50–77% reductions in hospitalizations (Gani et al. 2005).
Poultry vaccination and antiviral stockpiling policies reflect interpretations of the evidence base, responses to international agencies' recommendations, and local political imperatives and competing public health priorities. Three countries in South-East Asia have responded to the challenge of HPAI in different ways. Whilst Indonesia and Vietnam introduced poultry vaccination programmes, Thailand rejected this policy (but adopted other non-vaccine measures such as culling with compensation, and movement restrictions of poultry, for instance). By contrast, all three countries adopted similar policies for antiviral stockpiling in preparation for a global pandemic. This paper explores, through a comparative analysis of policy formulation, why different approaches were adopted in countries in one policy arena, whilst in another linked policy field, substantial similarities across all countries emerged.
Method
The conceptual framework for analysing two policies, poultry vaccination for HPAI and antiviral stockpiling for pandemic influenza in three countries in South-East Asia (Indonesia, Thailand and Vietnam), was adapted from Walt and Gilson (1994). Our retrospective analysis focused principally on the policy formulation process of the two defined policies. Policy content was determined through a review of policy documents. In the case of vaccination, though no explicit policy to vaccinate was introduced in Thailand, there was an explicit policy not to do so (Department of Livestock Development 2006). Thus, though Hardee et al. (2004) suggested that written policy documents should include a rationale, goals and objectives, programme measures, implementation arrangements, funding and other resources, and plans for monitoring and evaluation, we adopted a definition of policy based on official guidance on defined intervention, a purposive approach proposed by Green and Collins (2006). Our policy process analysis is focused on the formulation process in order to explore people and institutional relations, and how policies were arrived at or agreed upon and communicated (Sabatier and Jenkins-Smith 1993).
We used multiple qualitative research methods (Patton 2002): literature review of both primary and secondary documents, as well as in-depth interviews of key informants relevant to the policies. Primary documentary policy data were collected through a review of official documents including laws and regulations, government strategic plans, guidelines, ministerial minutes of meetings and correspondence between ministries and other agencies, and briefing papers. Secondary policy documentary data sources included case reports, business reports, academic documents, and newspaper and other media reports.
For in-depth interviews, stakeholders were selected through purposive sampling and snowball sampling (Hansen 2006) and included interviewees from ministries (health, agriculture, finance, and labour), policy makers at local and central levels, academics, public sector workers (doctors, veterinarians, public health specialists), non-governmental agencies, the private sector (representatives of the pharmaceutical industry and the poultry industry), United Nations (UN) agencies and donor agencies. Triangulation was ensured through interviewing a wide range of respondents and cross referencing with documentary data sources. Data were qualitatively analysed and key emerging themes were identified and consolidated in a framework that was checked and refined iteratively. Over 130 in-depth stakeholder interviews were conducted between March 2008 and November 2009 (Table 1). Informed consent was obtained from all interviewees. Where anonymity was sought, this is respected.
Table 1.
Interviewees from the three study countries (numbers in parentheses refer to the code identifier of each respondent)
| Institution affiliations | Indonesia | Thailand | Vietnam |
|---|---|---|---|
| National Advisory Committee | 2 (#1, #2) | 8 (#1, #7, #8, #9, #10, #12, #18, #26) | 2 (#26, #42) |
| Ministries of health | 9 (#3 – #13) | 5 (#2, #3, #4, #11, #38) | 5 (#1, #8, #20, #21, #25) |
| Ministries of agriculture | 3 (#14 – #16) | 4 (#28 – #31) | 7 (#2, #18, #19, #27, #28, #29, #30) |
| Academia | 1 (#52) | 5 (#13, #14, #27, #32 – #33) | 5 (#3, #4, #6, #7, #31) |
| Pharmaceutical and vaccine industry | 1 (#53) | 7 (#5, #6, #19, #20, #21, #22, #23) | 3 (#9, #32, #43) |
| Small-scale poultry producers (including fighting cocks) | 2 (#20, #35) | 2 (#16, #24) | 3 (#10, #24, #33) |
| Large-scale poultry producers | 1 (#54) | 1 (#17) | 2 (#5, #34) |
| International organizations | 1 (#17) | 2 (#15, #25) | 1 (#38) |
| Health service providers | 18 (#18, #21 – #28, #36 – #39, #45 – #49) | 0 | 6 (#11, #12, #22, #35, #36, #37) |
| Public health agencies | 0 | 0 | 1 (#13) |
| Veterinary service providers | 14 (#19, #29 – #34, #40 – #44, #50, #51) | 4 (#34 – #37) | 8 (#14, #15, #16, #17, #23, #39, #40, #41) |
| Total | 54 | 38 | 43 |
Data analysis was undertaken simultaneously with data collection (Glaser and Strauss 1967). The ‘framework analysis’ method was used to analyse the data. This consisted of familiarization, identifying a thematic framework, mapping and interpretation. A contact and content summary form was developed for each interview during familiarization (Rashidian et al. 2008). The initial thematic framework was developed using literature, prior thoughts developed during workshops, and interviews, research questions and a thematic guide (Arredondo and Orozco 2008). The initial thematic guide was developed through a series of meetings amongst researchers. Comparative analysis was conducted by comparing information gained from documentary review with information provided by interviewees (Patton 2002). The preliminary data analysis was supported by further analysis at completion of data collection. Data were collected on cards and catalogued across categories and themes. Figure 1 shows a schematic framework of the policy analysis methods.
Figure 1.
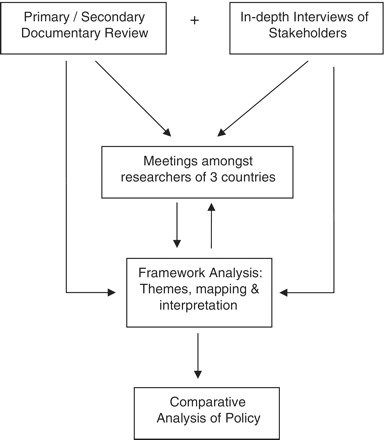
Schematic framework of policy analysis methodology
Relevant ethics committees in each country reviewed the study protocol and granted permission for the study.
Results
Poultry vaccination and antiviral stockpiling policies
Vaccine policy in each of the three countries differed over the period studied in terms of either formal or informal policies. Policy makers in Thailand considered the question of poultry vaccination in 2004 and the Office of the Prime Minister issued a statement, number 0411/365, on 17 September 2004, recommending that poultry vaccination should not be implemented (in Department of Livestock Development 2006). By contrast, Indonesia's vaccination policy, issued in February 2004 by the Director General for Animal Husbandry and Production, recommended vaccinations for all poultry deemed to be at risk in areas where HPAI was prevalent (Ministry of Agriculture 2009). Similarly, Vietnam, through its National Steering Committee for Avian and Human Influenza Control and Prevention, issued a policy statement, in July 2005, which recommended vaccination of all poultry (Government of Vietnam 2005a; Government of Vietnam 2005b). Thus Thailand's formal policy was different from the policies of Vietnam and Indonesia.
Table 2 summarizes the key points in policy analysis leading to the poultry vaccination policy in the three countries.
Table 2.
Comparative analysis of poultry vaccination policy of Indonesia, Vietnam and Thailand
| Vaccination |
No vaccination | ||
|---|---|---|---|
| Indonesia | Vietnam | Thailand | |
| Economic imperatives | Big local poultry producers influence policy to protect local industry from exports. | Small-scale producers want protection to prevent economic hardship from dead birds and culling. |
|
| Evidence focus | Poultry protection from the virus. | Evidence from poultry vaccination from China. |
|
| FAO position | FAO recommends. | FAO recommends. | FAO expert does not recommend due to management problems. |
| Input from human and animal health agencies | Absence of human health input. | Both human and animal health input. | Both human and animal health input but more weight on animal health side. |
By contrast, all three countries developed antiviral stockpiling policies that were both similar and consistent with international recommendations. Indonesia set a policy objective in 2006 to achieve population coverage for treatment of 0.5–1% of its population (Ministry of Health 2009; interview #6In160407). Similarly, Thailand's policy of March 2005 aimed to provide treatment coverage for 1% of the population (Ministry of Public Health 2005). In both countries, because of budget constraints, the priority allocation is focused on rapid containment through treatment of people exposed to poultry and health care workers exposed to the first cluster of human cases in the event of a pandemic. A stepwise increase in stockpiling was envisaged in Thailand, with year-on-year increases of 100 000 treatment courses being secured for 5 years (interviews #7Th100708, #11Th251208). Vietnam adopted an approach in April 2005 that mirrored Indonesia's and Thailand's, and by November 2005, the Administration of Pharmaceutical Management announced an agreement with Hoffman La-Roche to provide 25 million capsules, which is sufficient for about 0.3% of the population (Ministry of Health 2005a).
Policy formulation: poultry vaccination
Indonesia
Two key factors informed poultry vaccination policy in Indonesia. The first was economic: the economic consequences of infected poultry, the impact on domestic and international trade, and the costs to poultry producers and Indonesia's trade balance. The second factor was governance: Indonesia's decentralized policy-making process and policy implementation fundamentally informed the process of policy development in relation to poultry vaccination.
Compared with Thailand, the export poultry market for Indonesia is small whilst the internal domestic market is relatively large. The policy of vaccination was driven, in large part, by recommendations from government advisory experts on drugs for animal use, experts on communicable diseases, the office for animal quarantine, the pharmaceutical industry and lobbyists from both large- and small-scale poultry producers serving predominantly domestic markets (interviews #14In280809, #53In201109). Large-scale poultry producers, through industry associations and high-level contacts with government, were especially powerful lobbyists and effective at influencing vaccine policy (interview #54In241109). Small-scale producers, though having some influence through their industry associations, effectively influenced local media outputs, notably television and newsprint media. Producers were especially concerned that, with high poultry death rates from H5N1 and the consequences of culling decimating poultry flocks, their domestic markets would succumb to international producers wishing to exploit Indonesia's demand for poultry products (interviews #14In280809, #16In010809). One interviewee from the Ministry of Agriculture noted this and alluded to food security issues too (interview #14In280809): “If we implemented a culling process, we might not have sufficient supplies of chickens. The disease has spread extensively. A culling policy will not be favourable politically.”
Indeed, some large-scale producers had implemented vaccine use before the government formalized its position on poultry vaccination (interview #54In241109). Thus, pressure to vaccinate poultry was driven predominantly by poultry producers (interview #16In010809).
Culling was also potentially very costly to the government. Compensation to producers, if it was to be effective, had to be close to market values. ‘Stamping out’ (culling all poultry in the areas of the outbreaks) was not implemented since this policy has implications for the government to provide compensation which the government cannot afford (interview #52In021109). In other words, vaccination was considered more affordable than financial compensation for culling.
A further consideration for vaccination was the protection of the gene pool of Indonesian poultry, something threatened if the majority local breeds were culled (interview #16In010809). Eradication of HPAI had been considered by policy makers but concerns that it was unfeasible, largely because of cost considerations, meant that greater weight was given to vaccine implementation (interview #52In201109). The government's promotion of vaccination was supported by the UN agencies of the FAO and the OIE. The relationship between the FAO, its office located within the Ministry of Agriculture, and the Government was reported by several informants as being particularly close (interviews #52In201109, #14In280809, #16In010809). The FAO advocated a risk assessment for pandemic influenza prior to implementing a vaccination policy. Indonesia's limited vaccine implementation capacity was a cause of some concern because it would limit vaccination effectiveness, and also potentially pose a wider public health threat from persistent viral shedding. However, immediate domestic economic considerations within the animal health community weighed more heavily on policy makers' minds than human public health concerns of uncertain magnitude in an uncertain future. Scientific evidence was also to support a poultry vaccination policy. Research published in 2001 had shown that vaccination protected poultry from lethal infection with influenza A H5N1 but virus shedding could persist (Seo and Webster 2001).
Agencies concerned with human public health, notably the World Health Organization (WHO) and the Ministry of Health, were largely absent in the policy formulation process for poultry vaccination. However, one year after the policy was implemented, in 2005, concerns were being aired in the human public health community because of human cases of influenza A H5N1 occurring despite there being fewer poultry cases and even in areas where vaccination had been effectively implemented (Suroso 2006). The reason, it was suggested, that public health agencies were absent from the policy formulation process was the organizational silos that these agencies appeared to sit within, hindering cross-disciplinary and cross-institutional communication. These institutional silos persisted despite the National Committee on Avian Influenza including high-level representatives from several ministries, such as the Coordinating Minister for People's Welfare (Committee Chairman), the Coordinating Minister for Economic Affairs, the Minister of Agriculture, the Minister of Health, and some other related ministers, Commander of the Indonesian Army, Chief of the Indonesian Police and the Chairman of the Indonesian Red Cross.
Because of the devolved nature of governance in Indonesia, consistent and coherent policy implementation has become a major challenge (Forster 2009). Although the issue of vaccination had climbed up the policy agenda at central level, especially in the Ministry of Agriculture and the National Committee on Avian Influenza Preparedness, devolved decision-making, budgetary constraints and limited implementation capacity at district and regional agency levels meant an uneven implementation of policy across the country (Kromo, n.d.). These geographic disparities have been compounded by limitations in monitoring and evaluation capacity. Thus a formal policy of vaccination, driven by domestic economic concerns, has been only patchily implemented, constrained by economic, geographic, governance and infrastructural impediments. Eradication of influenza A H5N1 was deemed, it was implied, not feasible and a policy of mitigation given the virus’ endemic status has been adopted informally.
Vietnam
Vietnam's exposure to influenza A H5N1 had resulted in substantial economic impact prior to 2004. The poultry industry had been severely affected, with 45 million poultry being culled in 2003–04 (Pfeiffer et al. 2007), with an economic cost equivalent to a 0.1 percentage fall in gross domestic product (GDP) (Dinh et al. 2005). Poultry production in Vietnam is dominated by back-yard, small-scale producers. The export market is very small. These small-scale producers, along with community leaders, voiced their concerns about the culling response to influenza A H5N1 through local newsprint and television media in a manner similar to that witnessed in Indonesia. The principal concerns of producers were related to the economic hardships that resulted from diseased birds and culling, and the impact on poultry purchasing behaviour, because consumers were becoming wary of buying poultry, prices were falling and profit margins were declining (interview #34In112008).
As in Indonesia, the FAO played an important role in offering guidance and support to policy formulation in Vietnam. In 2004, the FAO recommended to the Ministry of Agriculture a unique policy of blanket vaccination based on the premise that disease would be controlled and virus shedding would be curtailed (FAO 2004; Vu 2009). Vaccination policy in China, a major vaccine producer, where reports of successes from vaccinating ducks were being generated, helped persuade stakeholders in the Department of Animal Health that an aggressive vaccination policy would be effective (interview #16In071209). These reports preceded FAO advice and ensured policy makers were becoming receptive to the notion of an important policy shift (Ministry of Agriculture 2008), a shift that put Vietnam in the spotlight in the international community. The proposal for blanket vaccination policy was, however, not universally accepted. Some scientific expert advisers to the Department of Animal Health were concerned on two counts (interview #32In081108). They were concerned, firstly, that safety issues (for poultry) had not been fully resolved, and secondly, that consumers’ perception of the quality of poultry would be detrimentally affected and demand for domestically produced products would fall. These concerns were overridden by the scientific position taken by the majority of advisers and the international community (FAO 2004).
Unlike Indonesia, stakeholders concerned with the human health consequences of influenza A H5N1, including high-level Ministry of Health personnel, were intimately involved in policy formulation and supportive of vaccinating poultry through ongoing policy dialogues between ministries as well as through their positions on the National Steering Committee for Avian and Human Influenza Control and Prevention. No concerns were raised about the potential public health consequences of ongoing viral shedding or the masking of disease in poultry (something that, it has been suggested, might make it difficult to detect outbreaks early). The institutional silos that separated human public health from animal welfare and economic considerations that were apparent in Indonesia were not present in Vietnam. The centralized and robust nature of governance arrangements in Vietnam ensured cross-ministerial communication (interview #26Vn100907). Substantial international financial support and powerful government advocacy ensured that implementation of policy was systematically and comprehensively applied, in contrast to Indonesia. Thus, the operational capacity, an element that the FAO suggested was an important consideration in poultry vaccination policy, was high and, unlike in Indonesia, has not threatened to undermine formal policy to date. Thus, Vietnam in contrast to Indonesia, with the considerable support of the international community, embraced the notion of eradication of influenza A H5N1 and deemed vaccination an important policy component if this goal was to be achieved. Economic domestic considerations facilitated this strategy whilst human public health concerns received little attention.
Thailand
As with Indonesia and Vietnam, the principal issue influencing vaccination policy in Thailand was the economic imperatives (Safman 2009). Public health assumed less importance, though stakeholders had greater voice than in Indonesia and Vietnam. Thailand, however, unlike Vietnam and to a lesser degree Indonesia, has a very substantial export market as well as a large domestic market for poultry. Thailand's poultry production is also, by contrast, dominated by industrial large-scale producers, who oppose poultry vaccination. On the other hand, vaccination supporters are predominantly rural people with backyard poultry production systems. Backyard poultry are reared for family consumption rather than trade. That noted, backyard poultry including fighting cocks, though of little national economic importance, retain an important traditional position in the cultural life of the country, particularly in rural communities.
The export economy played an important part in vaccination policy formulation. In 2003, the year prior to the H5N1 outbreaks, the value of chicken exports was approximately US$1 billion, and it declined by almost half in the following year when major importers banned chicken from Thailand during the outbreaks (Department of Disease Control 2008). If influenza A H5N1 was to become endemic in poultry in Thailand, export markets would suffer and impact substantially on GDP. Importers, especially from the European Union and Japan, the largest export markets for Thailand, were concerned that vaccination would hinder detection of influenza A H5N1, and thus would ban imports of vaccinated chickens (Manager Online 2004a; The Nation 2004). International trade would be impacted severely, noted one member of the National Advisory Committee (interview #18Th080409). Industry lobbyists, working both behind the scenes and through the mass media, helped ensure that policy makers remained aware of the concerns of the export industry. For small-scale producers as well as the backyard producers, some visibility to owners of fighting cocks was given through the support of some well-known celebrities, who voiced their support for the vaccine through mass media. ‘We are happy that we fight for the small producers and we accepted the government resolution, but we still believe in the effectiveness of vaccination’, one prominent celebrity was quoted (Manager Online 2004b).
In contrast to Indonesia and Vietnam, the FAO office in Bangkok was less supportive of a vaccine policy. Their concern was not that the vaccine per se could not be an effective tool in the control of influenza A H5N1 but, if implementation was not robust and comprehensive, then the policy may generate public health problems from viral shedding. Linked to this was uncertainty over when to stop vaccination and the development of a coherent exit strategy (interview #15Th100309). The FAO's concerns, though different from some poultry producers’ concerns, were strategically allied in advocating non-vaccination. An opposing voice in this debate was that of small-scale producers and owners of fighting cocks. Their interests were unrelated to international trade, but addressed the sustainment of long-held cultural traditions, traditions that were threatened by culling, as reflected by a supporter of backyard chicken production systems (interview #17Th300309). They also supported the poultry vaccination policy because of its impact on other diseases such as Newcastle, an opinion advanced, for example, by a fighting cock's owner (interview #24Th300309). Advocates of vaccination also highlighted scientific evidence used in support of the Verona Recommendations by OIE (2007), and evidence that vaccination in Mexico had helped reduce viral shedding (interview #16Th250309).
Stakeholders whose principal interests were in protecting human public health broadly took a similar position on vaccination policy. The Ministry of Public Health (MoPH) and Ministry of Agriculture and Cooperatives held frequent formal meetings under the Deputy Prime Minister's office. The concerns voiced by public health advisers and officials at the MoPH were similar to those voiced by Indonesia's public health stakeholders, that the possibility of continued viral shedding, especially in the absence of disease in poultry, might pose a human public health threat and raise the potential for re-assortment into a more dangerous strain (interview #7Th100708). However, unlike in Indonesia, these voices were heard, acknowledged and were in harmony with ministries beyond public health, including those that had responsibility for agriculture, and the Cabinet more broadly (interviews #7Th100708, #18Th080409). Other than small-scale producers, all others came to the same conclusion: that poultry vaccination was not the best policy response to the threat posed by avian influenza H5N1 and that policy should emphasize non-vaccine measures.
Policy formulation: antiviral stockpiling
Indonesia
Indonesia aligned its antiviral stockpiling policy with the WHO guidelines regarding a public health response through ensuring population coverage (WHO 2004). Given budgetary limitations, however, the policy was formulated to provide treatment for only 0.5–1% of the population. By 2006, Indonesia had procured 16 million oseltamivir capsules, equivalent to population coverage for treatment of about 0.75%. Economic imperatives rather than public health research evidence was the dominant influencing factor in target setting for population coverage (interviews #6In160407, #4In110407). Stocks were supplied through central government purchases, from a Japan-ASEAN (Association of Southeast Asia Nations) collaboration, and through WHO (interviews #17In050507, #10In110407; Ministry of Health 2009). The strategic objective of stockpiling of antivirals was explicitly rapid containment. As in other countries of South-East Asia, in contrast to more affluent countries in Europe and North America for example (Mounier-Jack and Coker 2006), a mitigation strategy that was dependent upon substantial population coverage with antiviral treatment courses was not considered feasible (Coker and Mounier-Jack 2006).
Vietnam
Following WHO's recommendation to reserve oseltamivir in the event of a pandemic, planning for importation, production and supply of the drug became a part of Vietnam's national strategic plan. The Ministry of Health calculated that approximately 10% of the population was likely to fall sick in the event of a pandemic. Vietnam's National Preparedness Plan in Response to Avian Influenza Epidemic H5N1 and Human Influenza Pandemic (Government of Vietnam 2005a) was prepared by the National Steering Committee and approved by the Government on 18 November 2005. This integrated plan outlined response measures under WHO's global pandemic alert phases and allocated responsibilities to ministries, the Peoples’ Committee at all levels, and other organizations. Based on this plan, the human and animal health sectors in Vietnam prepared specific action plans, and the National Plan of Action on Human Influenza Pandemic Prevention and Control in Vietnam was approved by Ministry of Health on 24 November 2005 (Ministry of Health 2005b). In preparation for antiviral stockpiling, based on calculations of population need and economic assessments, on 9 November 2005 the Administration of Pharmaceutical Management announced the government had purchased from Hoffmann La-Roche 25 million capsules (2.5 million treatment courses) of oseltamivir, in addition to the 2.5 million capsules (250 000 treatment courses) stockpiled with the support of the international community (Ministry of Health 2005a). Oseltamivir would be provided free of charge to patients. In 2005, Hoffmann La-Roche and Vietnam signed an agreement that licensed the domestic manufacture of oseltamivir in Vietnam.
Thailand
In 2005, the Thai Ministry of Public Health estimated that 10% of the population might succumb to the disease during a pandemic, and thus oseltamivir was needed for treatment. This figure was based on a combination of considerations including budgetary factors, an analysis of at-risk population demographics and evidence to support the efficacy of oseltamivir in a pandemic setting. Subsequently, the target of population coverage was reduced to 1% in the national strategic plan that was endorsed by the Cabinet (Wibulpolprasert 2005). Government budgetary constraints and domestic manufacturing capacity were the principal drivers behind this shift in emphasis. International aid in supporting national stockpiles is minimal in Thailand. The voice of the Ministry of Finance dominated the public health debate and strongly influenced the final strategic direction taken. The priority allocation is focused on rapid containment through treatment of people exposed to poultry and the health care workers who would be exposed to the very first human cases. In 2005 and 2006, 2.6 million capsules of oseltamivir (260 000 treatment courses) were bought from Hoffman La-Roche (interview #4Th230508). Domestic production capacity has since been developed and, since 2007, a policy was implemented to increase domestic production year-on-year by 100 000 treatment courses over 3 years (interview #11Th251208).
Discussion
In this research, we have explored the forces at play in three South-East Asian countries’ policy responses to HPAI, a public health challenge that is of international concern in a region where the disease has become endemic in some areas and episodic in others. Though all these countries have experienced HPAI, they have responded in different ways. This paper explores the mesh of power relations between institutions and actors in the HPAI response in each country. These actors included National Advisory Committees, large- and small-scale poultry producers with different vested interests (as evidenced notably in Thailand), international organizations, academia, and human public health and animal health institutions (as shown in Table 1). Through the lens of poultry vaccination and human antiviral policy, we have documented commonalities and divergences in responses that reflect institutional power relationships, national priorities, and the balance between overarching national economic and public health imperatives. The differences have implications for the development and sustainability of regional and global public health strategies for emerging infectious diseases.
Economic imperatives associated with poultry production, rather than public health imperatives, have been at the heart of poultry vaccine policy for HPAI in Indonesia, Thailand and Vietnam. These imperatives, in contrast to antiviral policy, have taken precedence over public health concerns and challenge the notion, through their divergence of approach, of both a regional strategy for HPAI as well as broader notions of a ‘One Health’ approach to emerging infectious diseases (King 2008). The development of vaccination policies was based upon strategic goals that were neither explicit nor regionally consistent. In Indonesia and Vietnam, vaccination was introduced largely because of domestic economic concerns and a particular reading of the evidence on vaccination that supported ‘control’ and favoured mitigation rather than eradication. By contrast, Thailand, with its substantial international trade in poultry, has adopted a policy goal of eradication. The evidence on vaccination was interpreted differently; not that mitigation might not be supported by vaccination, but that continued shedding of HPAI might result, and international export markets fears be realized. Though the poultry industry's economic imperatives appeared to be prioritized over human health concerns, the forces advocating this played out differently in different countries. In Thailand, for example, though their voices were not loud, the public health actors took part in the discourse. Likewise, in Vietnam, public health actors worked in close cooperation with their veterinary counterparts. However, in Indonesia, the voices of public health actors were largely muted. Likewise, the voices of multilateral agencies charged with agricultural and animal welfare matters were dominant in support of policy formulation, and this, it could be argued, challenged the coherence of a regional human public health strategy.
By contrast, the national domestic imperative in antiviral policy making across all three countries was public health. Economic (and other sectoral interests) were largely absent from the debate. All three countries adopted policies that were in accordance with WHO recommendations and aligned with most other countries’ policy approaches. Though the evidence base in support of antiviral stockpiling was fragile, a consensus was achieved which was coherent regionally. Budgetary constraints prevented stockpiling at the levels achieved in western countries.
Our research raises at least two potentially important questions. First, is coherence in policy making under the rubric of ‘One Health’ necessary? That is, should concerns regarding public health and the threat of the emergence of novel infectious diseases mean that public health voices and their authority be more pronounced further ‘upstream’, where the forces that enable these diseases to exploit new ecological niches play out? The attention the notion of ‘One Health’ is now receiving seems to suggest that the answer should be ‘yes’. An institutional framework to support this ‘One Health’ approach demands attention.
The second question that emerges is whether national policy differences, driven by common themes (economic imperatives in the case of poultry vaccination), challenge regional (and global) public health strategies? Does a policy of mitigation of HPAI in one country and of eradication in its neighbour threaten sustainable regional (and indeed, global) public health? The answer to this question is more challenging. Risk management of the pandemic threat, as opposed to risk assessment, is grounded in notions of national sovereignty (Fidler 2008). When sectors beyond public health are potentially affected by policy initiatives such as the economy, farming, industry and security, then it is difficult to envisage nation states in isolation adopting coherent policies. In South-East Asia there are a multitude of regional institutions that have endorsed regional cooperation, including the Association of Southeast Asia Nations (ASEAN), the Ayeyawady-Chao Phraya-Mekong Economic Cooperation Strategy (ACMECS), the Asia-Pacific Economic Cooperation (APEC), the Mekong Basin Disease Surveillance network (MBDS), as well as multilateral UN agencies and their regional offices. The challenge may be, as with the answer to the first question, making these institutions ‘work’ to ensure the balance of national, regional and global needs and interests are met.
Funding
This project, Pandemic influenza preparedness: policy analysis, grant number 104165-003, was conducted under the APEIR (Asian Partnership on Emerging Infectious Disease Research) and was funded through the International Development Research Centre (IDRC, www.idrc.ca). The funders played no role in design, data collection or analysis.
Conflict of interest
None declared.
Acknowledgements
The authors are grateful to Dr Chantana Padungtod of the Ministry of Public Health, Thailand, for her substantial technical expertise and support on this project.
References
- Arredondo A, Orozco E. Equity, governance and financing after health care reform: lessons from Mexico. International Journal of Health Planning and Management. 2008;23:37–49. doi: 10.1002/hpm.913. [DOI] [PubMed] [Google Scholar]
- Bangkok Business. KU says government should go for avian influenza vaccine. 2007 Bangkok, 25 May. Online at: http://www.bangkokbiznews.com, accessed 14 January 2008.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. New England Journal of Medicine. 2005;353:2209–11. doi: 10.1056/NEJMp058281. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture. Evaluasi pengendalian avian influenza tahun 2006 [Evaluation of AI control in 2006]. National Coordination Meeting for Avian Influenza Control, Surabaya, Campaign Management Unit of Directorate of Animal Health of the Ministry of Agriculture; Indonesia. 2006. [Google Scholar]
- Coker R, Mounier-Jack S. Pandemic influenza preparedness in the Asia-Pacific region. The Lancet. 2006;368:886–9. doi: 10.1016/S0140-6736(06)69209-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Disease Control, Ministry of Public Health. Lesson learned from the control of avian influenza and preparedness plan for pandemic influenza of the Ministry of Public Health (BE. 2547-2550) Thailand: Nonthaburi; 2008. [Google Scholar]
- Department of Livestock Development, Ministry of Agriculture and Co-operatives. Avian influenza control in Thailand, 2006. Bangkok, Thailand: Center for Avian Influenza Control; 2006. [Google Scholar]
- Dinh VT, Rama M, Suri V. The cost of avian influenza in Vietnam. 2005 Online at: http://go.worldbank.org/SXJV7V2B60, accessed 1 February 2011.
- FAO. Recommendation on the prevention, control and eradication of highly pathogenic avian influenza (HPAI) in Asia (proposed with the support of the OIE) Verona. Italy: Food and Agriculture Organization; 2004. [Google Scholar]
- Fidler DP. Influenza virus samples, international law, and global health diplomacy. Emerging Infectious Diseases. 2008;14:77–84. doi: 10.3201/eid1401.070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P. The political economy of avian influenza in Indonesia. 2009 STEPS Working Paper 17. Brighton, UK: STEPS Centre. Online at: http://www.steps-centre.org/PDFs/Indonesia.pdf, accessed 20 October 2009.
- Gani R, Hughes H, Fleming D, et al. Potential impact of antiviral drug use during influenza pandemic. Emerging Infectious Diseases. 2005;11:1355–62. doi: 10.3201/eid1109.041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B, Strauss AL. The Discovery of Grounded Theory. Chicago, IL: Aldine; 1967. [Google Scholar]
- Government of Vietnam. National Preparedness Plan in Response to Avian Influenza Epidemic H5N1 and Human Influenza Pandemic. 2005. Government decision No. 6719/VPCP-NN, 18 November, Vietnam. [Google Scholar]
- Government of Vietnam. Directive of the Prime Minister. 2005. Vaccinate Livestocks. No. 25/2005/CT-TTg, July 12, Vietnam. [Google Scholar]
- Green A, Collins C. Management and planning. In: Merson MB, Black RE, Mills AJ, editors. International Public Health: Diseases, Programmes, Systems and Policies. Sudbury, MA: Jones and Bartlet; 2006. pp. 553–94. [Google Scholar]
- Hansen E. Successful Qualitative Health Research: A Practical Introduction. Buckingham, UK: Open University Press; 2006. [Google Scholar]
- Hardee K, Feranil I, Boezwinkle J, Clarke B. The policy circle: a framework for analyzing the components of family planning, reproductive health, maternal health and HIV/AIDS policies. POLICY Working Paper Series No. 11. Washington, DC: USAID; 2004. [Google Scholar]
- King L. The convergence of human and animal health One World – One Health. 2008 UMN Workshop Minneapolis, MN, May 14, 2008. Online at: http://www.localactionglobalhealth.net/Portals/0/Convergence%20-%20Minnesota%20-%20May%2014%20-%202008%20-%20LONNIE%20KING%20-%20One%20World%20One%20Health%20-%20Presentation.pdf, accessed 20 October 2009.
- KOMNAS Presentation. 2008. 10th National Veterinary Conference of the Indonesian Medical Association. Bogor, 20 August 2008. Cited in Forster P. 2009. The political economy of avian influenza in Indonesia. STEPS Working Paper 17. Brighton, UK: STEPS Centre. Online at: http://www.steps-centre.org/PDFs/Indonesia.pdf, accessed 20 October 2009.
- Kromo. AI, Virus vs Vaksinasi [AI, Virus vs Vaccination] Majalah Poultry Indonesia, Jakarta. Online at: http://www.poultryindonesia.com/modules.php?name=News&file=print&sid=1125, accessed 25 August 2009.
- Manager Online. EU-Japan threaten to ban Thai chicken, if Thaksin uses vaccine. 2004 Bangkok, 13 September. Online at: http://www.manager.co.th, accessed 14 January 2008.
- Manager Online. To break AI deadlock: Watch out for illegal vaccine, people dead instead of chicken. 2004 Bangkok, 16 Septemer. Online at: http://www.manager.co.th, accessed 14 January 2008.
- Ministry of Agriculture. The contextual to develop poultry vaccination policy. Hanoi: Department of Animal Health, Vietnam; 2008. [Google Scholar]
- Ministry of Agriculture. Vaccine Policy & Vaccination Strategy for Avian Influenza. Directive of Director General of Animal Husbandry. 2009. No 30099/PD.620/F/9/2009, 30 September, Republic of Indonesia. [Google Scholar]
- Ministry of Health. Signing the Tamiflu manufacturing plant in Vietnam. 2005. Announcement on Tamiflu purchase from Roche. Decision No 8186/ QLD-ĐK, 9 November 2005, Vietnam. [Google Scholar]
- Ministry of Health. Action plan on prevention of H5N1. 2005. Decision No. 38/2005/QĐ-BYT, 24 November 2005, Vietnam. [Google Scholar]
- Ministry of Health. National Strategic Plan for Avian Influenza Control and Pandemic Influenza Preparedness 2006–2008. Jakarta, Indonesia: 2006. Bappenas. [Google Scholar]
- Ministry of Health. Presentation at AI National Committee Panel Expert Meeting. Jakarta, Indonesia: Secretary of Directorate General of Pharmaceutical Presentation; 2009. Provision of Oseltamivir. [Google Scholar]
- Ministry of Public Health. Ministerial memo, 21 March. Thailand: Nonthaburi; 2005. Research & development of oseltamivir production for pandemic influenza preparedness. [Google Scholar]
- Mounier-Jack S, Coker RJ. How prepared is Europe for pandemic influenza? Analysis of national plans. The Lancet. 2006;367:1405–11. doi: 10.1016/S0140-6736(06)68511-5. [DOI] [PubMed] [Google Scholar]
- OIE. Avian influenza vaccination: OIE information document, Verona Recommendations. 2007 Online at: http://www.oie.int/eng/info_ev/Other%20Files/A_Guidelines%20on%20AI%20vaccination.pdf, accessed 27 September 2009.
- Patton MQ. Qualitative Research and Evaluation Methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Pfeiffer DU, Minh PQ, Martin V, Epprecht M, Otte MJ. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. The Veterinary Journal. 2007;174:302–9. doi: 10.1016/j.tvjl.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Rashidian A, Eccles MP, Russell I. Falling on stony ground? A qualitative study of implementation of clinical guidelines’ prescribing recommendations in primary care. Health Policy. 2008;85:148–61. doi: 10.1016/j.healthpol.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Sabatier P, Jenkins-Smith H. Policy Change and Learning: An Advocacy Coalition Approach. Boulder, CO: Westview Press; 1993. [Google Scholar]
- Safman R. The political economy of avian influenza in Thailand. 2009 STEPS Working Paper 18. Brighton, UK: STEPS Centre. Online at: http://www.steps-centre.org/PDFs/Thailand.pdf, accessed 20 October 2009.
- Seo SH, Webster RG. Cross-reactive, Cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. Journal of Virology. 2001;75:2516–25. doi: 10.1128/JVI.75.6.2516-2525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suroso S. Referral system and current situation of human avian influenza. Presentation at Faculty of Public Health University of Indonesia; March 21; Depok, Indonesia. 2006. [Google Scholar]
- The Nation. Vaccination of fighting cocks will hit poultry export. 2004. 13 September, Thailand. [Google Scholar]
- UN System Influenza Coordinator and World Bank. Responses to avian influenza and state of pandemic readiness. Fourth Global Progress Report. New York: UN System Influenza Coordinator; 2008. [Google Scholar]
- Vu T. The political economy of avian influenza response and control in Vietnam. 2009 STEPS Working Paper 19. Brighton, UK: STEPS Centre. Online at: http://www.steps-centre.org/PDFs/Vitenam.pdf, accessed 20 October 2009.
- Walt G, Gilson L. Reforming the health sector in developing countries: the central role of policy analysis. Health Policy and Planning. 1994;9:353–70. doi: 10.1093/heapol/9.4.353. [DOI] [PubMed] [Google Scholar]
- Wibulpolprasert S, editor. The National Strategic Plan for Avian Influenza Control and Influenza Pandemic Preparedness in Thailand, 2005–2007. Bangkok, Thailand: OS Printing House; 2005. [Google Scholar]
- WHO. WHO guidelines on the use of vaccines and antivirals during influenza pandemics. Geneva: World Health Organization; 2004. [Google Scholar]
- WHO. Avian influenza: countries affected by outbreaks in birds. 2006 Online at: http://www.who.int/csr/disease/avian_influenza/avianinfluenza_factsheetJan2006/en/index.html#countries, accessed 27 September 2009.
- WHO. Guidelines for the management of a regional stockpile of oseltamivir. New Delhi: Regional Office for South-East Asia; 2006. [Google Scholar]
- WHO. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. 2009 Online at: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_07_01/en/index.html, accessed 27 September 2009.


