Abstract
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has important implications for the safety of participants in clinical trials and the research staff caring for them and, consequently, for the trials themselves. Patients with heart failure may be at greater risk of infection with COVID-19 and the consequences might also be more serious, but they are also at risk of adverse outcomes if their clinical care is compromised. As physicians and clinical trialists, it is our responsibility to ensure safe and effective care is delivered to trial participants without affecting the integrity of the trial. The social contract with our patients demands no less. Many regulatory authorities from different world regions have issued guidance statements regarding the conduct of clinical trials during this COVID-19 crisis. However, international trials may benefit from expert guidance from a global panel of experts to supplement local advice and regulations, thereby enhancing the safety of participants and the integrity of the trial. Accordingly, the Heart Failure Association of the European Society of Cardiology on 21 and 22 March 2020 conducted web-based meetings with expert clinical trialists in Europe, North America, South America, Australia, and Asia. The main objectives of this Expert Position Paper are to highlight the challenges that this pandemic poses for the conduct of clinical trials in heart failure and to offer advice on how they might be overcome, with some practical examples. While this panel of experts are focused on heart failure clinical trials, these discussions and recommendations may apply to clinical trials in other therapeutic areas.
Keywords: Heart failure, Clinical trials, COVID-19, Coronavirus
Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused an outbreak of pneumonia that has now led to a pandemic.1 By May 2020, the World Health Organization (WHO) had reported >5 million cases and >250 000 deaths to the ‘coronavirus disease 2019’ (COVID-19).2 COVID-19 is highly transmissible and spreads rapidly in the population. Many of those infected will develop a fever and persistent cough, and some will progress to severe lung injury.3,4 Acute respiratory failure may lead to severe complications or death, particularly in patients with pre-existing cardiovascular disease.1,4
The COVID-19 pandemic poses potential serious additional risks for participants and staff engaged in clinical trials; their safety is of key importance. From a patient perspective, potential risks entail not only additional exposure to infection during research visits, but also risks due to disruption of general clinical care or due to failure to monitor adequately the continuation or withdrawal of research interventions. The safety of patients and those who care for them is paramount, but we should also ensure, as far as possible, that clinical trials are still able to answer the questions they were designed to address.5,6
The importance of the social contract for clinical trials
Investigators, participants, and funders enter a ‘social contract’ when they decide to participate in a trial. Participants make the largest investment: their own health and well-being, time, and effort, often made over a period of years, should be valued immensely. Investigators and funders provide their intelligence, expertise, curiosity, questions, and trial designs. Investigators guide patient care and investigation during a trial, considering both trial-specific and general medical issues that arise, and funders provide opportunities, resources, and management expertise. The different stakeholders in this ‘social contract’ also have both expectations and motivations, some common and some diverse. Patients would like a better treatment for themselves and others in a similar situation, now or in the future. Investigators, physicians, and nurses want to help their patients, expand their professional knowledge and skills, and help answer important medical questions for their patients and the wider society. Commercial companies have specialist knowledge in several important areas that often exceeds that of all other parties. They may offer substantial resources, in terms of manpower, organization, equipment, expertise, and finances. However, a return of investment is needed in order to reward the stakeholders who have financed the research and to enable the company to invest in future research.
As physicians and trial leaders, we consequently have the moral and ethical duty to our patients and the many thousands of people currently participating in clinical trials to provide reliable results. We are responsible for the safety of trial participants, but must also ensure that the commitment of trial volunteers is worthwhile.
The integrity of clinical trials and the reliability of their results are essential for current and future generations of patients. This remains true for heart failure (HF) trials even during the COVID-19 crisis. When the COVID-19 epidemic has subsided, HF will still remain a major cause of morbidity and mortality.
Why is this consensus document needed?
Recognizing the unprecedented challenges facing clinical trials during this pandemic, many national or regional regulatory authorities have issued guidance on the conduct of clinical trials in the face of the COVID-19 crisis (Supplementary material online, Appendix S1). These efforts are important, but many clinical trials are international, crossing jurisdictional borders. A single document that provides general, internationally relevant guidance, that can be modified according to local circumstances and regulations being recognized, could help enhance the safety of trial participants and maintain the validity of trials and their results overall. Such a consensus document may also impact regional guidance. Accordingly, the Heart Failure Association of the European Society of Cardiology on 21 and 22 March 2020 conducted web-based meetings with expert clinical trialists in Europe, North America, South America, Australia, and Asia. The main objectives of this Expert Position Paper are to highlight the challenges that this pandemic poses for the conduct of clinical trials in HF, and to offer advice on how they might be overcome, with some practical examples. This expert panel acknowledges that suitable solutions will depend on many factors, including whether the trial has recently started or is nearing completion, the trial design, the type and timing of interventions, the nature of the patient population enrolled, the local and regional severity of the COVID-19 outbreak, and the local governing laws and healthcare resources. As the pandemic evolves, so will the recommendations.
Guidance to people considering participation in clinical trials
Patients not requiring urgent care should avoid hospitals or clinics whilst local regulations advise ‘social distancing’. HF patients are generally older and have a higher burden of comorbidities, which make them an especially high-risk group if they get a coronavirus infection. Every attempt should be made to reduce the risk of exposure. Some clinical trials might still be able to enrol new patients during the pandemic without increasing patient exposure, for example those recruiting patients with acute HF during the index hospital admission if no extra trial visits in hospitals or clinic will be required after discharge, or if the rest of the trial-related data collection can be accomplished remotely. However, in most circumstances, it will be difficult to recruit new participants into HF trials that are currently in progress, for many reasons, including the fact that research staff may be redirected to support clinical services or COVID-related research, and may, therefore, not be available to enrol patients in clinical trials.
Guidance to sponsors for initiating new clinical trials
Sponsors should assess the feasibility and safety of initiating new clinical trials until the evolution of the COVID-19 pandemic becomes clearer and the rate of infection is under control. Currently, only trials in HF that directly address specific issues relevant to COVID-19 (e.g. preventing infection or the consequences of infection) or that treat aspects of COVID-19 disease progression in HF patients (such the use of anti-inflammatory therapies) should be encouraged. The timing of when it will again be safe to initiate new clinical trials will be strongly influenced by local circumstances, rules, and regulations. For instance, the National Institute of Health Research in the UK has suspended all new clinical trials to prioritize COVID-19 research and to enable the redeployment of staff to clinical duties.7
Guidance for ongoing clinical trials at various stages
Pre-randomization screening
Recruiting new participants into clinical trials will be interrupted in most cases, or at least very severely curtailed, due to the additional risk of infection and the reduced availability of research staff, so that in most cases screening will cease. Whether it is advisable to contact patients by telephone to ask trial-related questions will depend on what ethical permissions have been obtained and the anticipated reaction of patients. Patients may be reassured by contact from a healthcare professional they know. However, it is also possible that patients may think it is inappropriate to be asked about trial participation when they or their family members might be in a crisis, whether that be medical or financial. This might depend on the nature of the research. Trials on protection from infection with or the complications of COVID-19 might be expected by patients and greeted with enthusiasm.
In some countries, the load on the healthcare system is now gradually decreasing, and authorities are considering reducing restrictions, whilst still maintaining social distancing. Depending on the region and the resilience of the healthcare system, it may be possible to restart patient recruitment even when social distancing measures are still recommended. These strategies will heavily depend on the local regulations and burden of COVID-19 on the healthcare system in a particular region, and will require frequent re-assessment of the situation. When follow-up visits for existing trials are again possible, then enrolment of new patients should also be possible. However, patients may be wary about agreeing to participate in clinical trials until they are confident that the COVID-19 pandemic is firmly under control.
Patient recruitment into international clinical trials will reflect the confidence that the sponsors have that a particular country has controlled not only the current risk of COVID-19 infection but also the risk of resurgence; some predict that the COVID-19 pandemic might recur in waves over the next few years. Funders and sponsors need to be confident that the necessary treatment can be delivered and follow-up measurements can be done, physically or remotely, and that clinical outcomes will not be determined by the risk of, or infection with, COVID-19. This may influence the geographic distribution of patient recruitment.
Continuation of enrolled participant follow-up
Permanent cessation of ongoing clinical trials is rarely the best course of action, unless they are nearing planned completion or have not yet started. Most trials should continue to follow patients who have already been enrolled provided this does not increase risk, even if enrolment of new participants is temporarily halted.
Globally almost two-thirds of deaths are due to the effects of chronic diseases such as HF.8,9 It is the ethical responsibility of the biomedical research community towards our patients to continue to seek better therapies for conditions that shorten life, even in these difficult times. Termination of a clinical trial may require patient contact, such as study close-out visits to the hospital, for final investigations and safety-related assessments, and occasionally to down-titrate and stop investigational drugs or to start conventional treatments safely. Hence, ending a trial could in some cases also increase the patient contact and local hospital research workload for a short period of time.
If there are circumstances where patient safety cannot be ensured, or trial data simply cannot be collected, the trial may need to be paused, or stopped altogether (e.g. if early in the course). Trials that require close and frequent follow-up or complex investigations require detailed discussions to determine the optimum strategy for follow-up. Trials of devices that have already been implanted and cannot be managed remotely may require follow-up to ensure safe and effective device performance (e.g. for implantable pulse generators where regular battery changes may be needed, and for neurostimulation devices that may require clinically supervised titration).
Lastly, the decision to restart enrolment into a trial should be re-assessed at regular intervals and will depend on epidemiological data pertinent to a given region or site and after review of the institutional capacity, policies, and local regulations.
The pre-randomization period in trials with run-in phases
There are special considerations for trials with run-in phases, and views are controversial on this. For instance, if the run-in phase requires optimization of guideline-recommended therapy, it can be argued that the risks of additional visits should be balanced against the advantages of optimizing therapy during the pandemic. However, if the run-in period is time limited and must be immediately followed by randomization and the active treatment phase, it is preferable not to commence the run-in at all, as a run-in phase that cannot lead to randomization serves no purpose. Also, when orderly and timely delivery of the investigational product and data collection cannot be ensured, the run-in phase cannot continue for the time being. Hence, consideration should be given to a moratorium on commencing patients in a run-in phase, until local staff can see a realistic prospect of successful and safe trial participation that is the aim of the run-in phase.
During the trial: by stakeholders
Sponsors/clinical or academic research organization
A ‘first do no harm’ commitment mandates that every effort is made to minimize the risk of infection for trial patients and trial staff by converting physical visits into virtual visits whenever possible. To ensure safe and documented study drug administration, investigational medicinal products (IMPs) may be mailed to the patient’s home address using dedicated couriers. Risk management plans for storage and back-up transportation modalities should be considered and documented on a case-by-case basis. This is of major importance, because it may be ill advised for patients to attend face-to-face visits. Furthermore, healthcare facilities may be overwhelmed, especially if research staff are reassigned to clinical duties or quarantined due to SARS-CoV-2 exposure.
Trialists
Physical visits should be reduced and/or deferred whenever feasible. Trial procedures and endpoints that can be adequately assessed using remote visits, such as symptoms or questionnaires (e.g. EQ-5D) that do not require an in-person visit (Table 1), can instead be documented by postal questionnaires or standardized scripted phone follow-up. Alternative solutions using validated tools (such as ‘apps’) are encouraged.10–12 Heart rate, heart rhythm, and blood pressure may all be potentially recorded by patients or carers, if appropriate equipment and training is available.13 Participants should be asked to comply with standard operating procedures developed by the trial management team to ensure uniform data collection. Face-to-face visits at home might be necessary if a blood test, a device check (that cannot be done remotely), or intravenous therapy is required. Patients would appreciate this (see patient statements in Supplementary material online, Appendix 2). However, in some regions, in-person visits may not be possible due to local laws and regulations.
Table 1.
Measurement of endpoints using home-based testing (the current approach for all these measurements is for the patient to attend the research centre and the research team to collect the data)
| Measurement/endpoint category | Example | Alternative method | Validity (high/medium/low/unknown) | Reference |
|---|---|---|---|---|
| Symptom status | NYHA class | Phone script; smartphone (app-based) self-assessment | Uncertain (patients and HCP score differently) | N/A |
| Quality of life | KCCQ, EQ-5D | Phone script; emailed link | High for EQ5D | N/A |
| Adherence | Pill count | Video link with patient | High/medium | N/A |
| Vital signs | Blood pressure | Home-based cuff | High | George et al.19 |
| Heart rate | Patient count; smartphones count; BP cuff count | High | De Ridder et al.17 | |
| Weight | Home-based scale | High | N/A | |
| Temperature | Home thermometer | High | N/A | |
| Oxygen saturation | Home pulse ox by plethysmography on smartphone | High | N/A | |
| ECG | Heart rate and rhythm; QRS | Apple watch; Kardia (Alivecor) | Medium/high; depending on technology and information required | Perez et al.18 |
| Exercise capacity | 6-min walk test | Home-based via app | Medium | Brooks et al.16 |
| Clinical outcomes | Hospitalization | Retrieve from EMR or central data repository | High | N/A |
KCCQ, Kansas City Cardiomyopathy Questionnaire; ECG, electrocardiogram; BP, blood pressure; EMR, electronic medical record; HCP, healthcare providers.
Data that are critical for addressing the primary hypothesis of the trial should be prioritized. A risk–benefit analysis should be applied to both primary and secondary endpoints, balancing the safety of participants and staff with the impact that missing data will have on the results and their interpretation. For example, specialized investigations including some blood tests, the investigation of mechanisms of action of the treatment under study, and recording of surrogate endpoints that are ancillary trial aims may need to be interrupted for a period of time or entirely sacrificed to improve patient safety. When the primary endpoint is a clinical event, such as hospitalization or death, this can usually be documented, although accurate ascertainment and reporting might be delayed.
Study site/research coordinators
When in-person visits are deemed essential, patients should be contacted via telephone first to screen for symptoms suggestive of viral illness. In addition, visits should be arranged so that patient waiting times and overlap are avoided by spreading visits over the working day. Non-essential companions should be discouraged from accompanying patients for their visits. Other trial processes that are not absolutely essential (such as resolving data queries) should be relaxed or, if of low value, omitted altogether to reduce the burden on research staff. Moreover, healthcare workers could unknowingly be a vector of coronavirus and, if SARS-CoV-2 testing is available, it should be performed on trial staff to improve patient safety. However, currently this is not possible in most places, and local circumstances and rules will prevail. Importantly, testing of patients and staff with known exposure to or symptoms of COVID-19 should be prioritized. Visitor logs and contact information—regularly maintained—should be collected to enable subsequent contact tracing if required. If personal protective equipment is available, it should be used by both patients and staff during any face-to-face encounter. However, personal protective equipment should be reserved for frontline healthcare staff where there are shortages. In addition, careful cleaning of all surfaces and equipment is required after each patient contact.
The number of staff that trial participants interact with should be minimized. Delegation logs where named individuals are approved to undertake certain duties in specific trials should be created to ensure as many team members cross-cover each other so that, in the case of staff absence, there is an alternative authorized person.
Case report forms (CFRs) should be changed so that COVID-19 is a drop-down box for missing visits, tests, and drug supply. A check list of screening questions to expedite the central endpoint committee deliberation may be helpful as well. Local, regional, and national rules take precedence and must be adhered to. It is understandable that different policies and laws will exist in different regions, meaning that the final decision taken will be locally determined. Patients must be informed about changes to the trial and planned follow-up as speedily as possible.
Clinical endpoint committee
We strongly believe adjudication of endpoints in the future should consider COVID-19 infection and its consequences. If a patient is known to be infected with COVID-19, this will assist interpretation and future analysis of results (e.g. sensitivity analyses based on COVID-19 status). Many regions have limited resources/test kits for COVID-19 diagnosis, and thus routine testing of trial participants may not be possible. However, testing may become less costly and much more widely available in most regions, perhaps as a rapid point of care test.14 When COVID-19 status cannot be assessed immediately, it might be possible to retain specimens for future testing providing this is permitted and suitable safeguards for healthcare staff are in place. Moreover, COVID-19 pneumonitis can cause signs and symptoms similar to HF, and may also cause acute systolic HF or myocarditis. Careful documentation of any tests of cardiac function (e.g. echocardiography or invasive haemodynamic measurements) or treatments directed at HF (e.g. intravenous diuretic, inotropic, or vasodilator agents) in the CRF might assist differentiation between acute respiratory distress syndrome and HF.
The event adjudication committee, blinded to assigned intervention, may be later asked to assess whether COVID-19 contributed to a presumed cardiovascular endpoint event, based on emerging evidence and publications. A graded classification of relatedness (e.g. proven, likely, or unlikely) may be useful for future trial analysis and could be added to the CRF. When a patient is hospitalized, determination of COVID-19 status is strongly advocated, particularly when hospitalization is part of the primary endpoint of the trial. Sites should be encouraged to focus on collection of narratives whenever possible, as these can be especially valuable in patients with complex disease and when information from the medical chart may be lacking for many structural, personnel, or expediting reasons. For example, phone interviews with a patient may uncover useful clinical information that should be documented in the patient’s hospital and trial notes.
Patients may avoid hospitals during the coronavirus pandemic, and hospitals may be too overwhelmed to admit HF patients in the way in which they would normally do. The clinical decision threshold for admitting a patient with decompensated HF may have changed during the COVID-19 pandemic, which may impact event rates for an uncertain period of time. Similarly, the proportions of deaths due to cardiovascular, non-cardiovascular, or uncertain cause may change due to COVID-19 infection. If true, then during the period of time of the COVID-19 crisis, the assumption that, in a HF trial, the majority of ‘undetermined cause’ deaths are probably cardiovascular in origin may need to be reconsidered.
Data monitoring committee
Independent data monitoring committees (DMCs) play a crucial role in the conduct of cardiovascular trials. Adjustments to the work of the DMC should be implemented. In trials in which 80–90% of the recruitment has been completed, the DMC may be asked to perform an interim analysis to assess if the study question has already been answered. Trials that have already answered the primary question (for futility, harm, or benefit) may be stopped and thus limit unnecessary exposure for patients and investigators. Similarly, if 90% or more of the anticipated events have accrued, the power of the trial may not be affected severely and thus a decision regarding stopping or continuing a trial which is about to end could be made. This also addresses the concern that the results of an almost complete trial might be changed by the impact of the epidemic. For example, if this epidemic had struck in 2014, the PARADIGM-HF trial could have been stopped well before March 2014 without adversely affecting the result and reducing the risk to patients and staff without exposing the trial to the risk of including many deaths due to infection.
Terminating a trial (as opposed to pausing recruitment with the possibility of future resumption)—which is not what we generally recommend—is not without risks for patients in some cases, as discussed above, and hence careful evaluation is required before making such a decision, case by case. If based on an interim analysis, appropriate statistical methods can be used (e.g. promising zone approach),15 but this cannot be applied post-hoc. Generally, the steering committee, blinded to study results, should provide relevant boundaries and related decision rules for the DMC to use in order to make recommendations about early stopping of the trial or not. The consequences of likely scenarios should be considered before looking at unblinded data. This process is important for maintaining the integrity of the clinical trial.16
Lastly, the DMC should be flexible regarding the ability of trial organizers to provide timely reports. There may be unavoidable delays in reporting serious adverse events and endpoints due to COVID-19-related measures.
Institutional review board
Institutional review board (IRB) policies should be adapted to support clinical trials in these extraordinary circumstances. As trial protocol modifications are expected to occur frequently, IRB policies should be adjusted to avoid the need for repeated reporting of protocol deviations. Flexibility may be considered for the timing of scheduled visits as long as they do not invalidate results; for example, if a face-to-face visit may be safely deferred, then this should be allowed provided there are no safety concerns (e.g. the IMP is out of date or cannot be supplied in acceptable condition, or at all). This consideration will vary depending on the trial design and study intervention. Waivers in eligibility criteria due to difficulty in performing evaluations should not be allowed. In addition, definitions of protocol deviations may need to be revisited if certain tests cannot be performed within an expected time frame for logistical reasons. If a COVID 19-related protocol modification is not considered to be eligible for a waiver, then expedited review will often be needed.
Post-trial/close-out
Sponsors/academic research organization
Sponsors should describe in appropriate sections of the clinical study report the measures that were taken to manage trial conduct during disruption of the study caused by COVID-19-related factors. Moreover, a listing of all patients affected by the COVID-19-related study disruption should be mentioned by unique participant number identifier and by investigational site, with an explanation of how it altered participation. Trial registration sites such as Clinicaltrials.gov should consider reporting all COVID-19-related changes to the clinical trials.
Trialists/Biostatisticians
Statistical analysis plans should be reviewed carefully and modified, if necessary, to reflect changes to the trial precipitated by the COVID-19 pandemic. Statistical approaches may change; power calculations may need to be revised, but rigorous methods of analysis, including the principles of the intention to treat approach, should prevail. COVID-19 status may or may not be important for the outcomes seen in a trial. It is unclear what to do with data acquired before and after a prolonged delay in the conduct of the trial. If a trial is temporarily halted, statistical analysis plans may need to be revised to determine how to calculate and manage events that occur during the waiting time. Delays in recruitment can also alter the temporal distribution of events, and hence power calculations may need revision.
It may also be useful to select a date to distinguish data collected before COVID-19 (‘BC’) and after COVID-19 (‘AC’). The date should be defined per centre, before and after the first documented COVID patient is admitted to the local hospital. This will allow post-COVID-19 sensitivity analyses to be done and ensure for which part investigators may have the greatest confidence in the integrity of the data. The date threshold must be specified (per site, per country, and/or for the trial overall) prior to looking at the trial data.
It may be useful to describe a pre–post COVID analysis for all trials that were running at the time of the start of the COVID-19 crisis. This is for several reasons: (i) the intervention might alter susceptibility to, or the consequences of, infection; (ii) there may be uncertainty about the cause of events; (iii) any benefit from the intervention may be overwhelmed or exaggerated by the impact of the virus; (iv) delivery of the intervention in the population studied may be affected; and, finally, (v) the general care or care specifically for HF may be affected (e.g. monitoring of laboratory values) for trial participants.
CROs/monitors
Electronic monitoring, i.e. records from the hospital, may provide an attractive alternative form of monitoring. At enrolment, patients may be consented for blanket electronic approval, and this can provide important information to improve future trials. In light of the COVID-19 pandemic, in some countries patient consent requirements (CROs) for certain data protection issues have been abandoned for all COVID-19-related data for clinical purposes.17
Specific examples from trials of heart failure
A few examples of actual recommendations given in a convenience sample of ongoing HF trials, for some of which the authors are primary investigators, are provided in the Supplementary material online, Appendix 3.
Concluding remarks
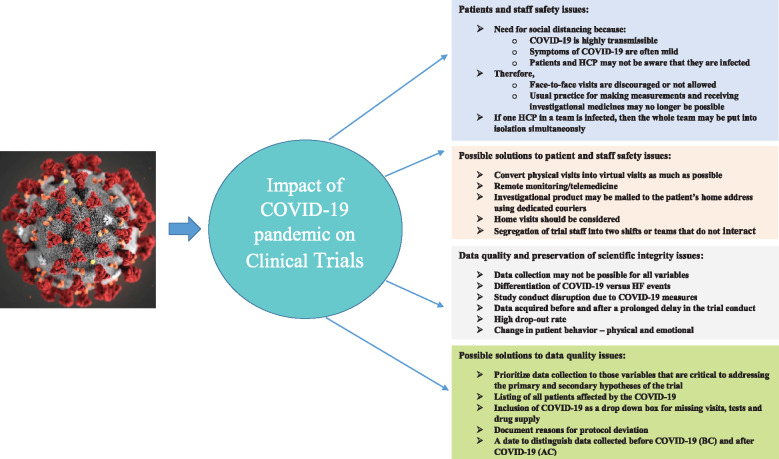
In summary, the COVID-19 pandemic has caused various issues related to the conduct of clinical trials (Figure 1). In these challenging times, it is our moral and ethical responsibility to keep trial participants safe and to safeguard the investment they have already made in ongoing clinical trials so that we can provide the answers that we have promised to them. Clearly, patients with HF are at serious risk if infected by COVID-19. Any additional risk from participation in clinical trials should be avoided or minimized. This should be a balance against the risk of not receiving optimal therapy, which is often only attained when patients participate in clinical trials when they receive expert, specialist protocol-driven care provided by the research team. It is important to remember the social contract between patients, trialists, and funders through which patients enter trials in good faith, so that they can help improve future healthcare and scientific knowledge. The recommendations we make may evolve over time as the epidemiology of COVID-19 remains dynamic.
Figure 1.
A summary of issues along with the possible solutions related to patient and staff safety in the conduct of clinical trials due to the COVID-19 pandemic. Problems related to data quality and perseveration of scientific integrity are also highlighted along with their possible solutions. HCP, healthcare providers.
Supplementary Material
Acknowledgements
We gratefully acknowledge the support of the patients Cynthia Chauhan, John Renesch, and Richard Jacob who gave feedback to us on this document. These patients were acquaintances or patients of some authors.
Conflicts of interest: Dr. Anker reports grants and personal fees from Vifor Int, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Novartis, personal fees from Servier, grants and personal fees from Abbott Vascular, personal fees from Impulse Dynamics, personal fees from Cardiac Dimensions, outside the submitted work; Dr. Butler reports personal fees from Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, V-Wave Limited, and Vifor, outside the submitted work; Dr Khan reports no disclosures; Dr. Abraham reports personal fees from Abbott, personal fees from Boehringer-Ingelheim, personal fees from Edwards Lifesciences, personal fees from Impulse Dynamics, personal fees from Respicardia, personal fees from Sensible Medical, personal fees from V-Wave, outside the submitted work; Dr. Bauersachs reports personal fees from Abbott, grants and personal fees from Abiomed, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from BMS, personal fees from Boehringer Ingelheim, grants and personal fees from CvRX, personal fees from Daiichi Sankyo, personal fees from Medtronic, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Servier, grants and personal fees from Vifor, grants and personal fees from Zoll, outside the submitted work; Dr. Bocchi reports personal fees from Servier, Astra-Zeneca, Novartis, non-financial support from Servier, Baldacci, grants from Jansen, Bayer/Merck, Astra-Zeneca, Boehringer Ingelheim, from null, outside the submitted work; Dr. Bozkurt reports personal fees from Bristol Myers Squibb, personal fees from scPharmaceuticals, personal fees from Baxter Healthcare Corporation, personal fees from Sanofi-aventis, personal fees from Relpysa, other from Abbott, outside the submitted work; Dr. Braunwald reports grants from Astra Zeneca, grants from Daiichi Sankyo, grants from Merck, grants from Novartis, personal fees from Amgen, personal fees from Cardurion, personal fees from MyoKardia, personal fees from NovoNordisk, personal fees from Verve, outside the submitted work; Dr Chopra reports no disclosures; Dr. Cleland reports personal fees from Abbott, grants and personal fees from Amgen, grants and personal fees from Bayer, personal fees and non-financial support from Medtronic, grants and personal fees from Novartis, grants and personal fees from Pharmacosmos, grants and personal fees from Vifor, grants and personal fees from BMS, grants and personal fees from Servier, outside the submitted work; Dr. Ezekowitz reports grants and personal fees from Amgen, grants and personal fees from Bayer, grants and personal fees from Merck/MSD, grants and personal fees from Cytokinetics, grants and personal fees from Novartis, grants and personal fees from Applied Therapeutics, grants and personal fees from American Regent, during the conduct of the study; Dr. Filippatos reports Committee Member in trials sponsored by Medtronic, Vifor, Servier, Novartis, BI, outside the submitted work; Dr. Friede reports personal fees from Novartis, personal fees from Bayer, personal fees from Janssen, personal fees from SGS, personal fees from Roche, personal fees from Boehringer Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Galapagos, personal fees from Penumbra, personal fees from Parexel, personal fees from Vifor, personal fees from BiosenseWebster, personal fees from CSL Behring, personal fees from Fresenius Kabi, personal fees from Coherex Medical, personal fees from LivaNova, outside the submitted work; Dr. Hernandez reports grants and personal fees from AstraZeneca, personal fees from Amgen, personal fees from Boston Scientific, grants from American Regent, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Merck, personal fees from Bayer, grants from Verily, outside the submitted work; Dr. Lam is supported by a Clinician Scientist Award from the National Medial Research Council of Singapore; has received research support from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic and Vifor Pharma; has served as consultant or on the Advisory Board/ Steering Committee Executive Committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scienticis, Corvia Medical, Cytokinetics, Darma Inc., Eko.ati Pte Ltd, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd, Roche Diagnostics, Stealth BioTherapeutics, The Corpus, Vifor Pharma and WebMD Global LLC; and serves as co-founder & non-executive director of Eko.ai Pte Ltd; Dr. Lindenfeld reports grants from Astra Zeneca, personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from CVRx, personal fees from Edwards Lifesciences, personal fees from Impulse Dynamics, grants from Sensible Medical, grants from Volumetrix, personal fees from VWave, during the conduct of the study; personal fees from Astra Zeneca, grants from Astra Zeneca, personal fees from Boehringer Ingelheim, personal fees from CVRx, personal fees from Edwards Lifesciences, personal fees from Impulse Dynamics, grants from Sensible Medical, grants from Volumetrix, personal fees from VWave, outside the submitted work; Dr. McMurray reports other from Bayer, non-financial support and other from Cardiorentis, non-financial support and other from Amgen, non-financial support and other from Oxford University/Bayer, non-financial support and other from Theracos, non-financial support and other from Abbvie, other from DalCor, other from Pfizer, other from Merck, non-financial support and other from Novartis, non-financial support and other from Glaxo Smith Kline (GSK), other from Bristol Myers Squibb (BMS), non-financial support and other from Vifor-Fresenius, non-financial support and other from Kidney Research UK (KRUK), non-financial support and other from Novartis, non-financial support and other from AstraZeneca, outside the submitted work; Dr. Mehra reports personal fees from Abbott, personal fees from Medtronic, personal fees from Janssen, personal fees from Mesoblast, personal fees from Portola, personal fees from Bayer, personal fees from Baim Institute for Clinical Research, personal fees from Levitiicus, personal fees from Fineheart, personal fees from NupulseCV, personal fees from Triple Gene, outside the submitted work; Dr. Metra reports personal fees from Abbott Vascular, Astra Zeneca, Bayer, Edwards Therapeutics, Livanova, Vifor pharma, outside the submitted work; Dr. Packer reports personal fees from Amgen, personal fees from Akcea, personal fees from AstraZeneca, personal fees from Amarin, personal fees from Actavis, personal fees from Abbvie, personal fees from Boehringer Ingelheim, personal fees from Cardiorentis, personal fees from Daiichi Sankyo, personal fees from Novartis, personal fees from ParatusRx, personal fees from Pfizer, personal fees from Sanofi, personal fees from Synthetic Biologics, personal fees from Theravance, outside the submitted work; Dr. Pieske reports personal fees from Bayer Healthcare, personal fees from Novartis, personal fees from Merck, personal fees from Astra Zeneca, personal fees from BMS, outside the submitted work; Dr. Pocock has nothing to disclose; Dr. Ponikowski reports personal fees and other from Novartis, grants, personal fees and other from Vifor Pharma, grants, personal fees and other from Servier, personal fees and other from BMS, personal fees and other from Boehringer Ingelheim, personal fees and other from Astra Zeneca, personal fees and other from Cibiem, personal fees and other from Renal Guard Solutions, personal fees and other from Impulse Dynamics, personal fees and other from Respicardia, personal fees from Berlin Chemie, outside the submitted work; Dr. Rosano has nothing to disclose; Dr. Teerlink reports personal fees and non-financial support from Abbott, personal fees from AbbVie, grants, personal fees and non-financial support from Amgen, personal fees from AstraZeneca, personal fees and non-financial support from Bayer, personal fees and non-financial support from Boerhinger-Ingelheim, grants, personal fees and non-financial support from Bristol-Myers Squibb, grants, personal fees and non-financial support from Cytokinetics, personal fees and non-financial support from EBR Systems, personal fees and non-financial support from Medtronic, personal fees and non-financial support from Merck, personal fees and non-financial support from Windtreee Therapeutics, grants from Department of Veterans Affairs, outside the submitted work; Dr. Tsutsui reports personal fees from MSD K.K., Astellas Pharma Inc., Pfizer Japan Inc., Bristol-Myers Squibb Company, Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Takeda Pharmaceutical Company Limited, Bayer Yakuhin, Ltd., Novartis Pharma K.K., Kowa Pharmaceutical Co. Ltd., Teijin Pharma Ltd., other from Nippon Boehringer Ingelheim Co., Ltd. Bayer Yakuhin, Ltd. Novartis Pharma K.K. Ono Pharmaceutical Co., Ltd., grants from Actelion Pharmaceuticals Japan Ltd. Japan Tobacco Inc. Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., IQVIA Services Japan, Omron Healthcare., Astellas Pharma Inc., Novartis Pharma K.K., Takeda Pharmaceutical Company Limited, Teijin Pharma Ltd., MSD K.K, outside the submitted work; Dr. Van Veldhuisen reports personal fees from Johnson & Johnson, personal fees from Novartis, outside the submitted work; Dr. Verma reports grants and personal fees from Boehringer-Ingelheim, grants and personal fees from AstraZeneca, personal fees from Eli Lilly, grants and personal fees from Janssen, personal fees from EOCI Pharmacomm Ltd, personal fees from Sun Pharmaceuticals, personal fees from Toronto Knowledge Translation Working Group, during the conduct of the study; grants and personal fees from Amgen, grants and personal fees from Bayer, grants from Bristol-Myers Squibb, personal fees from HLS Therapeutics, grants and personal fees from Merck, personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Sanofi, outside the submitted work; Dr. Voors reports personal fees from Amgen, personal fees from cytokinetics, personal fees from Boehringer Ingelheim, grants and personal fees from Roche , personal fees from Novartis, personal fees from AstraZeneca, personal fees from Bayer, personal fees from Myokardia, personal fees from Merck, personal fees from Bayer AG, outside the submitted work; Dr Wittes reports that the company for which she works, Statistics Collaborative, has contracts with many companies, some of which are developing treatments for heart failure; Dr. Zannad reports personal fees from Janssen, personal fees from Bayer, personal fees from Boston Scientific, personal fees from Amgen, personal fees from CVRx, personal fees from Boehringer, other from cardiorenal, personal fees from AstraZeneca, personal fees from Vifor Fresenius, personal fees from Cardior, personal fees from Cereno pharmacuetical , personal fees from Applied Therapeutics, personal fees from Merck, other from CVCT, personal fees and other from Novartis, outside the submitted work; Dr. Zhang has nothing to disclose; Dr Seferovic reports medtronic honorarium for lecture, Abbott honorarium for lecture, Servier honorarium for lecture, Astra Zeneca honorarium for lecture, Respicardia honorarium for lecture, Boehringer Ingelheim consultancy agreement and honorarium for lecture, Novartis consultancy agreement and honorarium for lecture, Vifor Pharma consultancy agreement; Dr. Coats reports personal fees from Astra Zeneca, Bayer, Menarini, Novartis, Nutricia, Servier, Vifor, Actimed, Cardiac Dimensions, CVRx, Enopace, Faraday, Gore, Impulse Dynamics, Respicardia, Stealth Peptides, V-Wave, Corvia, Arena, ESN Cleer, outside the submitted work
References
- 1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Li M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JTK, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z.. Early transmission dynamics in Wuhan, China of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (19 April 2020).
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDermott MM, Newman AB.. Preserving clinical trial integrity during the coronavirus pandemic. JAMA 2020;doi: 10.1001/jama.2020.4689. [DOI] [PubMed] [Google Scholar]
- 6. Ledford H. Coronavirus shuts down trials of drugs for multiple other diseases. Nature 2020;580:15–16. [DOI] [PubMed] [Google Scholar]
- 7. Thornton J. Clinical trials suspended in UK to prioritize covid-19 studies and free up staff. BMJ 2020;368:1172. [DOI] [PubMed] [Google Scholar]
- 8. http://www.euro.who.int/__data/assets/pdf_file/0004/185215/Leading-causes-of-death-in-Europe-Fact-Sheet.pdf (25 March 2020).
- 9. https://www.fightchronicdisease.org/sites/default/files/docs/GrowingCrisisofChronicDiseaseintheUSfactsheet_81009.pdf (25 March 2020).
- 10. Brooks GC, Vittinghoff E, Iyer S, Tandon D, Kuhar P, Madsen KA, Marcus GM, Pletcher MJ, Olgin JE.. Accuracy and usability of a self-administered 6-minute walk test smartphone application. Circ Heart Fail 2015;8:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Ridder B, Van Rompaey B, Kampen JK, Haine S, Dilles T.. Smartphone apps using photoplethysmography for heart rate monitoring: meta-analysis. JMIR Cardio 2018;2:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP.. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;38:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. George J, Macdonald T.. Home blood pressure monitoring. Eur Cardiol 2015;10:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-first-emergency-use-authorization-point-care-diagnostic (25 March 2020).
- 15. Shih WJ, Li G, Wang Y.. Methods for flexible sample-size design in clinical trials: likelihood, weighted, dual test, and promising zone approaches. Contemp Clin Trials 2016;47:40–48. [DOI] [PubMed] [Google Scholar]
- 16. https://www.ema.europa.eu/en/documents/scientific-guideline/points-consider-implications-coronavirus-disease-covid-19-methodological-aspects-ongoing-clinical_en.pdf (28 March 2020).
- 17. https://www.hhs.gov/sites/default/files/hipaa-and-covid-19-limited-hipaa-waiver-bulletin-508.pdf (25 March 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.