Abstract
Objectives. To document clinical characteristics of influenza-like illness, reported use of health preventive measures and attitudes towards vaccination among patients with influenza-like illness in general practice during the influenza pandemic in 2009.
Methods. Cross-sectional survey in general practice. Patients, who were identified as having influenza-like illness during the peak of the influenza pandemic activity in Norway, were eligible for inclusion in the study. A questionnaire was sent 2–4 weeks after the patients visit to the GP with influenza-like illness diagnosis during October to December 2009, from general practices in Norway. A sample of responders >18 years also had a blood test to check for serological response to the pandemic H1N1 virus.
Results. Questionnaires were sent to 1324 patients, and 357 (27%) were returned. Fever (91% versus 49%, P < 0.01), cough (85% versus 73%, P = 0.016) and gastrointestinal symptoms (58% versus 38%, P < 0.01) were more frequent in the age group <18 years compared to older patients. Serological H1N1 responses were analysed in 72 patients; 34 (47%) were positive (haemagglutination inhibition assay titres ≥40). There were no statistically significant differences in symptoms between seropositive and seronegative patients. Women reported better adherence to personal protective measures, such as hand washing and cough etiquette than men. Women were also more concerned about possible adverse effects of the pandemic influenza vaccine than men.
Conclusions. Discrimination between influenza and other viral upper respiratory tract infections is difficult in daily clinical practice, even during an influenza pandemic. A gender difference was found in reported precautions to prevent influenza.
Keywords: GP, pandemic influenza H1N1, prevention and control, primary health care, vaccination
Background
In April 2009, a novel influenza A (H1N1) virus infected hundreds of persons in Mexico and the USA and spread rapidly throughout the globe.1 The influenza A (H1N1) virus was characterized as a pandemic strain with a low level of immunity in man;2 however, older individuals (>60 years of age) had some degree of protection, due to circulating antibodies from previous influenza H1N1 viruses.3–5 The clinical course of H1N1 influenza appeared to be mild in most cases, but individual cases of severe complications and deaths were observed in healthy young individuals, pregnant women and obese people.5–7 The pandemic virus spread rapidly in Norway in October with consultations with GPs for influenza-like illness (ILI) peaking in November 2009.8 During the pandemic, the Norwegian health authorities recommended vaccination against the pandemic H1N1 virus.9 Also, public health campaigns to encourage the use of preventive measures such as regular hand washing and good cough hygiene occurred during the pandemic.
Primary care plays a major role in the response to a pandemic.10 During influenza outbreaks, patients consult their GP or out-of-hours services for treatment and advice, and further referral is generally not necessary. Hence, general practice is a suitable setting for recruitment of influenza patients for research. Diagnosis of influenza is primarily based upon symptoms, clinical signs and epidemiological knowledge. Fever with cough when prevalence of influenza is high in the community has been shown to have a positive predictive value of 80%.11 The usefulness of serological testing in the clinical setting is limited, due to time delay in analysis.
At the start of the pandemic, it was suggested that the new pandemic strain would give a slightly different clinical picture compared to seasonal influenza.12 It is a challenge for GPs to accurately diagnose patients with influenza among all other patients with other types of ILI.
We conducted a cross-sectional survey in general practice based on patients with ILI during the peak of the influenza pandemic activity in Norway. The first aim of this research was to investigate symptoms and clinical course among patients diagnosed with ILI in general practice. The second aim was to explore whether symptoms could be differentiated between serological negative and positive cases (restricted by design to patients >18 years of age). A third aim was to investigate patients’ attitudes towards vaccination and reported use of health preventive measures.
Methods
In Norway, the municipalities organize primary health care, including general practice. General practice is organized as a registered list system, which provides each citizen with one GP. If secondary health care is needed, referral from primary care is mandatory. Emergency medical service is usually provided by the patient’s GP during office hours and by out-of-hours service provided by GPs on duty.
We invited GPs in five municipalities in Hordaland County; Bergen (256 600 inhabitants), Austevoll (4571 inhabitants), Lindås (14 286 inhabitants), Meland (6631 inhabitants) and Kvam (8360 inhabitants)13 to register all patients with clinical influenza in the period of October 26 to December 31 2009. In Bergen, we restricted the invitation to larger practices with three or more GPs; in the other municipalities, we invited all GPs. Fifty-five GPs participated in this study, and they had a total of 63 808 patients on their lists. Practice size ranged from ∼400 to 2000 registered patients for individual GPs. No power calculations were performed as this is a descriptive study of clinical practice in the primary care setting. The patient population of 55 GPs were regarded as sufficient for this purpose. The municipalities selected were chosen for logistical reasons.
In collaboration with the research group, the chief municipal medical officer of each municipality mailed a letter to all GPs in July 2009, and they were requested to use International Classification of Primary Care (ICPC-2) code ‘R80 Influenza-like illness’ in their medical records when diagnosing a patient with influenza or influenza-like illness. For all other influenza-related contacts to GPs’ offices, they were encouraged to use other diagnostic codes. GPs were asked to diagnose influenza in line with clinical recommendations and usual practice. Patients given ICPC-2 diagnosis R80 were later identified by an electronic search in the GPs’ medical records. The search was performed weekly by the GP or other health care provider in the office. After the study was finished, a manual search was performed in 15 of 55 GPs’ medical records to investigate the accuracy of diagnostic procedures.
Patients given an ILI diagnosis were sent a questionnaire 2–4 weeks after their first encounter with the GP’s office. The questionnaire consisted of four pages and was divided into the following sections: (i) self-reported symptoms of influenza including duration of symptoms; (ii) information about treatment, type of consultation and frequency of visit to health care providers as well as advice and preventive measures taken during the pandemic; (iii) history of seasonal and pandemic vaccination; statements concerning vaccination and influenza disease (pre-formulated motives/barriers) and (iv) demographics and co-morbidities. The questionnaire was designed for the purpose of this study, and there was thus no time for a validation process.
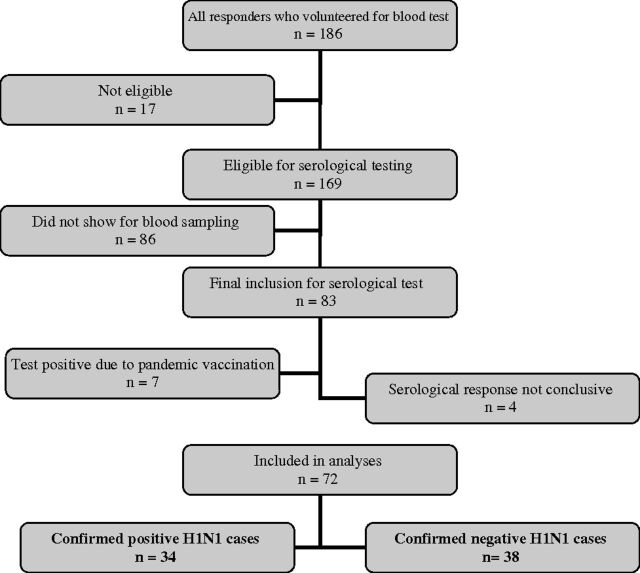
All patients >18 years of age receiving the questionnaire were also asked to volunteer to give a blood sample to measure serological response to the pandemic H1N1 virus. If the patient did not give information about the date of sickness or did not donate a blood sample in the appropriate time window (see below), he/she was rejected for this part of the study. The blood sample was collected at the GP’s practice and sent to the Influenza Centre at the University of Bergen. The blood sample was collected 4–10 weeks after the first contact and analysed by the haemagglutination inhibition assay (HAI), which measures the specific serological pandemic influenza H1N1 response.8 The geometric mean haemagglutination inhibition titre was calculated for each subject and titres <10 were assigned a value of 5 for calculation purposes and considered negative. Samples with HAI titres ≥40 were considered seropositive and deemed protective. Titres between 10 and 39 were considered possible influenza H1N1 and excluded in statistical analyses. Furthermore, if a subject reported pandemic vaccination prior to ILI (in the questionnaire), the sample was excluded in the statistical analyses. Figure 1 shows a flow chart for selection of patients for serological analysis.
FIGURE 1.
Flow chart of cases with serological test; 72 cases were included for final statistical analysis
The statistical analyses were performed using SPSS version 17.0. Univariate and bivariate statistics were used to characterize the sample as well as stratified analysis where relevant. For the analyses of statements concerning influenza vaccines and influenza disease; variables were stratified according to gender and age group. Age group was dichotomized at 40 years of age based on the results of preliminary analyses. Pearson chi-square test was used to check for differences between groups. P-values <0.05 were considered statistically significant.
The study was approved by the Regional Committee for Medical Research Ethics. All patients provided written informed consent.
Results
During the 10-week-study period, a total of 1324 patients (54% females) were given the influenza diagnosis in GPs’ records. Data were available for age and gender for patients of 50 of 55 participating GPs (Table 1). Twenty-nine per cent were in age group 0–17 years, 44% in age group 18–39 years, 22% in age group 40–59 years and 5% in age group >60 years old.
TABLE 1.
Demographic characteristics for all invited (N = 1324) and for participants included in the study (N = 357) and by serological response in a subsample (n = 72)a
| Variable | All invited (N = 1324)b |
All participants (N = 357) |
Serologically tested cases (n = 72) |
|||||||
| Total with blood test | Positive H1N1 (n = 34) |
Negative H1N1 (n = 38) |
P-value | |||||||
| N | % | n | % | n | n | % | n | % | ||
| Age | NS | |||||||||
| 0–17 years | 359 | 29 | 106 | 30 | 0* | – | – | – | – | |
| 18–40 years | 577 | 44 | 127 | 36 | 32 | 18 | 56 | 14 | 44 | |
| >40 years | 275 | 27 | 122 | 34 | 40 | 16 | 40 | 24 | 60 | |
| Missing | 2 | 0 | ||||||||
| Gender | NS | |||||||||
| Male | 577 | 46 | 146 | 41 | 24 | 12 | 50 | 12 | 50 | |
| Female | 665 | 54 | 210 | 59 | 48 | 22 | 46 | 26 | 54 | |
| Missing | 1 | 0 | 0 | |||||||
| Smoking status | NS | |||||||||
| Smoker | 71 | 20 | 19 | 7 | 37 | 12 | 63 | |||
| Non-smoker | 286 | 80 | 53 | 27 | 51 | 26 | 49 | |||
| Level of education | NS | |||||||||
| Primary and secondary school | 57 | 16 | 7 | 2 | 29 | 5 | 71 | |||
| High school | 84 | 23 | 22 | 11 | 50 | 11 | 50 | |||
| Higher education <4 years | 61 | 17 | 18 | 7 | 39 | 11 | 61 | |||
| Higher education >4 years | 78 | 22 | 25 | 14 | 56 | 11 | 44 | |||
| Missing | 77 | 22 | 0 | |||||||
Confirmed H1N1 was defined as a serum HAI titre of ≥40 and negative H1N1 (<10) as HAI titres = 5. NS, not statistically significant. Number (n) and proportion of responders. *, not applicable. Only cases ≥18 years old were asked to provide blood samples.
Percentage is calculated vertically (within the characteristic) for all invited and all participants, while it is calculated horizontally (within each level of the characteristic) for the serologically tested subsample.
Eighty-two patients missing due to lack of data on gender and age.
Of the 1324 mailed questionnaires, 357 (27%) were returned and completed. Of the responders, 30% were aged ≤17 years, median age was 32 years and 59% were female (Table 1). In the age group over 40 years, 76 (62%) participants were female. In the age group <40 years, 133 (57%) were female. Daily or occasional smoking was reported by 20%. A higher degree of education was reported by 39%. Antiviral therapy with oseltamivir was reported by 140 patients (39%) and was positively associated with the age group ≤40 years old (44% versus 31%, P = 0.021). Patients receiving antiviral therapy did not have significantly different symptoms or duration of influenza disease compared to patients not reporting this treatment (data not shown). Of the 169 patients asked to provide a sample for serological testing, 83 showed up and 72 were included in the final analysis (Figure 1). Among these, 38 were serologically negative. The median age for confirmed cases was 47 years old (range, 18–69), and for negative cases, 44 years old (range, 24–89). Only two patients had co-morbidities (chronic lung disease), both among confirmed cases. Hospitalization was reported in two patients.
Fatigue (94%), headache (79%), cough (77%) and myalgia/arthralgia (76%) were the most commonly reported symptoms among all participants. Fever and/or feverishness were reported in 94% of patients, whereas fever alone (elevated temperature to >38°C) was reported by only 61%. The median duration of illness was 7 days; the median duration of specific symptoms varied from 2 to 8 days (Table 2). Fever (91% versus 49%, P < 0.01), cough (85% versus 73%, P = 0.016) and gastrointestinal (GI) symptoms (58% versus 38%, P < 0.01) were more frequent in the age group <18 years of age, whereas myalgia/arthralgia (81% versus 65%, P < 0.01) and feverishness (69% versus 41%, P < 0.01) were more common among individuals ≥18 years old. Other symptoms were similarly distributed between these age groups.
TABLE 2.
Reported signs, median length of symptoms and duration of illness
| Variable | All cases (N = 357) |
Serologically tested cases (n = 72) |
|||||||
| Confirmed H1N1 (n = 34) |
Negative H1N1 (n = 38) |
||||||||
| N | % | Symptom length (days) | n | % | Symptom length (days) | n | % | Symptoms length (days) | |
| Fever and/or feverishnessa | 336 | 94 | – | 33 | 97 | – | 37 | 97 | – |
| Fatigue | 336 | 94 | 6 | 32 | 94 | 7 | 37 | 97 | 7 |
| Headache | 283 | 79 | 4 | 26 | 76 | 4 | 31 | 82 | 5 |
| Cough | 274 | 77 | 8 | 28 | 82 | 13 | 26 | 68 | 7 |
| Myalgia/athralgia | 272 | 76 | 4 | 25 | 74 | 4 | 32 | 84 | 6 |
| Sore throat | 236 | 66 | 5 | 19 | 56 | 5 | 29 | 76 | 7 |
| Elevated temperature (>38°C) | 219 | 61 | 3 | 19 | 56 | 3 | 17 | 45 | 3 |
| Feverishness | 217 | 61 | 4 | 23 | 68 | 3 | 29 | 76 | 4 |
| Rhinitis | 174 | 49 | 5 | 15 | 44 | 7 | 19 | 50 | 4 |
| Dyspnoea | 170 | 48 | 5 | 14 | 41 | 5 | 21 | 55 | 7 |
| GI-symptomsb | 157 | 44 | 2 | 15 | 44 | 2 | 16 | 42 | 3 |
| Other serious infection or symptoms | 50 | 14 | NA | 6 | 18 | NA | 6 | 16 | NA |
| Pneumonia | 12 | 3 | NA | 2 | 6 | NA | 1 | 3 | NA |
| Number of illness days | |||||||||
| Mean (range) | 8 | (1–31) | 9 | (3–23) | 9 | (3–21) | |||
| Median | 7 | 9 | 7 | ||||||
Number (n) and proportion of patients. NA, not applicable. Variable not included in questionnaire. Pearson chi-square test was conducted to check for differences in symptoms between H1N1 confirmed and negative cases; all were statistically non-significant.
Reported measured temperature >38°C and/or subjective feeling of fever/chills. Median length not calculated.
GI symptoms: vomiting, diarrhoea, nausea, stomach pain or other complains related to GI system.
The most common symptoms for patients with confirmed H1N1 were fatigue (94%), cough (82%) and headache (76%). Only 56% of patients with confirmed H1N1 reported elevated temperature (>38°C), but 97% did report either elevated temperature and/or subjective feeling of fever. There were no statistically significant differences in symptoms between groups of serologically confirmed positive and negative patients for H1N1 infection. However, patients with confirmed H1N1 influenza reported a much longer period of a cough (median length 13 days versus 7 days for negative cases). Also, patients with confirmed H1N1 influenza reported median length of influenza disease (period of feeling ill from influenza) slightly longer than confirmed negative cases; 9 days versus 7 days, respectively.
Table 3 shows self-reported adherence to different preventive measures and medical advice. Women reported more use of cough hygiene (64% versus 51%, P = 0.015), hand washing (87% versus 73%, P < 0.01) and use of paper tissue (55% versus 34%, P < 0.01) than men. Facemasks and gloves were rarely used and there was no gender-associated difference in this respect. There was no statistically significant difference according to age group (data not shown).
TABLE 3.
Self-reported adherence to advice during the pandemic
| Type of advice | Male (n = 146) |
Female (n = 210) |
P-value | ||
| n | % | n | % | ||
| Medical advice | |||||
| Washed hands often and thoroughly | 107 | 73 | 182 | 87 | <0.01 |
| Used hand disinfection several times daily | 38 | 26 | 73 | 35 | 0.080 |
| Coughed into elbow | 75 | 51 | 135 | 64 | 0.015 |
| Used paper tissues | 50 | 34 | 115 | 55 | <0.01 |
| Kept distance from people | 106 | 73 | 164 | 78 | 0.234 |
| Used gloves when in contact with other persons | 3 | 2 | 2 | 1 | 0.385 |
| Wore facemask | 5 | 3 | 11 | 5 | 0.417 |
| Folk remedy | |||||
| Stopped shaking hands | 27 | 18 | 44 | 21 | 0.568 |
| Drank more than normal | 62 | 42 | 130 | 62 | <0.01 |
| None of above/did not follow any advice | 12 | 8 | 9 | 4 | 0.121 |
N = 356, one case missing.
Table 4 shows statements concerning attitudes towards vaccination and influenza disease divided according to gender and age. Women were more concerned about possible adverse events of the pandemic influenza vaccine than men (64% versus 45%, P < 0.01). Patients in age group ≤40 years of age reported more concerns for pandemic vaccine adverse effects (61% versus 48%, P = 0.017) as well as for seasonal vaccine adverse effects (24% versus 15%, P = 0.047) compared to the older age group. Willingness to accept vaccination next year was strongly associated with the higher age group (41% for age >40 years versus 18% for age ≤40 years of age, P < 0.01).
TABLE 4.
Number (n) and proportion of persons who agreed to statements concerning influenza vaccines and influenza disease
| Statement | Female |
Male |
P | ≤40 years of age |
>40 years of age |
P | ||||
| n | % | n | % | n | % | n | % | |||
| Vaccines do not protect well against influenza | 42 | 21 | 18 | 13 | 0.050 | 31 | 14 | 28 | 24 | 0.013 |
| I am concerned that the vaccine against swine flu may have serious side effects | 134 | 64 | 64 | 45 | <0.01 | 140 | 61 | 57 | 48 | 0.017 |
| I am concerned that the regular vaccine against seasonal flu may have serious side effects | 50 | 24 | 25 | 17 | 0.123 | 56 | 24 | 18 | 15 | 0.047 |
| Society should not use large amounts of money on influenza vaccination for whole population | 69 | 33 | 43 | 30 | 0.578 | 75 | 33 | 37 | 31 | 0.792 |
| It is positive that all inhabitants in Norway will be offered influenza vaccination | 179 | 86 | 118 | 83 | 0.384 | 195 | 85 | 101 | 85 | 0.945 |
| Influenza disease strengthens the immune system in healthy individuals, and they should not be vaccinated | 89 | 43 | 79 | 56 | 0.023 | 116 | 51 | 52 | 44 | 0.245 |
| Influenza is a benign disease for healthy individuals, and they do not need vaccination | 133 | 65 | 98 | 70 | 0.338 | 158 | 70 | 73 | 61 | 0.121 |
| I have a great risk of getting influenza later on | 33 | 16 | 24 | 17 | 0.842 | 33 | 14 | 24 | 21 | 0.148 |
| I have a small risk getting influenza because I just had influenza | 105 | 51 | 79 | 56 | 0.418 | 120 | 53 | 63 | 53 | 0.893 |
| I have a small risk of getting influenza because I hardly ever getting sick | 67 | 33 | 56 | 39 | 0.208 | 76 | 33 | 47 | 40 | 0.243 |
| I have a great risk getting seriously ill if I catch influenza again | 23 | 11 | 10 | 7 | 0.192 | 17 | 7 | 16 | 13 | 0.073 |
| I will accept influenza vaccination next year to protect myself against seasonal influenza | 51 | 25 | 41 | 28 | 0.422 | 41 | 18 | 50 | 41 | <0.01 |
Alternatives were dichotomized as agree/partly agree versus disagree/donot know.
Discussion
The most common symptoms reported by the 357 participants with ILI were fever, fatigue, headache and cough. There were significant differences in symptoms reported by individuals <18 years of age compared to the age group ≥18 years. Hardly any differences were found between patients with or without serologically confirmed influenza A (H1N1), except a longer period of coughing in patients with positive H1N1 serology as well as longer duration of illness. Women reported more frequent use of hand washing and cough hygiene than men. Women were also more concerned about the side effects of the pandemic influenza vaccine, and this was also the case for individuals in the age group ≤40 years of age as compared to older individuals.
There is limited data on clinical characteristics of patients in general practice with influenza A (H1N1) diagnosis.14,15 The majority of previous studies on the influenza pandemic are performed in hospital settings or emergency units.7,16 Several studies have been conducted on behavioural responses and attitudes towards vaccination in health care personnel during the pandemic17–20 and in the general public.21,22 To the best of our knowledge, this is the first study on behavioural responses during the influenza pandemic in general practice patients.
The main strength of our study is that we have information from >300 patients in general practice during the peak of the pandemic. However, we still lack statistical power for some analyses of subgroups in our material. Recall bias was minimized by mailing questionnaires a very short time after the acute disease.
A limitation of this study is the low response rate of 27%. The low response rate may influence the internal validity of the results and therefore also threaten the generalizability. A reminder to non-responders might have increased the response rate, but we had no opportunity to trace non-responders. However, the true response rate among patients with actual ILI is probably higher. Manual control in medical records of 15 GPs revealed that 4 GPs (and probably their administrative assistants) had applied the influenza diagnosis code (ICPC R80) inappropriately for the purpose of this study. For example, it was used for situations such as influenza in the family, concerns about vaccination, etc. as well as own influenza symptoms. Accordingly, some individuals not suffering from ILI received questionnaires. However, we do not have data to exactly quantify the response rate among patients with actual ILI.
In the general population in Norway, ∼56% of laboratory confirmed influenza cases were aged ≤19.23 This may be an indication of the actual age distribution of influenza cases, but the number may be biased in many ways. In our material, only 30% of patients were in the age group 0–17 years. Children are less likely to answer questionnaires, if not assisted by a parent, and assistance is essential for the youngest patients. Hence, there may be some selection bias in our study according to age. Patients who experienced mild influenza symptoms probably did not consult their GP during the pandemic, and we may have lost mild and subclinical cases of influenza. Most employees had 7 days of sick leave for ILI without a doctor’s certificate according to advice from the Norwegian Minister of Health. On 5 November 2009, neuraminidase inhibitors were released as over the counter drugs for patients presenting with ILI, which supported patients’ self-care, and they were thus less likely to contact their GP for treatment and advice. Also, some patients were probably missed to out-of-office duties managed by GPs, as reflected by local data reported that there were 440 consultations with emergency doctors in Bergen city during 1 week at the peak of the pandemic and 46 new hospital admissions.8 Our material still reflects the situation for patients seeking a GP consultation for influenza, with the exception of the age distribution.
We found a difference in symptoms between age groups among all ILI patients. Individuals <18 years of age reported fever, cough and GI symptoms in higher proportions than those in age group ≥18 years, whereas myalgias/arthralgias and subjective feeling of fever were more frequent among older patients. Among adults who had a serological test, there were no statistically significant differences between those with confirmed H1N1 influenza and those with negative results. The influenza A (H1N1) was characterized by typical flu-like symptoms (fever, cough, headache, fatigue and myalgia) and up to 50% had GI symptoms according to one review paper.5 Our results supports that GI symptoms were more common among ILI patients, and we found that children <18 years of age were particularly prone to have such symptoms. A study published from the Netherlands, reported a positive predictive value of 76% for diagnosis made by the GP during periods of high influenza activity.24 However, results in our study suggest that influenza is not clinically easy to distinguish from other viral causes of ILI, even during an influenza pandemic.
Belongia et al.25 did not find differences in clinical manifestations between pandemic and seasonal strains. Shiley et al.7 found that cough was more common among patients with pandemic H1N1 influenza in comparison to seasonal influenza (98% versus 83%. P = 0.007). Patients with positive H1N1 serology in our study reported longer period of coughing (13 versus 7 days) and higher percentage of coughing (82% versus 68%) compared with patients testing negative for H1N1 influenza, although the differences were not statistically significant. Finally, among the patients in our material, the rate of hospitalization, as a measure of clinical seriousness, was very low (<1%). This may mirror the reality or may be related to low statistical power or even selection bias.
In our material, 39% of patients were treated with oseltamivir, although this did not seem to affect the clinical course of ILI as compared to patients not treated with oseltamivir. The use of oseltamivir for the whole Norwegian population in 2009 was 6% (57.49/1000 inhabitants).26 The proportion of oseltamivir treatment is relatively high in our material as compared to the population. This may be due to selection of patients who actually see a GP for ILI, and also it may reflect a selection bias among patients answering the questionnaire. Among the 392 early cases of confirmed H1N1 virus in UK, 92% reported treatment with oseltamivir.27 Interpretation of differences in antiviral medication between these countries may be related to higher availability of oseltamivir in UK compared to the area this study was conducted.
Prior to and during the pandemic, the Norwegian Institute for Public Health informed the public about specific behavioural measures to avoid viral spread and prevent disease. Specific advices such as frequent hand washing, avoiding public places when ill, use of hand disinfectant and avoid viral spread by droplets by coughing in the elbow room were thoroughly announced to the public by different ways. Women in our study reported more frequent use of preventive measures than men, such as hand washing, coughing in elbow room and use of paper tissue. Previous studies have also shown that women are more likely to follow behavioural recommendations during influenza pandemics, such as hand washing and other measures to prevent viral transmission.20–22,28 The use of facemasks was not promoted actively in Norway during the 2009 influenza pandemic. Only 4% of participants in our study reported the use of facemask, despite free availability at the pharmacy as compared to studies from Asia were up to 90% wear masks during flu epidemics.22 A systematic review could not find evidence that facemasks protect against being infected during an outbreak of influenza.29 Attitudes towards vaccination and influenza disease may be better explored by the use of qualitative methods. However, our results give an indication of attitudes among ILI patients, and our findings are in line with findings from previous studies from other settings.17,19,20,30
Our study showed that women more often reported concern about side effects of vaccination than men, and a similar tendency was associated with younger age. A study by Wong et al.30 showed that factors associated with willingness to accept pandemic vaccination among community nurses was age ≥40 years and previous seasonal influenza vaccination. In another study, from Turkey, female health care providers were more concerned about vaccine safety than men.20
We conclude that important finding in our study is that discrimination between influenza and other viral upper respiratory tract infections is difficult in daily clinical practice, even during an influenza pandemic. Women reported better adherence to personal protective measures, such as hand washing and cough etiquette than men. Individuals ≤40 years of age reported more concerns about possible adverse effects of pandemic and seasonal vaccines compared to people in the age group >40 years of age. These results may be useful for health care planners when designing health protective campaigns in the future.
Declaration
Funding: this work was supported by Norwegian Medical Association’s Funds for Research in General Practice and the Norwegian Directorate of Health.
Ethical approval: Regional Committee for Medical Research Ethics (2009/1334).
Conflict of interest: none.
References
- 1.World Health Organization. Influenza A(H1N1)—update 6. 2009. http://www.who.int/csr/don/2009_04_30_a/en/index.html (accessed on 19 August 2011) [Google Scholar]
- 2.World Health Organization. What is the Pandemic (H1N1) 2009 Virus? 2010. http://www.who.int/csr/disease/swineflu/frequently_asked_questions/about_disease/en/ (accessed on 19 August 2011) [Google Scholar]
- 3.Zimmer SM, Burke DS. Historical perspective—emergence of influenza A (H1N1) viruses. N Engl J Med. 2009;361:279–85. doi: 10.1056/NEJMra0904322. [DOI] [PubMed] [Google Scholar]
- 4.Miller E, Hoschler K, Hardelid P, et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 5.Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine. 2010;28:4895–902. doi: 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 7.Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol. 2010;31:676–82. doi: 10.1086/653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhun AS, Akselsen PE, Sjursen H, et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine. 2010;29:266–73. doi: 10.1016/j.vaccine.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 9.Waalen K, Kilander A, Dudman S, et al. High prevalence of antibodies to the 2009 pandemic influenza A(H1N1) virus in the Norwegian population following a major epidemic and a large vaccination campaign in autumn 2009. Euro Surveill. 2010;15(31) pii=19633 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19633 (accessed on 19 August 2011) [PubMed] [Google Scholar]
- 10.Opstelten W, van Steenbergen JE, van Essen GA, van der Sande MA. Threat of an influenza pandemic: family physicians in the front line. BMC Fam Pract. 2009;10:11. doi: 10.1186/1471-2296-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temte JL, Prunuske JP. Seasonal influenza in primary care settings: review for primary care physicians. WMJ. 2010;109:193–200. [PubMed] [Google Scholar]
- 12.Hauge SH, Dudman SG, Borgen K, et al. [Disease caused by the new influenza A(H1N1) virus] Tidsskr Nor Laegeforen. 2009;129:1736–9. doi: 10.4045/tidsskr.09.0748. [DOI] [PubMed] [Google Scholar]
- 13.Statistics Norway. Population statistics pr. 10-01-01 in Norway. 2010. http://www.ssb.no/emner/02/01/10/folkemengde/arkiv/tab-2010-03-11-14.html (accessed on 19 August 2011) [Google Scholar]
- 14.Michiels B, Thomas I, Van Royen P, Coenen S. Clinical prediction rules combining signs, symptoms and epidemiological context to distinguish influenza from influenza-like illnesses in primary care: a cross sectional study. BMC Fam Pract. 2011;12:4. doi: 10.1186/1471-2296-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitsch-Osuch A, Kuchar E, Gyrczuk E, et al. Clinical manifestations of influenza caused by A/H1N1 virus among children and teenagers consulted in general practice. Eur J Med Res. 2010;15(suppl 2):105–7. doi: 10.1186/2047-783X-15-S2-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandsaeter BJ, Pillgram M, Berild D, et al. Hospitalised patients with suspected 2009 H1N1 Influenza A in a hospital in Norway, July–December 2009. BMC Infect Dis. 2011;11:75. doi: 10.1186/1471-2334-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virseda S, Restrepo MA, Arranz E, et al. Seasonal and Pandemic A (H1N1) 2009 influenza vaccination coverage and attitudes among health-care workers in a Spanish University Hospital. Vaccine. 2010;28:4751–7. doi: 10.1016/j.vaccine.2010.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opstelten W, van Essen GA, Heijnen ML, Ballieux MJ, Goudswaard AN. High vaccination rates for seasonal and pandemic (A/H1N1) influenza among healthcare workers in Dutch general practice. Vaccine. 2010;28:6164–8. doi: 10.1016/j.vaccine.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Kaboli F, Astrakianakis G, Li G, et al. Influenza vaccination and intention to receive the pandemic H1N1 influenza vaccine among healthcare workers of British Columbia, Canada: a cross-sectional study. Infect Control Hosp Epidemiol. 2010;31:1017–24. doi: 10.1086/655465. [DOI] [PubMed] [Google Scholar]
- 20.Savas E, Tanriverdi D. Knowledge, attitudes and anxiety towards influenza A/H1N1 vaccination of healthcare workers in Turkey. BMC Infect Dis. 2010;10:281. doi: 10.1186/1471-2334-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowling BJ, Ng DM, Ip DK, Liao Q, Lam WW, Wu JT, et al. Community psychological and behavioral responses through the first wave of the 2009 influenza A(H1N1) pandemic in Hong Kong. J Infect Dis. 2010;202:867–76. doi: 10.1086/655811. [DOI] [PubMed] [Google Scholar]
- 22.Lau JT, Griffiths S, Choi KC, Lin C. Prevalence of preventive behaviors and associated factors during early phase of the H1N1 influenza epidemic. Am J Infect Control. 2010;38:374–80. doi: 10.1016/j.ajic.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norwegian Institute of Public Health. Norwegian Surveillance System for Communicable Diseases (MSIS) 2010. http://www.msis.no/. (accessed on 19 August 2011) [Google Scholar]
- 24.van Elden LJ, van Essen GA, Boucher CA, et al. Clinical diagnosis of influenza virus infection: evaluation of diagnostic tools in general practice. Br J Gen Pract. 2001;51:630–4. [PMC free article] [PubMed] [Google Scholar]
- 25.Belongia EA, Irving SA, Waring SC, et al. Clinical characteristics and 30-day outcomes for Influenza A 2009 (H1N1), 2008-2009 (H1N1), and 2007-2008 (H3N2) infections. JAMA. 2010;304:1091–8. doi: 10.1001/jama.2010.1277. [DOI] [PubMed] [Google Scholar]
- 26.The Norwegian Institute of Public Health. Norwegian Prescription Database. http://www.norpd.no/ (accessed on 19 August 2011) [Google Scholar]
- 27.McLean E, Pebody RG, Campbell C, et al. Pandemic (H1N1) 2009 influenza in the UK: clinical and epidemiological findings from the first few hundred (FF100) cases. Epidemiol Infect. 2010;138:1531–41. doi: 10.1017/S0950268810001366. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Cheong HK, Son DY, Kim SU, Ha CM. Perceptions and behaviors related to hand hygiene for the prevention of H1N1 influenza transmission among Korean university students during the peak pandemic period. BMC Infect Dis. 2010;10:222. doi: 10.1186/1471-2334-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowling BJ, Zhou Y, Ip DK, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. 2010;138:449–56. doi: 10.1017/S0950268809991658. [DOI] [PubMed] [Google Scholar]
- 30.Wong SY, Wong EL, Chor J, et al. Willingness to accept H1N1 pandemic influenza vaccine: a cross-sectional study of Hong Kong community nurses. BMC Infect Dis. 2010;10:316. doi: 10.1186/1471-2334-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]