This editorial refers to ‘Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019’†, by S. Shi et al., on page 2070.
The coronavirus (COVID-19) pandemic due to SARS-CoV-2 has taken hold all over the world. Our Chinese colleagues, who were on the pandemic frontline, are now starting to describe the clinical features and cardiovascular presentations seen in these patients and particularly the signals provided by biomarkers.1–4 In this issue of the European Heart Journal, the group from Wuhan Renmin University describe, in one of the larger cohorts (n = 671) reported to date, the importance of increases in cardiac troponin I (cTnI) in patients with a COVID-19 infection who were sick enough to require respiratory support.1 The authors found that a cTnI value of >0.026 ng/mL (AUC 0.92) was the optimal threshold for predicting in-hospital mortality, which is within the normal population range for this assay (<0.04 ng/mL). Overall, 62 (9.2%) patients died in hospital, of whom 53 (85% of deaths) had a cTnI >0.026 ng/mL and 47 (76%) had values >0.04 ng/mL. Mortality was 50% (53 of 106) for patients with a cTnI >0.026 ng/mL compared with just nine deaths (1.6%) amongst 565 patients with a lower cTnI. In multivariable analysis, older age and cardiovascular comorbidities were associated with higher cTnI. These findings are consistent with prior reports.2–4
There are some issues about how cTnI values were used in this analysis that should be appreciated. Only single samples, mostly obtained on admission, were used. Many more patients would have had an elevated cTnI at some point during their hospitalization. Multiple sampling to identify changes in values, reflecting progression or resolution of disease, will probably add to the prognostic significance of cTnI.5 The hospital used the Siemens Ultra assay, a non-high sensitivity assay. It is very likely that when high sensitivity troponin (hscTn) assays are used, the proportion with increased values will be even greater. The optimal value for prognostic classification was reported to be 0.026 ng/mL which is within the normal range (<0.04 ng/mL) for this assay. Had an hscTn assay been used, this confusing overlap might have been avoided. Clinicians should also appreciate that, in general, cTnT is more likely to be elevated for reasons other than ischaemia when compared with cTnI6 and that hscTnT may predict outcome more strongly in these circumstances.7 The reasons for this are unclear, but clinicians reading these articles need to consider this issue in extrapolating these data to their local practice. These factors in aggregate have probably led to underestimation of the frequency and magnitude of cTn increases based on the methods used in this study.
There are many reasons why troponin elevations might occur during a COVID infection (Figure 1) although this was not the focus of the current study. Patients with cardiovascular diseases (CVD) and risk factors such as hypertension and diabetes are at high risk of developing severe manifestations of COVID-19. Whether this reflects a greater susceptibility to, or more severe consequences of, infection is currently unclear. There is much speculation over the role of the angiotensin-converting enzyme 2 (ACE2) receptor for CoV-2 cellular docking which may be influenced by CVD and/or its treatment.8 Increases in cTn are common in patients with chronic stable CVD, especially when complicated by heart failure, and portend a worse prognosis.9 Modest elevations in cTn during COVID infections may just be a surrogate marker for the underlying severity of pre-existing CVD.
Figure 1.
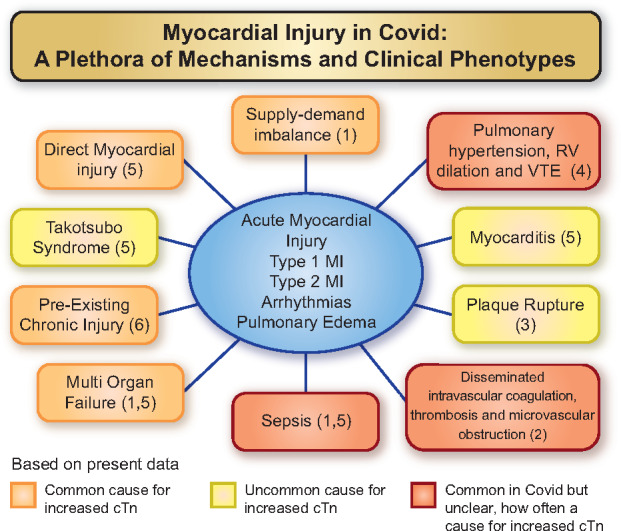
Possible mechanisms for myocardial injury (an increased cTn value) in patients with COVID-19. Based on present data, orange boxes denote common aetiologies. Yellow boxes denote uncommon causes, and red boxes denote situations that are common with COVID-19 disease and may have a role in causing cardiac injury in some patients. MI, myocardial infarction; RV, right ventricular; VTE, venous thromboembolism.
Increases in cTn may also reflect acute myocardial injury. In some cases, this will be due to acute atherosclerotic plaque disruption, coronary thrombosis, and epicardial coronary occlusion (a so-called type 1 event). However, in critically ill patients, supply–demand imbalance leading to myocardial injury is more common,10 due to the metabolic stress of the infection because of tachycardia, hypoxia, acidosis, and hypotension with or without substantial pre-existing epicardial coronary disease. When this is accompanied by clinical evidence of myocardial ischaemia, it is termed type 2 myocardial infarction. When myocardial ischaemia is not present, the proper term for the increased cTn even with a rising pattern is ‘acute myocardial injury’ as per the Universal Definition of Myocardial Infarction.11
In data published a decade ago, long before we knew about COVID, increases in non-hscTn were found to be common (42%) in patients with severe respiratory infections, and predicted a higher mortality.12 Increases in cTn are unsurprising in patients who are close to death but, importantly, increases in cTn improved prediction of outcome for those in the grey zone of intermediate risk. Thus, the patients for whom cTn provided unique information was not the group with very high APACHE or SOFA scores but those with lower scores where an elevated troponin told the physician that the risk was greater than the clinical picture suggested. Indeed, in the present study, cTnI appeared to be of little prognostic value in older people who were uniformly at high risk. Its value was in younger patients with markedly elevated cTn values (figure 5B, right hand panel in Shaobo et al.1). Patients with severe respiratory disease and an elevated cTnI generally require treatment of the underlying lung injury rather than an invasive coronary intervention.13 This is a difficult issue for some cardiologists, who believe that whenever troponin is elevated, angiography is obligatory. The COVID-19 pandemic provides a unique opportunity to change that sort of thinking.
This issue may be vexing to cardiologists because of the well-reported temporal relationship between antecedent viral infections of the respiratory tract and the subsequent development of myocardial infarction.14 However, at present, it appears that type 1 myocardial infarction is not common in this COVID-19 pandemic.1–4 Perhaps patients are choosing not to come to hospital for medical advice. Perhaps social distancing is reducing the risk of events. However, the inflammatory response may not be benign. Infection with SARS-CoV-2 impairs endothelial function and haemostatic balance, thus increasing thrombin activity, reducing plasminogen activator inhibitor-1 (PAI-1) activity, and accelerating production of fibrin degradation products and D-dimer.15 Endothelial inflammation, vascular oedema, and disseminated intravascular coagulation may lead to microvascular dysfunction and occlusion.15 These factors may exacerbate myocardial oxygen supply/demand imbalance due to hypoxia and tachycardia. Such an effect would be also be synergistic with the presence of fixed coronary stenoses, pulmonary hypertension due to lung injury, hypoxia, and pulmonary embolism. They could, at least in theory, also lead to coronary thrombosis. These findings are very similar to those reported previously with acute respiratory distress syndrome.16 In addition, with systemic infection, inflammation in many organs is expected.
Whether the severe inflammatory response driven by a cytokine storm in COVID destabilizes coronary plaques leading to rupture and intracoronary thrombus formation remains speculative. Recently, there have been newspaper reports of sudden cardiac death. Many of these may have been due to pulmonary emboli which are said to be common.17 They could also be due primarily to an arrhythmia or, less likely, some could be due to acute coronary occlusion. Although arrhythmias have been described in the present and other studies of COVID-19, it is not clear that, in the absence of drugs that prolong the QTc interval which some now use as therapy, the incidence of these are greater than one might expect from patients with any severe respiratory infection leading to myocardial injury.12,18
There are other potential causes of acute myocardial injury. Widespread inflammation and cell death probably account for the dramatic increases in serum ferritin observed in severe COVID-19. The increase in cTn may often reflect the heart’s ‘share’ of the generalized inflammation rather than organ-specific myocarditis. In addition, although only documented in case reports, other diseases seen in the critically ill can also occur, such as Takotsubo cardiomyopathy.19 Case reports also provide anecdotal evidence of myocarditis in patients with COVID-19.20 However, there are no robust published data on the prevalence and severity of concomitant myocarditis using sophisticated imaging or detailed pathological analyses. Based on present information, the incidence of myocarditis may not be high. The cases that do occur are reminiscent of the cases of acute myocarditis mimicking myocardial infarction reported many years ago.21 Pulmonary hypertension, either due to lung injury and hypoxia or due to pulmonary embolism and thrombosis, may place a severe load on the right ventricle, leading to myocardial damage and troponin release, and the high frequency of venous thrombo-embolism recently described.17
In conclusion, serial measurements of troponin might be useful in predicting risk in patients who are not already severely ill. Whether small increases in troponin reflect disproportionate myocardial involvement in the systemic inflammatory response to infection, COVID-19, or other organisms, is uncertain. Many of the increases in cTn observed are likely to reflect myocardial injury due to pre-existing disease and/or in response to critical illness. The inflammatory and coagulopathic stress responses may be non-specific or more marked with coronavirus. Organ-specific myocarditis may be uncommon. More research is required to determine whether acute myocardial injury is a marker or driver of clinical outcomes and whether it leaves a legacy of myocardial dysfunction, fibrosis, scar, and arrhythmia. Our Chinese colleagues have made an essential first step on this important path of research that will help us understand this new disease and improve patient care.
Conflict of interest: A.S.J. presently or in the past has consulted for most of the major diagnostic companies. J.G.F.C. has consulted for Abbott and Roche. H.A.K. has consulted for Roche Diagnostics.
Footnotes
† doi:10.1093/eurheartj/ehaa408.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Shaobo S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z.. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vestergaard KR, Jespersen CB, Arnadottir A, Soletormos G, Schou M, Steffensen R, Goetze JP, Kjoller E, Iversen KK.. Prevalence and significance of troponin elevations in patients without acute coronary disease. Int J Cardiol 2016;222:819–825. [DOI] [PubMed] [Google Scholar]
- 7. Arnadottir A, Vestergaard KR, Pallisgaard J, Soletormos G, Steffensen R, Goetze JP, Iversen K.. High-sensitivity cardiac troponin T is superior to troponin I in the prediction of mortality in patients without acute coronary syndrome. Int J Cardiol 2018;259:186–191. [DOI] [PubMed] [Google Scholar]
- 8. Nicin l, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Walther T, Zeiher AM, Dimmeler S.. CoV-2 receptor ACE2 in human hearts. Eur Heart J 2020;inpress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E; PEACE Investigators. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61:1240–9, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Babuin L, Vasile VC, Rio Perez JA, Alegria JR, Chai HS, Afessa B, Jaffe AS.. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients Crit Care Med 2008;36:759–765. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, BAX JJ, Morrow DA, White HD, the Executive Group on behalf of the Joint ESC/ACC/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 12. Vasile VC, Chai HS, Khambatta S, Afessa B, Jaffe AS.. Significance of elevated cardiac troponin T levels in critically ill patients with acute respiratory disease. Am J Med 2010;123:1049–1058. [DOI] [PubMed] [Google Scholar]
- 13. Sandoval Y, Jaffe AS.. Type 2 myocardial infarction. J Am Coll Cardiol 2019;73:1846–1860. [DOI] [PubMed] [Google Scholar]
- 14. Kwong JC, Schwartz KL, Campitelli MA.. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018;378:345–353. [DOI] [PubMed] [Google Scholar]
- 15. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS.. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia: a review. Crit Care Med 2006;34:871–877. [PubMed] [Google Scholar]
- 17. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH.. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sode BF, Dahl M, Nordestgaard BG.. Myocardial infarction and other co-morbidities in patients with chronic obstructive pulmonary disease: a Danish nationwide study of 7.4 million individuals. Eur Heart J 2011;32:2365–2372. [DOI] [PubMed] [Google Scholar]
- 19. Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C.. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu H, Ma F, Wei X, Fang Y.. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franz WM, Remppis A, Kandolf R, Kubler W, Katus HA.. Serum troponin T: diagnostic marker for acute myocarditis. Clin Chem 1996;42:340–341. [PubMed] [Google Scholar]


