Sir,
The coronavirus disease 2019 (COVID-19) pandemic, caused by the SARS-CoV-2 coronavirus, emerged in Wuhan, China in December 2019 and spread worldwide in early 2020. By 20 April 2020, it had already infected 2 440 528 people and killed 167 592 patients. Numerous clinical studies on COVID-19 and its treatment have been launched in many countries within a short period of time.1 In a recent review up to 7 March 2020, 115 clinical trials testing antiviral drugs were analysed but the landscape of clinical trials is evolving rapidly and new studies are registered every day.2
In the current situation, it is critical that medical doctors and health institutions have immediate access to the protocols, design and results of these clinical trials in order to fight this major global health emergency.3 To facilitate this common effort, we have developed a unique platform that provides a comprehensive overview of the global landscape of COVID-19 clinical research activity. This platform, freely accessible at https://covid.inato.com/analysis, centralizes available data about clinical studies from the WHO International Clinical Trials Registry platform (https://www.who.int/ictrp/en/), ClinicalTrials.gov, chictr.org.cn and clinicaltrialsregister.eu. The platform is updated every day and displays studies in a user-friendly website, providing a live review of registered clinical studies and their main design features in real time. For every clinical study, it is possible to access the clinical registry webpage with full study details and up-to-date results whenever they are published. In addition, Anticovid provides interactive maps and charts summarizing the main data about the existing clinical studies, such as the drug(s) used, the countries involved and the cumulative number of patients per therapeutic class.
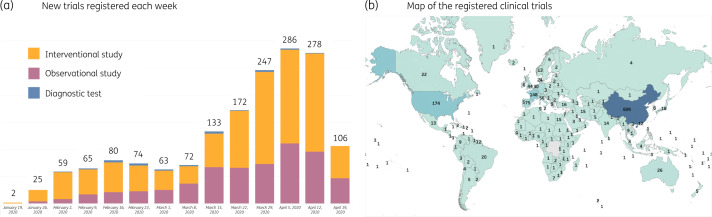
As of 26 April 2020, a cumulative total of 1708 studies had been registered, taking place in 67 countries on all continents (Figure 1). At this stage, China was still the country with the highest number of registered clinical studies (609, 36% of the total). The majority of studies were interventional (n = 1039; 61%) but a substantial proportion of the studies were observational (n = 595; 35%). Among the 1039 interventional studies, 562 studies (54%) studied pharmacological treatments. The most studied therapeutic classes were (hydroxy)chloroquine (n = 165/562; 29%), other antiviral agents (n = 115; 20%), immunomodulatory agents including interleukin inhibitors, Janus kinase (JAK) inhibitors and corticosteroids (n = 91; 16%) and traditional Chinese medicine (n = 53; 9%) (Figure 2). Other therapeutic classes included interferons (n = 36, 6%), blood (n = 50; 9%) or cell-based therapy (n = 22; 4%). Median anticipated sample size was found to be highly variable across studies and therapeutic classes (Figure 2) and quite low on average (123 patients). Considering the total number of patients to be enrolled, chloroquine or hydroxychloroquine (n = 169 052 patients) and other antiviral agents (n = 158 841 patients) were the two classes reaching the highest number. A total number of 44 private companies, cumulating 53 studies, were found to be involved as primary sponsors. For interventional studies, death was the primary outcome most frequently registered (n = 288/1039; 28%) followed by recovery time or length of hospital stay (n = 133; 13%) and ventilation mode (n = 113; 11%). The principal limitation of this platform is that data in clinical registries regarding study design, dose or duration of treatments being assessed, or primary outcomes, are often lacking—as has been described in a recent review.2 This restricts the information available for the platform and currently prevents more detailed analysis.
Figure 1.
(a) New trials registered each week and (b) map of the registered clinical trials (as displayed on the Anticovid platform for COVID-19 on 26 April 2020).
Figure 2.
(a) Median recruitment target for the five main treatments, (b) total recruitment target for the five main treatments, (c) total cumulative planned number of patients to be enrolled and (d) breakdown of trials by planned number of patients to be enrolled (as displayed on the Anticovid platform for COVID-19 on 26 April 2020).
The global clinical research effort against the COVID-19 pandemic is evolving at an unprecedented pace and scale (312 trials per month on average to date) and the first therapeutic guidelines have been produced in record time.4 As a benchmark for comparison, 190 clinical studies per year addressing HIV have been registered on ClinicalTrials.gov, 116 per year addressing influenza and 6 per year for dengue (data not shown). Coordination is incomplete and it is likely that there are both unmet needs and redundancies. The very high number of planned enrolments for clinical trials is worrying, even more so when we see trials stopped early because of a lack of new inclusions. Such a platform makes it possible to quickly see that the trials on hydroxychloroquine currently plan to include more patients than for all other antivirals together, while the interest of this treatment for COVID-19 is increasingly debated.5
This ongoing live review of the strategies that are registered in official clinical registries of clinical studies is an important asset for researchers and methodologists. It is, to date, the only one to our knowledge that centralizes this amount of information in a user-centred design. We will continue hosting, managing and upgrading the platform throughout the COVID-19 pandemic, given its potential usefulness for medical doctors, health authorities and drug sponsors.
Funding
Anticovid is an open-access platform developed by Inato. The development of Anticovid and this study was supported by internal funding.
Transparency declarations
Nathan Peiffer-Smadja, Erwan Sallard and François-Xavier Lescure have no financial links with Inato and no other conflicts of interest to declare. Bruno Vegreville is employed by Inato. Jean-David Zeitoun reports being an advisor for several consulting firms in connection with the pharmaceutical industry (Cepton, Oliver Wyman, Roland Berger, TBWA, Havas). He also reports speaking fees from a manufacturers’ professional association, consulting fees from IQVIA, Ferring, Pierre Fabre, AbbVie, AstraZeneca, Biogen, Boehringer Ingelheim, and Johnson & Johnson. He is a personal investor in approximately 20 digital companies, medtech companies or biotech companies, and as a limited partner in an investment fund. He is also a shareholder and advisory board member in several medtech companies. He reports being founding partner at Inato. Philippe Ravaud is a minority shareholder of Inato and has no other link to disclose, either within or outside the submitted work.
Author contributions
Conceptualization: N. Peiffer-Smadja and J. D. Zeitoun. Data collection, analysis and interpretation: N. Peiffer-Smadja, B. Vegreville and J. D. Zeitoun. Writing original draft: N. Peiffer-Smadja and E. Sallard E. Writing—review and editing: N. Peiffer-Smadja, E. Sallard, F. X. Lescure, J. D. Zeitoun and P. Ravaud.
References
- 1. Sanders JM, Monogue ML, Jodlowski TZ. et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020; doi:10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 2. Belhadi D, Peiffer-Smadja N, Yazdanpanah Y. et al. A brief review of antiviral drugs evaluated in registered clinical trials for COVID-19. medRxiv2020; doi:10.1101/2020.03.18.20038190.
- 3. Baden LR, Rubin EJ.. Covid-19—the search for effective therapy. N Engl J Med 2020; 382: 1851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IDSA. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. [DOI] [PMC free article] [PubMed]
- 5. Mahevas M, Tran V-T, Roumier M. et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. medRxiv2020; doi:10.1101/2020.04.10.20060699.