Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus disease 2019 (COVID-19), employs angiotensin-converting enzyme 2 (ACE2) for cellular entry.1
The effects of ACE inhibitors (ACEis) and angiotensin receptor blockers (ARBs) on ACE2 expression and activity are unclear. Concerns arose about possible interactions between renin–angiotensin–aldosterone system (RAAS) inhibitors and SARS-CoV-2 infectivity and virulence.2 An opposite hypothesis1 suggests that SARS-CoV-2 down-regulates ACE2 expression after cell entry, increasing RAAS activation and angiotensin II levels. Early evidence suggested that angiotensin II serum levels are increased in COVID-19 patients, and directly correlated with viral load and lung injury,3 while large observational studies did not detect harmful associations between ACEi/ARB therapy and the likelihood of COVID-19 or in-hospital mortality.4,5 However, no evidence is available on the effect on mortality of ACEi/ARB continuation or discontinuation in hospitalized COVID-19 patients.
Consecutive patients with confirmed COVID-19 admitted to Humanitas Clinical and Research Hospital (Rozzano-Milan, Lombardy, Italy) up to 1 April 2020 were enrolled in a prospective registry, which complies with the Declaration of Helsinki and was approved by our institutional Ethics Committee.
Discontinuation was defined as ACEi/ARB interruption at the time of hospital admission in patients known to be taking ACEis/ARBs at home. Patients suspending ACEis/ARBs during hospitalization for clinical reasons (e.g. hypotension, worsening renal function, etc.) were considered as the ACEi/ARB continuation group. The primary outcome was mortality. The odds ratio (OR) and 95% confidence interval (CI) were calculated with binary logistic regression. Clinical follow-up was censored at the date of death or discharge. ORs for mortality were adjusted with multivariable logistic backward regression analysis. The following covariates were included in the model (only vital parameters and laboratory values obtained within 24 h since hospital admission were included): age >75 years, body mass index >30 kg/m2, body temperature >37.5°C, estimated glomerular filtration rate (eGFR) <60 mL/min/m2, diabetes mellitus, chronic obstructive pulmonary disease, left ventricular ejection fraction <50%, malignancy, heart rate >100 b.p.m., respiratory rate >20 breaths/min, cardiac involvement [high sensitivity troponin I (hs-TnI ≥19.6 ng/L) and brain natriuretic protein (BNP) ≥100 pg/mL], C-reactive protein, white blood cells >10 000/mm3, and D-dimer (per 1 unit increase, ng/mL).
A total of 397 patients with COVID-19 were included; 14.1% continued ACEi/ARB therapy, 29.5% discontinued ACEis/ARBs at time of hospitalization, and 56.4% did not take these drugs at home.
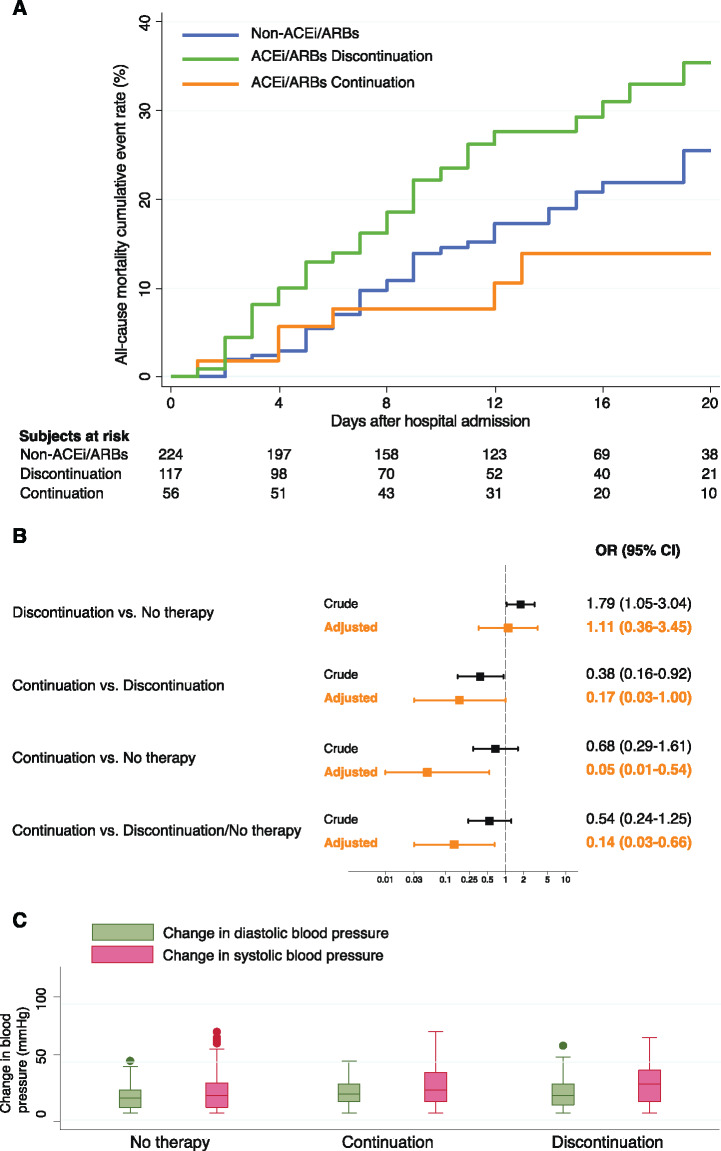
Mortality rates were 12.5% in the continuation group, 27.4% in the discontinuation group, and 17.4% in patients not taking ACEis/ARBs at home (P = 0.036). Cumulative event rates for mortality are displayed in Figure 1A. Crude and adjusted mortality risk estimates according to ACEi/ARB therapy are displayed in Figure 1B. Risk of mortality was significantly reduced in patients who continued ACEis/ARBs as compared with those who discontinued, those not taking ACEi/ARB therapy, and both these groups pooled. Changes in blood pressure values during hospitalization were similar between groups (Figure 1C).
Figure 1.
Impact of ACEi/ARB therapy on mortality. (A) Kaplan–Meier curves for mortality, stratified by ACEi/ARB therapy; (B) risk of mortality stratified by ACEi/ARB therapy; (C) box plots of blood pressure changes during hospitalization, stratified by ACEi/ARB therapy.
This is the first study investigating continuation and discontinuation of ACEis/ARBs in COVID-19 patients. Our findings can be summarized as follows: (i) 44% of hospitalized COVID-19 patients are on ACEi/ARB therapy at hospital hospitalization; (ii) approximately two-thirds of these patients discontinued ACEis/ARBs at hospital admission; and (iii) ACEi/ARB continuation during hospitalization was shown to be protective against mortality.
Due to hypothetical safety concerns,2 prior to any recommendations from scientific societies, some front-line physicians switched patients from ACEis/ARBs to other antihypertensive drugs.6
A large case–control study has shown improved survival in COVID-19 patients taking ACEis.5 However, there are no data evaluating the prognostic role of ACEi/ARB continuation/discontinuation in hospitalized COVID-19 patients. By showing a significantly lower risk of mortality in the continuation group after adjustment for several covariates, our findings support continuation of ACEi/ARB therapy during COVID-19 hospitalization.
Potential mechanisms of an ACEi/ARB-mediated protective effect include reduced severity of COVID-19 pneumonia, preserved hypoxic vasoconstriction, limited deterioration of renal function, and protection against myocardial injury.1 Moreover, ACEi/ARB discontinuation may lead to decompensations of pre-existing chronic heart failure and blood pressure instability.1 However, we did not observe a significant difference in changes of blood pressure values between groups during hospitalization.
Our study has some limitations. First, we are unaware of the reasons leading to ACEi/ARB discontinuation. However, this would not have changed our findings. Moreover, despite some significant differences in comorbidities and vital parameters between groups, covariate adjustment strengthened the results observed at unadjusted comparisons. Finally, the observational nature of our registry does not prove a causal relationship between ACEi/ARB continuation and reduced mortality. Nevertheless, our findings add urgently needed evidence in support of in-hospital ACEi/ARB continuation in COVID-19 patients.
In conclusion, COVID-19 patients who continue ACEis/ARBs have a lower risk of mortality compared with those discontinuing ACEis/ARBs at the time of hospitalization or not takiing these drugs at home, suggesting that ACEis/ARBs could have a therapeutic role in COVID-19 patients. Our findings support recommendations of cardiovascular societies of not suspending ACEis/ARBs in COVID-19 patients.
Conflict of interest: none declared.
References
- 1. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD.. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L.. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G.. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehra MR, Desai SS, Kuy SR, Henry TD, Patel AN.. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020; doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang (13 May 2020).