Abstract
Introduction
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) share their target receptor site with the SARS-CoV-2 virus, that may cause ACE2 receptor up-regulation which raised concerns regarding ACEI and ARB use in COVID-19 patients. However, many medical professional societies recommended their continued use given the paucity of clinical evidence, but there is a need for an updated systematic review and meta-analysis of the latest clinical studies.
Methods and results
A search was conducted on PubMed, Google Scholar, EMBASE, and various preprint servers for studies comparing clinical outcomes and mortality in COVID-19 patients on ACEIs and/or ARBs, and a meta-analysis was performed. A total of 16 studies were included for the review and meta-analysis. There were conflicting findings reported in the rates of severity and mortality in several studies. In a pooled analysis of four studies, there was a statistically non-significant association of ACEI/ARB use with lower odds of developing severe disease vs. non-users [odds ratio (OR) = 0.81, 95% confidence interval (CI): 0.41–1.58, I2=50.52, P-value = 0.53). In a pooled analysis of six studies, there was a statistically non-significant association of ACEI/ARB use with lower odds of mortality as compared with non-users (OR = 0.86, 95% CI = 0.53–1.41, I2 = 79.12, P-value = 0.55).
Conclusion
It is concluded that ACEIs and ARBs should be continued in COVID-19 patients, reinforcing the recommendations made by several medical societies. Additionally, the individual patient factors such as ACE2 polymorphisms which might confer higher risk of adverse outcomes need to be evaluated further.
Keywords: COVID-19, Angiotensin-converting enzyme inhibitor, Angiotensin receptor blocker, Meta-analysis, Mortality, Clinical severity
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-COV2) causes coronavirus disease (COVID-19), a potentially fatal disease that is of immense global public health concern. The initial cases were reported in December 2019 in Wuhan, China.1 Since then, there have been 3 041 764 confirmed COVID-19 patients in the world as of 27 April 2020, with a total of 211 167 deaths. The USA has the greatest number (988 189) of confirmed cases, with a total of 56 259 deaths. Most cases were diagnosed in New York (291 996), with a total of 22 668 deaths.2
Several studies, including a recent meta-analysis, have reported that co-existing conditions, including hypertension, cardiac diseases, cerebrovascular diseases, and diabetes, were common among patients with COVID-19 who had severe illness, were admitted to the intensive care unit (ICU), received mechanical ventilation, or died than among patients who had mild illness.3,4
Notably, of the most frequent comorbidities reported in these studies of patients with COVID-19, hypertension in particular is often treated with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs).5 This could theoretically result in an up-regulation of ACE2, which is an active binding target for SARS-CoV-2 virus,6 in the epithelial cells of the lung, intestine, kidney, and blood vessels.
Although this raised concerns regarding the use of these drugs in COVID-19 patients, several animal studies presented conflicting findings regarding increased ACE2 expression due to ACEIs and ARBs, and previous human studies suggested that administration of an ACEI/ARB does not increase ACE2 expression.7 In light of these findings and a paucity of clinical outcome studies, many professional cardiovascular and hypertension societies including the European Society of Cardiology, Italian Society of Pharmacology, International Society of Hypertension (ISH), European Society of Hypertension, Joint American Heart Association/American College of Cardiology/American Heart Failure Association, and others recommended the continued use of ACEIs/ARBs in COVID-19 patients.8–12
However, since the conception of these recommendations, several clinical studies have been conducted which evaluated the association of ACEIs and ARBs with clinical severity and mortality outcomes in COVID-19 patients. Therefore, the medical literature was systematically reviewed, and a meta-analysis was performed of the current clinical studies evaluating the safety and efficacy of ACEs and ARBs in COVID-19 patients.
Methods
Literature search
A literature search was conducted on the PubMed/MEDLINE database using keywords, i.e. ‘ACE inhibitors AND COVID’ and ‘ARB AND COVID’. We applied search filters to include humans and English language studies published up to 1 May 2020. Additional papers of possible interest were identified by examining references of pertinent review articles and searching Google Scholar and preprint servers such as MedRxiv and BioRxiv. We included studies which evaluated clinical severity and mortality outcomes for patients with COVID-19 on an ACEI, an ARB, or both.
We excluded those studies which were in vitro or conducted in animal models, as well as those human studies which evaluated only ACE expression levels (Figure 1).
Figure 1.
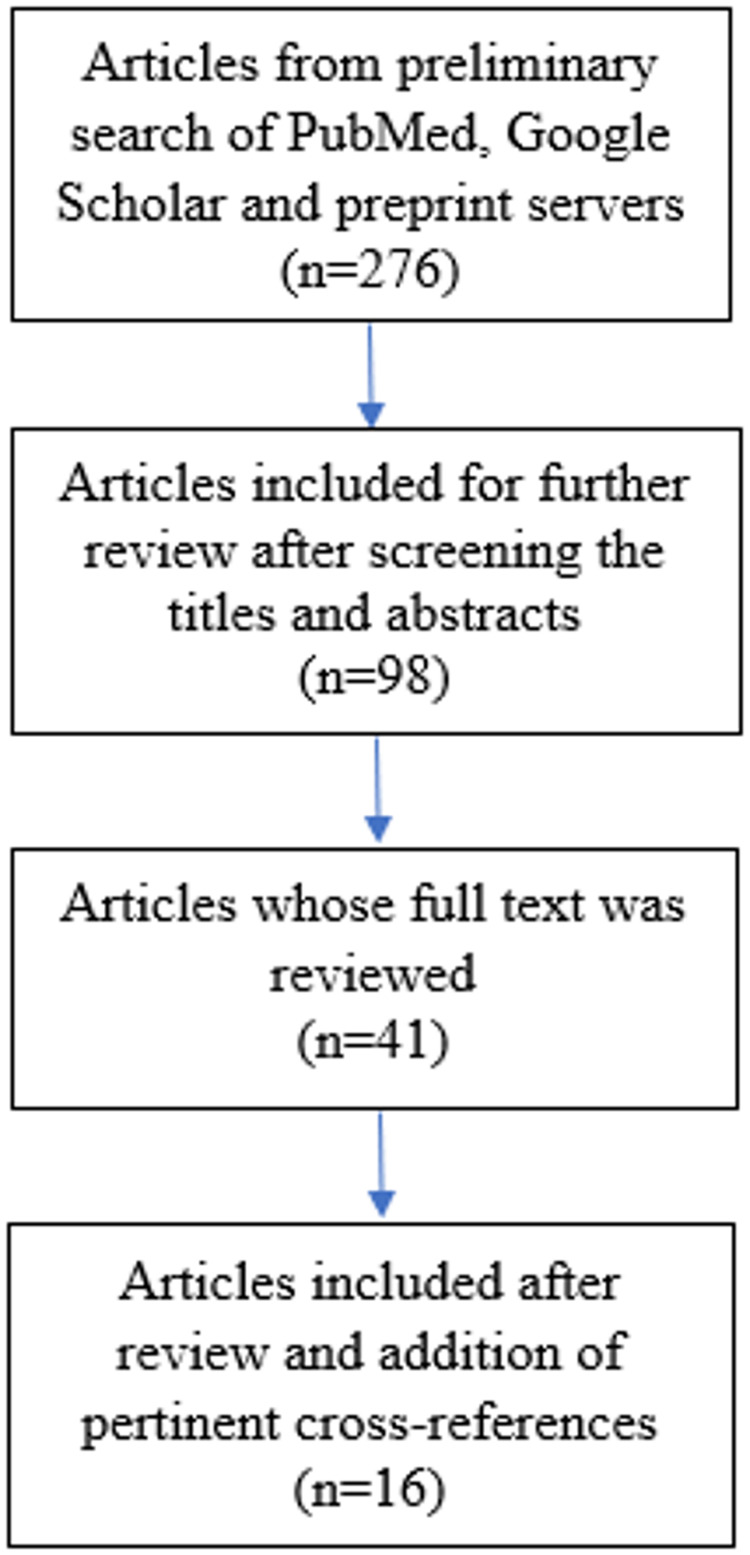
Flow diagram for study selection.
Data extraction
Information on the demographics, comorbidities, and pharmacotherapy with ACEIs, ARBs, and other drugs, clinical severity outcomes, and mortality was extracted.
Statistical analysis
The meta-analysis for severity and mortality was conducted for four and six peer-reviewed studies, respectively, using the comprehensive meta-analysis (CMA) software version 3 (Biostat Inc., Englewood, NJ, USA). The studies were assessed for methodological quality based on the Newcastle–Ottawa Scale (NOS).13 The NOS has eight criteria and generates scores ranging from 0 (high risk of bias) to 9 (low risk of bias). Studies with NOS scores of >7 were regarded as high quality. Heterogeneity was assessed using the Higgin’s I2 test, and the choice of fixed or random effects model was made based on the calculated heterogeneity. The publication bias was reported by using funnel plots. We reported the findings based on both a fixed and random effects model derived from the heterogeneity of the studies.
Results
A total of 276 articles were found in the search. Based on the screening of titles of the articles, 178 were excluded as they did not meet the inclusion criteria. Further, abstracts of 98 articles were read and, subsequently, the full text of 41 articles were reviewed. Of these, 16 articles were shortlisted which compared the clinical and/or mortality outcomes of COVID-19 patients on an ACEI or ARB with non-users.14–29 Finally, these 16 studies were included for review and, out of these, six and four studies were included in the meta-analysis of mortality and severity outcomes in COVID-19 patients on an ACEI/ARB, respectively (Table 1).
Table 1.
Demographic and clinical characteristics of the patients of the included studies
| Study (month year) | Country | No. of patients | Age (median, years) | Sex males | HTN | DM | Other comorbidities | ACEI/ARB usage |
|---|---|---|---|---|---|---|---|---|
| Meng et al. (March 2020) | China | 417 | 64.5* (IQR = 55.8–69.0) | 24* | 42† | 6* | 17* | |
| Richardson et al. (April 2020) | USA | 5700 | 63 (IQR = 52–75) | 3437 | 3026 | 1808 |
|
413‡ |
| Li et al. (April 2020) | China | 1178 | 55.5 (IQR = 38–67) | 545 | 362 | 203 |
|
115* |
| Liu et al. (March 2020) | China | 511 | 65.2 (mean) (SE = 10.7)* | 43* | 78 | NA | NA | 22* |
| Zhang et al. (April 2020) | China | 3430 | 57 (IQR = 45–65) | 1675 | 1128 | 388 |
|
188* |
| Feng et al. (April 2020) | China | 476 | 53 (IQR = 40–64) | 271 | 113 | 49 |
|
33* |
| Guo et al. (March 2020) | China | 187 | 58.50 (mean) (SD = 14.66) | 91 | 61 | 28 |
|
19 |
| Bean et al. (April 2020) | UK | 205 | 62.95 (mean) (SD = 19.94) | 106 | 105 | 62 | CAD/HF: 30 | 46 |
| Yang et al. (April 2020) | China | 251 | 66 (IQR = 61–73)* | 62* | 126 | 55 |
|
43 |
| Zeng et al. (April 2020) | China | 274 | 60 (mean) (SD = 15) | 150 | 75 | 42 |
|
28* |
| Ip et al. (April 2020) | USA | 3017 | NA | NA | 1584 | NA | NA | 460 |
| Yan et al. (April 2020) | China | 49 277 | 48.75§ (mean) (SD = 14.19) | 311§ | 137 | 60 | CD/CVD: 16 | 58 |
| Mancia et al. (May 2020) | Italy | 37 031 | 68 (mean) (SD = 13) | 23 329 | NA | NA |
|
15 375 |
| Mehra et al. (May 2020) | Asia, Europe, North America | 8910 | 49 (mean) (SD = 16) | 5346 | 2346 | 1272 | COPD: 225 | 1326 |
| Reynolds et al. (May 2020) | USA | 12 594¶ | 49 (IQR = 34 –63) | 5226 | 4357 | 2271 |
|
1110 |
| Dauchet et al. (May 2020) | France | 288** | NA | 179 | 105†† | 40 |
|
62‡‡ |
HTN, hypertension; DM, diabetes mellitus; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; IQR, interquartile range; CHD, coronary heart disease; AV block, atrioventricular block; CAD, coronary artery disease; HF, heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; ESRD, end-stage renal disease; CVD, cerebrovascular disease; SE, standard error; NA, not applicable; CD, cardiovascular disease; SD, standard deviation; RD, respiratory disease; KD, kidney disease, MI, myocardial infarction.
Reported for hypertensive patients.
Nine out of total 51 hypertensive patients were excluded in subsequent analysis because they were not on any antihypertensive drugs during hospitalization.
Home medication reconciliation information was available for 2411 of the 2634 patients who were discharged or who died by the study end.
Calculated for 610 COVID 19 patients out of total of 49 277.
Patients tested for COVID-19.
Patients aged over 35 years suspected of or diagnosed with COVID-19.
Patients on antihypertensive treatment.
Reported for COVID-19-positive patients (187 out of 288 suspected of or diagnosed patients).
All the included studies compared clinical severity-related outcomes in COVID-19 patients on an ACEI or ARB with non-users. However, there was non-uniformity in the definition of the severe outcomes amongst the studies. THe studies by Meng et al., Li et al., Liu et al., Feng et al., and Yang et al. were all conducted in China and defined clinical severity of COVID 19 based on guidelines established by the National Health Commission of the People’s Republic of China (7th edition).30 Of these, Meng et al., Liu et al., and Feng et al. reported that patients on an ACEI/ARB had lower rates of severe outcomes as compared with non-users, whereas Li et al. and Yang et al. reported equivalent results. Additionally, a study in France by Dauchet et al. also reported equivalent results. However, none of these studies performed adjustments for covariates or a matched analysis14,20,22–24,27 (Table 2). Based on the NOS, Meng et al., Richardson et al., Li et al., Feng et al., Guo et al., Yang et al., and Zeng et al. were lower quality studies, whereas the studies conducted by Liu et al., Zhang et al, Bean et al., Yan et al., Mancia et al., Mehra et al., and Reynolds et al. had high methodological quality.
Table 2.
Comparison of clinical severity and mortality outcomes in COVID-19 patients on an ACEI and/or ARB vs. non-users
| Study (month year) | No. of patients on an ACEI | No. of patients on an ARB | No. of patients on ACEI/ARB | No. of patients not on an ACEI/ARB | Severe outcomes on ACEI/ARB vs. no ACEI/ARB | Mortality on an ACEI/ARB vs. no ACEI/ARB |
|---|---|---|---|---|---|---|
| Meng et al. (March 2020) | 2 | 15 | 17 | 25 (HTN) | 23.5% vs. 48%* | 0% vs. 4% |
| Richardson et al. (April 2020) | 168 | 245 | 413 | 953 |
|
32.7% (ACEI) vs. 30.6% (ARB) vs. 26.7% (no ACEI/ARB) |
| Li et al. (April 2020) | NA | NA | 115 | 247 |
|
|
| Liu et al. (March 2020) (HTN, n = 78) | 3 | 19 | 22 | 17† | NA | |
| Zhang et al. (April 2020) | 31§ | 157§. | 174¶ | 348¶ |
|
Adjusted HR = 0.37 (95% CI = 0.15–0.89), P-value = 0.03 |
| Feng et al. (April 2020) | 8 | 27 | 33 | 62** | NA | |
| Guo et al. (March 2020) | NA | NA | 19 | 168 | Use of ACEIs/ARBs was higher in patients with elevated TnT levels (21.1% vs. 5.9%) | 36.8% vs. 25.6% |
| Bean et al. (April 2020) | 37 | 9 | 46 | 159 | 13.5% (ACEI) vs. 44.4% (ARB) vs. 27.7% (no ACEI/ARB)†† | NA |
| Yang et al. (April 2020) | NA | NA | 43 | 83 | 4.7% vs. 13.3%; P-value = 0.216 | |
| Zeng et al. (April 2020) | NA | NA | 28 | 47 |
|
7% vs. 11% |
| Ip et al. (April 2020) | 277 | 219 | 460 | 669 | NA | 27%, P-value = 0.001 (ACEI) vs. 33%, P-value = 0.12 (ARB) vs. 30% (ACEI/ARB) vs. 39% (no ACEI/ARB) |
| Yan et al. (April 2020) | 5 | 53 | 58 | NA | NA | |
| Mancia et al. (May 2020) | 8071 | 7304 | 15 375 | NA |
|
Included with critical or fatal outcomes |
| Mehra et al. (May 2020) | 770 | 556 | 1326 | NA | NA |
|
| Reynolds et al. (May 2020) | 627 | 664 | 1110 | 1101 | Included with severe outcomes | |
| Dauchetcet al. (May 2020) | 31*** | 31*** | 62*** | 23*** |
|
NA |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; HTN, hypertension; ICU, intensive care unit; NA, not applicable; OR, odds ratio; CI, confidence interval; HR, hazard ratio; ARDS, acute respiratory distress syndrome; TnT, troponin T; SPR1, standardized prevalence ratio (R1, North of France population reference).
Severity of COVID-19 patients according to the National Health Commission of the People’s Republic of China guidelines.
Not on any antihypertensive drug.
Odds ratio with reference to patients not on any antihypertensive.
Before matching.
After matching.
Other regimens.
Primary endpoint being death or transfer to a critical care unit for organ support within 7 days of symptom onset.
The criteria were based on the American Thoracic Society and Infectious Diseases Society of America.
Odds ratio of severe vs. non-severe.
Severe COVID-19 was defined as admission to the intensive care unit, the use of non-invasive or invasive mechanical ventilation, or death.
Reported for COVID-19-positive patients (187 out of 288 suspected of or diagnosed patients).
Richardson et al. and Zhang et al. compared the rates of invasive ventilation and found that they were slightly higher or equivalent in patients on an ACEI/ARB as compared with non-users, respectively. In addition, Richardson et al. also reported slightly higher rates of ICU admissions in patients on an ACEI (21.4%) and an ARB (20.8%) as compared with non-users (14.8%). Zhang et al. reported that the patients on an ACEI/ARB had lower rates of septic shock [hazard ratio (HR) = 0.32, P-value = 0.01] and acute respiratory distress syndrome (ARDS) (HR = 0.65, P-value = 0.07) as compared with non-users.15,21 On the other hand, Guo et al. found that patients with elevated troponin T (TnT) levels were more frequently on an ACEI/ARB (21.1% vs. 5.9%) (25) (Table 2).
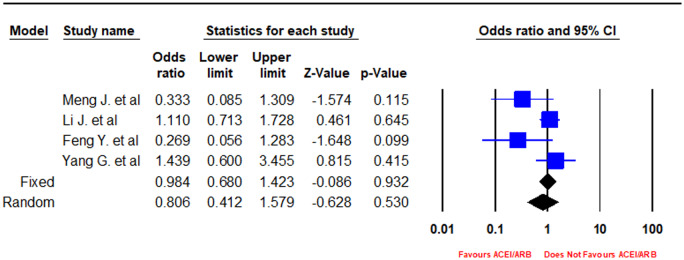
In a pooled analysis of four peer-reviewed studies, there was a statistically non-significant association of an ACEI/ARB with lower odds of developing severe disease vs. non-users (OR = 0.81, 95% CI 0.41–1.58, I2 = 50.52, P-value = 0.53) (Figures 2 and 3).
Figure 2.
Forest plot depicting meta-analysis of clinical severity based on Chinese guidelines in COVID-19 patients on an angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB).
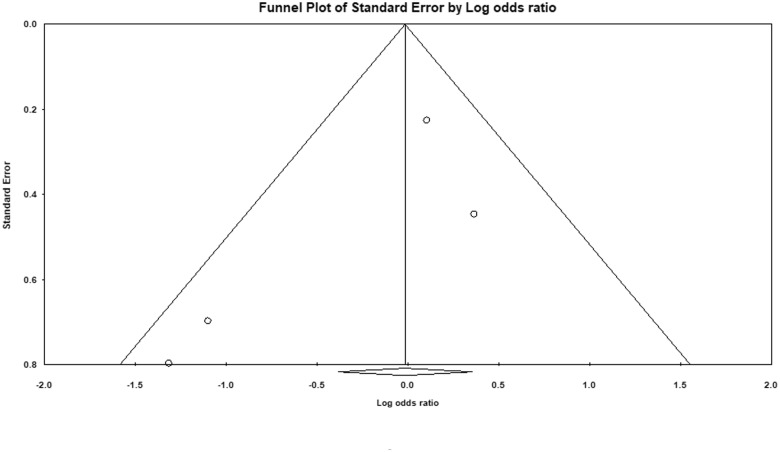
Figure 3.
Funnel plot depicting publication bias for studies evaluating clinical severity based on Chinese guidelines in COVID-19 patients on an angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB).
Mortality outcomes were assessed in nine studies, namely Meng et al., Richardson et al., Li et al., Zhang et al., Guo et al., Yang et al., Zeng et al., Ip et al., and Mehra et al. In addition, Bean et al. looked at composite primary endpoints including death or transfer to critical care for organ support within 7 days of symptom onset. Mancia et al. reported patients who received assisted ventilation or died as having a critical or fatal infection. Meng et al., Li et al., Yang et al., Zeng et al., and Ip et al. reported lower rates of mortality in ACEI/ARB users vs. non-users in an unadjusted analysis. Zhan et al. performed matching and an adjusted analysis of 522 patients in which they found that the rate of mortality was statistically significantly lower in patients on an ACEI/ARB as compared with non-users (HR = 0.37, P-value = 0.03]. Mehr et al. reported lower mortality in patients on an ACEI vs. no ACEI (OR = 0.33, 95% CI = 0.20–0.54). Similarly, Bean et al. found lower rates of their primary endpoint of death or critical care transfer in patients on ACEIs as compared with non-users (13.5% vs. 27.7%). Mancia et al. found a lower rate of critical or fatal outcomes in patients on an ACEI vs. no ACEI (OR = 0.91, 95% CI 0.69–1.21) and in patients on an ARB vs. no ARB (OR = 0.83, 95% CI 0.63–1.10). Similarly, Reynolds et al. found a slightly lower rate of severe outcomes which included admission to the ICU, the use of non-invasive or invasive mechanical ventilation, or death in patients on an ACEI/ARB (24.8%) vs. no ACEI/ARB (24.9%)14,15,17–19,21,22,25–29 (Table 2).
On the contrary, Guo et al. and Richardson et al. reported higher rates of mortality in patients on ACEIs/ARB as compared with non-users. Richardson et al. included 168 hypertensive patients on ACEIs, 245 on ARBs, and 953 patients on neither an ACEI nor an ARB, and reported 32.7, 30.6, and 26.7% mortality rates, respectively15,25 (Table 2).
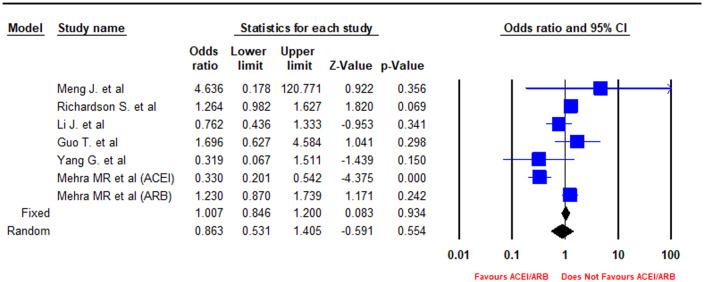
In a pooled analysis of six peer-reviewed studies, there was a statistically non-significant association of ACEIs/ARBs with lower odds of mortality as compared with non-users (OR = 0.86, 95% CI = 0.53–1.41, I2 = 79.12, P-value = 0.55) (Figures 4 and 5) The sensitivity of the pooled results of clinical severity and mortality outcomes to the removal of each study is reported in Supplementary material online, Figures S1–S4.
Figure 4.
Forest plot depicting meta-analysis of mortality outcomes in COVID-19 patients on an angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB).
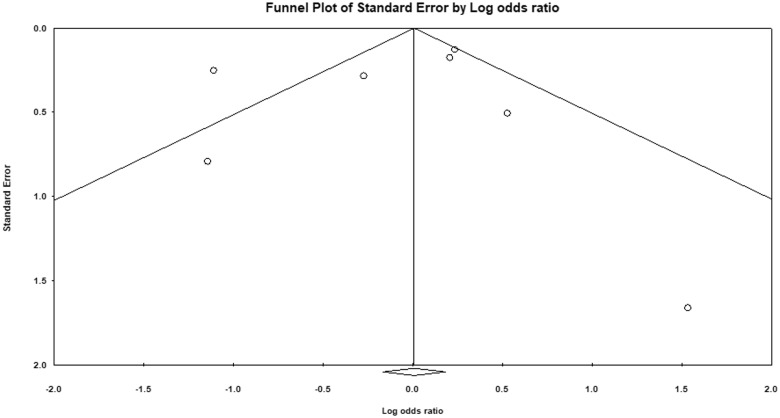
Figure 5.
Funnel plot depicting publication bias for studies evaluating mortality outcomes in COVID-19 patients on an angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB).
Discussion
Despite the worldwide implementation of public health measures such as social distancing, contact tracing, and mass testing to aid in the control of COVID-19, the global cases have risen to >3 million, and >200 000 patients had lost their lives by 27 April 2020,2,31 which further requires attention. Several studies have reported increased rates of COVID-19-associated mortality in patients with significant comorbidities such as hypertension, cardiovascular disease, chronic kidney disease, or heart failure.3,4 Although ACEIs and ARBs are commonly prescribed to treat some of these comorbidities, the fact that the ACE2 receptor is the main binding site for entry of SARS-CoV-2 caused concerns regarding the use of ACEIs and ARBs in such patients.5,32
Several evidence-based consensus and position statements were formulated by various cardiovascular and hypertension societies which recommended continued use of ACEIs and ARBs in COVID-19 patients, citing the lack of any contrary clinical evidence.8–12 Since then, however, several clinical studies have evaluated the association of ACEIs and ARBs in COVID-19 patients and comorbidities.
It is imperative to accurately predict clinical outcomes of COVID-19 patients, especially those with comorbidities and taking an ACEI or ARB, to decide whether to continue or switch to another medication. However, there were conflicting findings reported in several studies, as Meng et al., Liu et al., Feng et al., Zhan et al., Mancia et al., and Reynolds et al. reported that patients on an ACEI or an ARB had lower rates of severe outcomes, whereas Richardson et al. and Zhang et al. reported higher or equivalent rates of invasive ventilation, respectively. In addition, Richardson et al. reported a higher rate of ICU admissions in patients on ACEIs/ARBs as compared with non-users, and Guo et al. found that patients on ACEIs/ARBs had higher rates of cardiovascular disease and elevated TnT levels. It is pertinent to note that none of the above studies performed adjustment for covariates or matching for analysis, undermining the statistical strength of their results to a certain extent.14,15,17,19,21,23–25 However, Zhang et al. did perform matching and an adjusted analysis of 522 patients in which they found slightly higher rates of ICU admissions in patients on an ACEI (21.4%) or an ARB (20.8%) as compared with non-users (14.8%).21 In our random effects meta-analysis, a pooled analysis of four peer-reviewed studies conducted in China revealed that there was a statistically non-significant association (OR = 0.81, 95% CI 0.41–1.58, I2=50.52, P-value = 0.53) of ACEI/ARB use with lower odds of developing severe disease defined as per the Chinese COVID-19 guidelines in patients on ACEIs/ARBs vs. non-users (Figures 2 and 3).
Similarly, there were also conflicting results on the rate of mortality reported by the various clinical studies. Meng et al., Li. et al., Zhang et al., Yang et al., Zeng et al., and Ip et al. reported lower rates of mortality in ACEI/ARB users vs. non-users. whereas Guo et al. and Richardson et al. reported higher rates of mortality in patients on ACEIs/ARBs as compared with non-users.14,15,21,22,25,27–29 Zhang et al. again performed matching and adjustment in assessing the mortality outcomes, strengthening their conclusions regarding safety of ACEI/ARB use; however, a large sample size study conducted in New York in >1000 COVID-19 patients by Richardson et al. raised concerns of worse mortality outcomes with ACEI/ARB use and cannot be overlooked.15,21 In a pooled analysis of six peer-reviewed studies, there was a statistically non-significant association of ACEI/ARB use with lower odds of mortality as compared with non-users (OR = 0.86, 95% CI = 0.53–1.41, I2 = 79.12, P-value = 0.55) (Figures 4 and 5).
Several hypotheses have been put forward explaining the positive and negative aspects of ACEI/ARB administration in COVID-19 patients. Positive effects include ACE2 receptor blockade, disabling viral entry into the heart and lungs, and an overall decrease in inflammation secondary to ACEIs/ARBs, suggesting that the use of an ACEI might be protective against respiratory complications. Negative effects include a possible retrograde feedback mechanism, by which ACE2 receptors are up-regulated, which may lead to severe pneumonia, increasing the risk of severe outcomes and mortality.33 Individuals with ACE2 polymorphisms have an increased genetic predisposition for an increased risk of SARS-CoV-2 infection and may have harmful effects of ACEIs/ARBs.34 This aspect is worth considering and needs to be evaluated in future studies.
To the best of our knowledge, this systematic review is a comprehensive exploration and analysis of existing literature in this topic to date. Our review has limitations in its rigour due to the scarce existing data and diverse study types available. The rapidly emerging knowledge base of COVID-19 presents the possibility that a few studies (particularly unpublished/under peer review) remain uncaptured. However, we have tried our best to mitigate this by allowing broad search terms and by including many databases and repositories. We have also tried to comprehensively review and analyse the existing data.
Considering the inconsistent clinical studies and conflicting hypotheses, it is essential to evaluate the clinical outcomes in COVID-19 patients on ACEIs/ARBs in future large studies, particularly randomized controlled trials, and additionally evaluate the association of clinical outcomes with ACE2 polymorphisms. Based on this, there are ongoing trials studying the effect of losartan (an ARB) in patients with COVID-19 in outpatient and inpatient settings (NCT04311177 and NCT04312009).35,36
Conclusion
It is concluded that ACEIs and ARBs should be continued in COVID-19 patients, reinforcing the recommendations made by several medical societies. Additionally, the individual patient factors such as ACE2 polymorphisms which might confer higher risk of adverse outcomes need to be evaluated further.
Author contributions
A.G.: conception and design, data acquisition and analysis, drafting the manuscript, and final approval of the manuscript. M.O.: data acquisition, drafting and final approval of the manuscript.
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Conflicts of interest: none declared.
Data availability statement: The data underlying this article are available in the article and in its online Supplementary material.
Supplementary Material
References
- 1. Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol 2020;18:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Map – Johns Hopkins Coronavirus Resource Cente. https://coronavirus.jhu.edu/map.html
- 3. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y.. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD.. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rehan HS, Grover A, Hungin APS.. Ambiguities in the guidelines for the management of arterial hypertension: Indian perspective with a call for global harmonization. Curr Hypertens Rep 2017;19:17. [DOI] [PubMed] [Google Scholar]
- 6. Wan Y, Shang J, Graham R, Baric RS, Li F.. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020;94:e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sriram K, Insel PA.. Risks of ACE inhibitor and ARB usage in COVID‐19: evaluating the evidence. Clin Pharmacol Ther 2020;doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Society of Cardiology Council on Hypertension. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. 2020https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 9. Trifirò G, Crisafulli S, Andò G, Racagni G, Drago F.. Should patients receiving ACE inhibitors or angiotensin receptor blockers be switched to other antihypertensive drugs to prevent or improve prognosis of novel coronavirus disease 2019 (COVID-19)? Drug Saf 2020;43:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The International Society of Hypertension. A statement from the International Society of Hypertension on COVID-19. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/
- 11.European Society of Hypertension. ESH STATEMENT ON COVID-19. https://www.eshonline.org/spotlights/esh-statement-covid-19/
- 12.Heart Failure Society of America; American College of Cardiology; American Heart Association. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19 – American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-COVID-19
- 13. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 14. Meng J, Xiao G, Zhang J, He X, Ou M, Bi J, Yang R, Di W, Wang Z, Li Z, Gao H, Liu L, Zhang G.. Renin–angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 2020;9:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KWand the Northwell COVID-19 Research ConsortiumBarnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP.. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan H, Valdes AM, Vijay A, Wang S, Liang L, Yang S, Wang H, Tan X, Du J, Jin S, Huang K, Jiang F, Zhang S, Zheng N, Hu Y, Cai T, Aithal GP.. Role of drugs affecting the renin–angiotensin–aldosterone system on susceptibility and severity of COVID-19: a large case–control study from Zheijang Province, China. medRxiv 2020;2020.04.24.20077875. [Google Scholar]
- 17. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G.. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN.. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS.. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dauchet L, Lambert M, Gauthier V, Poissy J, Faure K, Facon A, Yelnik C, Panaget S, Plagnieux T, Verfaillie F, Mathieu D, Goldstein P, Meirhaeghe A, Amouyel P, on behalf of the Lille COVID-19 study group. ACE inhibitors, AT1 receptor blockers and COVID-19: clinical epidemiology evidences for a continuation of treatments. The ACER-COVID study. medRxiv 2020;2020.04.28.20078071. [Google Scholar]
- 21. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H.. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;26:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Wang X, Chen J, Zhang H, Deng A.. Association of renin–angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020;e201624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Huang F, Xu J, Yang P, Qin Y, Cao M, Wang Z, Li X, Zhang S, Ye L, Lv J, Wei J, Xie T, Gao H, Xu K-F, Wang F, Liu L, Jiang C.. Anti-hypertensive angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. medRxiv 2020;2020.03.20.20039586. [Google Scholar]
- 24. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F, Lu Y, Liu X, Chen Y, Li X, Li Y, Summah HD, Lin H, Yan J, Zhou M, Lu H, Qu J.. COVID-19 with different severity: a multi-center study of clinical features. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med 2020;201:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z.. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bean D, Kraljevic Z, Searle T, Bendayan R, Pickles A, Folarin A, Roguski L, Noor K, Shek A, O’Gallagher K, Zakeri R, Shah A, Teo J, Dobson RJB.. Treatment with ACE-inhibitors is associated with less severe disease with SARS-Covid-19 infection in a multi-site UK acute Hospital Trust. medRxiv 2020;2020.04.07.20056788. [Google Scholar]
- 27. Yang G, Tan Z, Zhou L, Yang M, Peng L, Liu J, Cai J, Yang R, Han J, Huang Y, He S.. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID-19 patients with hypertension: a single center retrospective study. Hypertension 2020;doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 28. Zeng Z, Sha T, Zhang Y, Wu F, Hu H, Li H, Han J, Song W, Huang Q, Chen Z.. Hypertension in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. medRxiv 2020;2020.04.06.20054825. [Google Scholar]
- 29. Ip A, Parikh K, Parrillo JE, Mathura S, Hansen E, Sawczuk IS, Goldberg SL.. Hypertension and renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. medRxiv 2020;2020.04.24.20077388. [Google Scholar]
- 30.China National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). 2020. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html
- 31. Matrajt L, Leung T.. Evaluating the effectiveness of social distancing interventions to delay or flatten the epidemic curve of coronavirus disease. Emerg Infect Dis 2020;26:doi: 10.3201/eid2608.201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rico-Mesa JS, White A, Anderson AS.. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep 2020;22:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losartan for Patients With COVID-19 Not Requiring Hospitalization – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04311177
- 36.Losartan for Patients With COVID-19 Requiring Hospitalization – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04312009
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.