Abstract
Background
There is no proven antiviral or immunomodulatory therapy for coronavirus disease 2019 (COVID-19). The disease progression associated with the proinflammatory host response prompted us to examine the role of early corticosteroid therapy in patients with moderate to severe COVID-19.
Methods
We conducted a single pretest, single posttest quasi-experiment in a multicenter health system in Michigan from 12 March to 27 March 2020. Adult patients with confirmed moderate to severe COVID were included. A protocol was implemented on 20 March 2020 using early, short-course, methylprednisolone 0.5 to 1 mg/kg/day divided in 2 intravenous doses for 3 days. Outcomes of standard of care (SOC) and early corticosteroid groups were evaluated, with a primary composite endpoint of escalation of care from ward to intensive care unit (ICU), new requirement for mechanical ventilation, and mortality. All patients had at least 14 days of follow-up.
Results
We analyzed 213 eligible subjects, 81 (38%) and 132 (62%) in SOC and early corticosteroid groups, respectively. The composite endpoint occurred at a significantly lower rate in the early corticosteroid group (34.9% vs 54.3%, P = .005). This treatment effect was observed within each individual component of the composite endpoint. Significant reduction in median hospital length of stay was also observed in the early corticosteroid group (5 vs 8 days, P < .001). Multivariate regression analysis demonstrated an independent reduction in the composite endpoint at 14-days controlling for other factors (adjusted odds ratio: 0.41; 95% confidence interval, .22 – .77).
Conclusions
An early short course of methylprednisolone in patients with moderate to severe COVID-19 reduced escalation of care and improved clinical outcomes.
Clinical Trials Registration
Keywords: Corticosteroids, outcomes, COVID-19, SARS-COV-2, coronavirus
In this multicenter quasi-experimental study of 213 patients, we demonstrate early short course of methylprednisolone in moderate to severe COVID-19 patients reduced the composite endpoint of escalation of care from ward to Intensive Care Unit, new requirement for mechanical ventilation, and mortality.
BACKGROUND
As of 9 April 2020, the United States has over 400 000 cases of confirmed coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Most patients will have mild illness, but older persons and those with comorbidities may develop severe disease necessitating hospitalization and time in the intensive care unit (ICU) [2, 3]. The disease pathophysiology presents in 2 distinct overlapping phases, the initial pathogenic viral response followed by host inflammatory response with grades of severity associated with distinct clinical findings [4, 5]. The pathological progression in severe COVID-19 includes an excessive and unregulated proinflammatory cytokine storm resulting in immunopathological lung injury, diffuse alveolar damage with the development of acute respiratory distress syndrome (ARDS), and death [6–9].
In the absence of any proven antiviral therapy, the current clinical management is primarily supportive care, supplemental oxygen, and mechanical ventilatory support [1, 10]. Adjunctive therapy with immunomodulatory agents targeting the inflammatory cytokine storm are being evaluated [5, 10]. Studies of corticosteroid therapy for phylogenetically similar coronavirus infections showed no benefit and potential harm [11]. Despite the frequent use in treating patients with COVID-19 in China, the role of corticosteroids is undefined [3, 5, 10–13]. A more recent observational study reported improved outcomes in patients with COVID-associated ARDS that received corticosteroids [14].
We postulated that early treatment with a short course of corticosteroids in patients with COVID-19 may attenuate the excessive host respiratory and systemic inflammatory responses. We report the clinical characteristics and early outcomes of patients with COVID-19 receiving short courses of methylprednisolone.
METHODS
Study Population
Consecutive patients hospitalized from 12 March 2020 through 27 March 2020 were eligible for inclusion if they were ≥18 years, had confirmed COVID-19 infection, with radiographic evidence of bilateral pulmonary infiltrates, and required oxygen by nasal cannula, high-flow nasal cannula (HFNC), or mechanical ventilation. Patients were excluded if they were transferred from an out-of-system hospital, died within 24 hours of presentation to the emergency department (ED), or were admitted for <24 hours. A confirmed case of COVID-19 was defined as a patient that had a positive reverse-transcription polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 in a nasopharyngeal sample tested by the Michigan Department of Health and Human Services (MDHHS) or the Henry Ford Health System (HFHS) centralized clinical microbiology laboratory. Beginning 16 March 2020, testing for hospitalized patients was performed by the centralized clinical microbiology laboratory.
Patients were risk stratified by severity of symptoms on presentation to the hospital as mild, moderate, or severe COVID-19. Patients without hypoxia or exertional dyspnea were considered to have mild COVID-19. Patients with mild COVID-19 were treated with symptom relief only and not admitted to the hospital. Patients who presented with infiltrates on chest radiography and required supplemental oxygen by nasal cannula or HFNC were classified as having moderate COVID-19. Patients who had respiratory failure requiring mechanical ventilation were classified as having severe COVID-19.
Study Design
This was a multicenter quasi-experimental study at HFHS, composed of 5 hospitals in southeast and south-central Michigan. The study was approved by the institution’s Investigational Review Board (13739) with waiver of consent. Patients in the standard of care (SOC) group from 12 March 2020 through 19 March 2020 were compared to an early corticosteroid group that included patients from 20 March 2020 through 27 March 2020.
Patients in both study groups received standard care, composed of supplemental oxygen, HFNC, invasive ventilation, antibiotic agents, antiviral agents, vasopressor support, and renal-replacement therapy, as determined by the primary team. Patients who progressed to ARDS were managed with SOC [15].
Intervention Standard of Care
Patients with moderate or severe disease who presented to HFHS within the first week of the COVID epidemic in Detroit were initially treated with supportive care with or without a combination of lopinavir-ritonavir and ribavirin or hydroxychloroquine according an institutional guideline developed by Infectious Diseases Physicians and Pharmacists. The institutional guidelines were developed by consensus, and based on the available literature, experience from Wuhan, China, and other centers around the world affected by COVID-19 before Michigan. Intravenous (IV) remdesivir compassionate use was requested for eligible mechanically ventilated patients. On 17 March 2020 lopinavir-ritonavir with ribavirin was removed from the COVID-19 institutional protocol [16]. Steroids were considered on a case-by-case basis.
Early Corticosteroid Group
As a result of observed poor outcomes, clinical rationale based on immunology, clinical course of COVID-19, and more recently best available evidence, the HFHS early corticosteroid protocol was developed (Supplementary Materials) [14, 15, 17]. We hypothesized that early corticosteroids would combat the inflammatory cascade leading to respiratory failure, ICU escalation of care, and mechanical ventilation. The early corticosteroid protocol was incorporated in the institutional COVID-19 guidelines on 20 March 2020. Patients with confirmed influenza infection were not recommended to receive early corticosteroids. The decision to prescribe hydroxychloroquine and early corticosteroids was at the discretion of the primary medical team.
Moderate COVID-19 was treated with hydroxychloroquine 400 mg twice daily for 2 doses on day 1, followed by 200 mg twice daily on days 2–5. Patients with moderate COVID-19 who required 4 liters or more of oxygen per minute on admission, or who had escalating oxygen requirements from baseline, were recommended to receive IV methylprednisolone 0.5 to 1 mg/kg/day in 2 divided doses for 3 days. Patients who required ICU admission were recommended to receive the above regimen of hydroxychloroquine and IV methylprednisolone 0.5 to 1 mg/kg/day in 2 divided doses for 3–7 days. ICU patients were also evaluated for tocilizumab on a case-by-case basis. Oral switch was performed to prednisone at a ratio of 1 to 1 when determined clinically appropriate by the primary medical team.
Data Collection
Data were ascertained from each institution’s electronic medical record and recorded in a standardized electronic case report form. Demographic data, information on clinical symptoms or signs at presentation, and laboratory and radiologic results during admission were collected. All laboratory tests and radiologic assessments, including plain chest radiography and computed tomography of the chest, were performed at the discretion of the treating physician.
Study Definitions
We ascertained coexisting conditions from electronic medical record and physician documentation. The National Early Warning Score (NEWS) was collected to evaluate baseline illness severity based on vital signs obtained in the ED [18]. Additionally, the quick Sequential Organ Failure Assessment was used to evaluate severity of illness of included patients based on ED vitals and examination [19]. All patients were followed for at least 14 days after initial presentation. Patient data were censored on 9 April 2020.
OUTCOME MEASURES
Primary Endpoint
The primary composite endpoint was escalation to an ICU from a general medical unit, progression to respiratory failure requiring mechanical ventilation after hospital admission, or in-hospital all-cause mortality. Patients directly admitted to the ICU from the emergency room were evaluated for the latter 2 outcomes, and those requiring mechanical ventilation in the emergency room were evaluated for mortality.
Secondary Endpoints
Secondary endpoints included development and severity of ARDS, days to ventilator liberation, shock, acute kidney injury (AKI), and length of hospital stay (LOS). LOS was reported only for patients who were discharged alive within the 14-day minimum follow-up period. ARDS was diagnosed and classified according to the Berlin definition [20]. AKI was diagnosed according to the Kidney Disease: Improving Global Outcomes definition [21].
Statistical Analysis
Continuous variables were reported as median and interquartile range (IQR) and compared using the Mann-Whitney test or t-test, as appropriate. Categorical data were reported as number and percentage (no., %) and compared using the χ 2 test or Fisher exact test, as appropriate. No imputation was made for missing data points. The sample size was derived from all eligible consecutive hospitalized patients during the study period. A 2-sided α < 0.05 was considered statistically significant. Bivariate and multivariable logistic regression analysis was planned a priori to test the association between the composite endpoint and exposure to the corticosteroid protocol. Covariates in the bivariate analysis with a P-value <.2 and clinical rationale were included in a multivariable regression model that was restricted to a subject-to-variable ratio of 10:1. To evaluate the implementation timing of the in-house RT-PCR SARS-CoV-2 testing a post hoc sensitivity analysis were conducted on the composite outcome. A nonequivalent dependent variable, receipt of, and time to empiric antibiotic therapy for pneumonia, was utilized to account for potential maturation in the management of COVID-19. Statistical analysis was performed using IBM SPSS version 25 (Chicago, IL) and SAS 9.4 (Cary, NC).
RESULTS
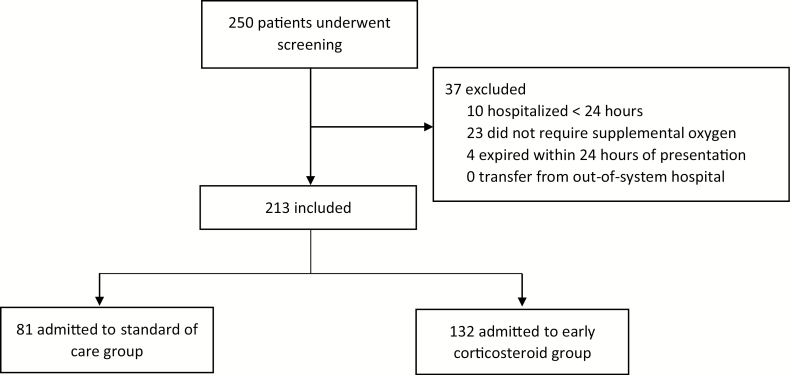
Two-hundred and fifty consecutive patients were evaluated for inclusion. Ten were hospitalized for ≤24 hours, 23 did not require oxygen by nasal cannula, HFNC, or mechanical ventilation, and 4 expired within 24 hours of admission (Figure 1). Two-hundred and thirteen patients were included, 81 (38%) in the SOC group and 132 (62%) in the early corticosteroid group. The median age of the SOC group and early corticosteroid group was 64 (IQR: 51.5, 73.5) and 61 (IQR: 51, 72) years, respectively. Black patients comprised 61.7% of the SOC group and 79.5% of the early corticosteroid group (P = .005). Of the comorbid conditions evaluated, chronic obstructive pulmonary disease was more frequent in the SOC group compared to the early corticosteroid group (18.5% vs 9.1%; P = .045). The presenting COVID-19 symptoms, baseline severity of illness, and other demographics are presented in Table 1. One patient had a concomitant influenza infection in the early corticosteroid group.
Figure 1.
Number of patients screened and included in the trial.
Table 1.
Baseline Demographics and Clinical Characteristics of Study Patients
| Characteristics | Total (n = 213) |
SOC (n = 81) | Early CG (n = 132) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Median age (IQR), y | 62 (51–62) | 64 (51.5–3.5) | 61 (51–72) | .400 |
| Male sex, no. (%) | 109 (51.2) | 41 (50.6) | 68 (51.5) | .899 |
| Black race, no. (%) | 155 (72.8) | 50 (61.7) | 105 (79.5) | .004 |
| Median body mass index (IQR) - kg/m2 | 32 (27.3–38.7) | 30 (25–39) | 33.2 (28.9–38.5) | .007 |
| Coexisting conditions, no. (%) | ||||
| Asthma | 33 (15.5) | 16 (19.8) | 17 (12.9) | .180 |
| Chronic kidney disease | 98 (46) | 41 (51.9) | 57 (43.5) | .240 |
| Chronic obstructive pulmonary disease | 27 (12.7) | 15 (18.5) | 12 (9.1) | .045 |
| Congestive heart failure | 26 (12.2) | 10 (12.5) | 16 (12.2) | .951 |
| Coronary artery disease | 38 (17.8) | 18 (22.2) | 20 (15.2) | .192 |
| Diabetes | 105 (49.3) | 37 (45.7) | 68 (51.5) | .411 |
| Hypertension | 158 (74.2) | 62 (76.5) | 96 (72.7) | .925 |
| Malignancy | 24 (11.3) | 11 (13.6) | 13 (9.9) | .405 |
| Smoking history | 88 (41.3) | 40 (49.4) | 48 (36.4) | .062 |
| Symptoms | ||||
| Cough, no. (%) | 158 (74.2) | 62 (76.5) | 96 (72.7) | .536 |
| Fever, no. (%) | 150 (70.4) | 57 (70.4) | 93 (70.5) | .989 |
| Myalgia, no. (%) | 85 (39.9) | 32 (39.5) | 53 (40.2) | .926 |
| Shortness of breath, no. (%) | 148 (69.5) | 50 (61.7) | 98 (74.2) | .054 |
| Median duration of symptoms (IQR), d | 5 (3–7) | 5 (2–7) | 6 (3–7) | .107 |
| Severity of illness in ED | ||||
| Median qSOFA (IQR) | 1 (0–1) | 1 (0–1) | 1 (0–1) | .850 |
| Median NEWS (IQR) | 7 (4–10) | 7 (4–10) | 7 (4–9) | .668 |
| Requiring mechanical ventilation in ED, no. (%) | 22 (10.3) | 10 (12.3) | 12 (9.1) | .448 |
| Direct admission to ICU, no. (%) | 26 (12.2) | 11 (13.6) | 15 (11.4) | .631 |
Abbreviations: CG, corticosteroid group; ED, emergency department; ICU, intensive care unit; IQR, interquartile range; NEWS, National Early Warning Score; qSOFA, quick Sequential Organ Failure Assessment; SOC, standard of care.
Overall, corticosteroids use was 56.8% and 68.2% in the SOC group and early corticosteroid group, respectively (P = .094). The early corticosteroid group had a greater proportion of corticosteroids initiated within 48 hours of presentation (12.4% vs 41.7%, P < .001), with a median time to initiation of 2 days (IQR: 1–3, range 0–8) as compared to 5 days (IQR: 3–7, range 1–9) in the SOC. The median time to hydroxychloroquine initiation was greater in the SOC group compared to the early corticosteroid group (3 [IQR: 1, 4] vs 1 [IQR: 0, 2] days, P < .126). Additional treatment characteristics are described in Table 2.
Table 2.
Treatments Received by Groups
| Treatment | Total (n = 213) |
SOC (n = 81) | Early CG (n = 132) | P value |
|---|---|---|---|---|
| Antimicrobials | ||||
| Empiric antibiotic prescribed for pneumonia, no. (%) | 163 (76.5) | 65 (80.2) | 98 (74) | .316 |
| Median time to empiric antibiotics (IQR), d | 1 (0–1) | 1 (0–1) | 0 (0–1) | .631 |
| Median duration of antimicrobials (IQR), d | 4 (2–5) | 5 (3–5) | 3 (2–5) | .009 |
| Hydroxychloroquine use, no. (%) | 161 (75.6) | 57 (70.4) | 104 (78.8) | .167 |
| Median time to hydroxychloroquine initiation (IQR), d | 2 (1–3) | 3 (1–4) | 1 (0–2) | .126 |
| Lopinavir/ritonavir and ribavirin use, no. (%) | 10 (4.7) | 9 (11.1) | 1 (0.76) | .001 |
| Remdesivir use, no. (%) | 5 (2.3) | 5 (6.2) | 0 (0) | .004 |
| Tocilizumab use, no. (%) | 14 (6.6) | 8 (10.1) | 6 (4.5) | .126 |
| Corticosteroid treatment | ||||
| Median time to steroid initiation from admission (IQR), d | 2 (1–4) | 5 (3–7) | 2 (1–3) | <.001 |
| Corticosteroids received in first 48 h, no. (%) | 65 (30.5) | 10 (12.4) | 55 (41.7) | <.001 |
| Corticosteroids received at any time, no. (%)a | 136 (63.8) | 46 (56.8) | 90 (68.2) | .094 |
| Methylprednisolone use, no. (%) | 129 (94.9) | 43 (93.5) | 86 (95.5) | .688 |
| Median methylprednisolone dose (IQR), mg | 40 (40–50) | 40 (40–50) | 40 (35–50) | .851 |
| Oral prednisone switch, no. (%) | 7 (5.4) | 5 (11.6) | 2 (2.3) | .041 |
| Median duration of corticosteroids (IQR), db | 3 (3-3) | 3 (3-3) | 3 (3-3) | .812 |
Abbreviations: CG, corticosteroid group; IQR, interquartile range; SOC, standard of care.
aRefer to Figure S1, supplemental materials for description of timing.
b29 patients received greater than 3 days; Early corticosteroid group 20 (22.2), 9 SOC (19.5).
The primary composite endpoint occurred at a significantly lower rate in the early corticosteroid group compared to the SOC group (34.9% vs 54.3%, P = .005). A significant reduction in each of primary composite endpoints was also noted (Table 3). In the sensitivity analysis subgroup, after the implementation of the in-house RT-PCR SARS-CoV-2 testing, 34.9% (46 of 132 patients) and 55% (33 of 60 patients) experienced the primary composite endpoint in the early corticosteroid group and SOC group, respectively (P = .009). After adjustment for male sex, NEWS of ≥7, and age ≥60, early corticosteroid initiation was independently associated with a reduction in the composite endpoint at 14 days (adjusted odds ratio: 0.41; 95% confidence interval [CI], (.22 – .77) (Table S2, Supplemental Materials).
Table 3.
Outcomes in Standard of Care and Early Corticosteroid Group
| Outcomes | SOC (n = 81) | Early CG (n = 132) | Odds Ratio (CI) | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| Primary composite outcome, no. (%) | 44 (54.3) | 46 (34.9) | .45 (.26–.79) | .005 |
| Death, no. (%) | 21 (26.3) | 18 (13.6) | .45 (.22–.91) | .024 |
| Respiratory failure requiring mechanical ventilation, no. (%)a | 26 (36.6) | 26 (21.7) | .47 (.25–.92) | .025 |
| Escalation from GMU to ICU, no. (%)b | 31 (44.3) | 32 (27.3) | .47 (.25–.88) | .017 |
| Secondary outcomes | ||||
| Overall mechanical ventilation, no. (%) | 36 (44.4) | 38 (28.8) | .51 (.28–.90) | .020 |
| ARDS, no. (%) | 31 (38.3) | 33 (26.6) | .040 | |
| Mild | 3 (3.7) | 1 (0.76) | .125 | |
| Moderate | 8 (9.9) | 9 (6.8) | .307 | |
| Severe | 20 (24.7) | 23 (17.4) | .201 | |
| Median duration of mechanical ventilation (IQR), d | 8 (4–13) | 7 (4–9) | .558 | |
| Median time to extubation (IQR), d | 8 (4–13) | 7 (4–9) | .558 | |
| Shock, no. (%) | 19 (23.5) | 17 (12.6) | .069 | |
| Acute kidney injury, no. (%) | 42 (51.9) | 59 (44.7) | .310 | |
| Median hospital length of stay (IQR), d | 8 (5–14) | 5 (3–7) | <.001 | |
| Discharged from hospital, no. (%) | 51 (62.2) | 88 (66.7) | .584 | |
| Remain hospitalized, no. (%) | 9 (11.1) | 26 (19.7) | .102 | |
| Remain intubated, no. (%) | 7 (8.6) | 13 (9.8) | .771 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CG, corticosteroid group; GMU, general medical unit; ICU, intensive care unit; IQR, interquartile range; SOC, standard of care.
aA total of 10 and 12 patients were not included in this analysis because they required mechanical ventilation in the emergency department in the SOC and early corticosteroid groups, respectively.
bA total of 11 and 15 patients were not included in this analysis because they were directly admitted to the ICU in the SOC and early corticosteroid groups, respectively.
The median LOS was significantly reduced from 8 days in the SOC group, to 5 days in the early corticosteroid group (P < .001). ARDS occurred in 38.3% and 26.6% in the SOC group and early corticosteroid group, respectively (P = .04). Outcomes at 14 days also included 9 (11.1%) of SOC patients remaining admitted as compared to 26 (19.7%) of early corticosteroid patients. Table 3 describes additional outcomes before and after implementation of the early corticosteroid protocol.
DISCUSSION
In this quasi-experimental study, hospitalized patients with moderate to severe COVID-19 that received an early short course of methylprednisolone had a reduced rate of the primary composite endpoint of death, ICU transfer, and mechanical ventilation, with a number needed to treat of 8 to prevent 1 patient transfer for mechanical ventilation. The reduction in ICU transfer and requirement for mechanical ventilation represents a potential intervention to reduce critical care utilization during the COVID-19 pandemic [22, 23]. The median reduction of hospital LOS by 3 days observed with the use of corticosteroids could positively impact hospital capacity during the COVID-19 surge.
Corticosteroids are not routinely recommended in patients with COVID-19 without an alternate indication or presence of ARDS [11, 15, 24]. Data are conflicting; corticosteroid use in previous viral respiratory illnesses has demonstrated delayed viral clearance and increased mortality [11, 25]. On the contrary, short-course corticosteroids in some reports are beneficial and safe in critically ill patients with SARS-CoV-2 and were not found to be an independent risk factor of prolonged viral RNA shedding [17, 26, 27]. These discordant findings may be explained by the observational nature of the studies, heterogeneity in patient acuity, inconsistent dosing regimens and duration, and timing of initiation of therapy [10, 17]. Corticosteroids were used in 11–35% of nonsevere and 45–72% of severe COVID-19 cases in China; however, the benefits and risks remain undefined [2, 6, 12–14]. A mortality benefit (hazard ratio, 0.38; 95% CI, .20–.72) with the use of methylprednisolone was reported in 1 retrospective cohort study of COVID-19 patients with ARDS [14].
COVID-19 can progress from mild to severe illness characterized by an initial viral infection phase followed by pulmonary inflammation, and then a hyper-inflammation phase [4, 6, 9]. The pulmonary phase is associated with progressive dyspnea and radiographic findings of pneumonia [4]. Symptom onset to dyspnea and ARDS development occurs between a median of 5–7 days and 8–12 days, respectively [6, 12, 28]. The present study findings support that timing is key. An early course of corticosteroid, specifically methylprednisolone, at the onset of dyspnea, may attenuate progression to the hyper-inflammation phase that requires escalation of care in patients with COVID-19. In this study, 3 days of early corticosteroids were administered at a median 2 days into hospitalization and 8 days from symptom onset. However, the administration of a 3-day course of corticosteroids later in the disease course (median 5 days after hospitalization), as occurred in our SOC group, did not appear to confer the same benefit. Hydroxychloroquine with or without azithromycin, remdesivir, and lopinavir/ritonavir with ribavirin were prescribed at similar frequency between groups. These agents have demonstrated mixed efficacy results for COVID-19 in placebo controlled-trials, with hydroxychloroquine being the most promising at this time [10]. Immunomodulatory agents, such as tocilizumab, were infrequently used in this study.
This study has several limitations. Given the pandemic nature of the disease, a pragmatic quasi-experimental design was used, and there are some differences in the baseline characteristics of the comparator groups. The potential for regression to the mean and maturation is an inherent limitation to all quasi-experiments. On 16 March 2020, rapid on-site RT-PCR testing for SARS-CoV-2 testing was implemented, and some of the SOC group experienced delayed diagnosis and treatment. However, the observed association was unchanged in the sensitivity analysis. A nonequivalent dependent variable, empiric antibiotic therapy for pneumonia, suggested no difference in management of COVID-19. Some of the SOC group received corticosteroids after initiation of the updated COVID-19 institutional treatment protocol. Steroids initiated in this group were started significantly later. Additionally, guideline adherence in the early corticosteroid group was not universal, and may have been subject to channeling bias. Prone ventilation was attempted in a single SOC group patient and then not utilized again until late March. This may have impacted development of the primary outcome in the subset patients who required mechanical ventilation. Finally, the study has a limited follow-up period of 14 days and may be subject to lead-time bias, similar to other recent reports. As of 9 April 2020, 51 (62.9%) of patients in the SOC cohort and 88 (66.7%) of patients in the early corticosteroid cohort were discharged from the hospital. As a result, outcomes for those patients are not known. Anecdotally, we observed hyperglycemia but no severe corticosteroid related adverse effects (ie, gastrointestinal hemorrhage), and data collection is ongoing.
In conclusion, early use of a short course of methylprednisolone, an inexpensive and readily available agent, in patients with moderate to severe COVID-19 may prevent progression of disease and improve outcomes. These findings are crucial given the ongoing COVID-19 pandemic and ICU bed and mechanical ventilator shortages. Research is urgently needed to further define the role of corticosteroids in patients with COVID-19 at a high risk of clinical deterioration, identified early in the disease course using prognostic markers or clinical prediction tools.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Eric Scher, MD,4 for his leadership and commitment to education in the field of Academic Internal Medicine. Krishna Modi, MD,4 Rebecca Bussa, Kelly Curran, MD,4 Abigail Entz, MD,4 Hafsa Abdulla, MD,4 and Charles Hammond, MD,4 for data collection and review. The authors thank the entire front-line staff members of the Henry Ford Health System for dedicated and compassionate patient care during the COVID-19 outbreak.
1Infectious Diseases, Henry Ford Hospital, Detroit, Michigan, USA, 2Pulmonary Medicine, Henry Ford Hospital, Detroit, Michigan, USA, 3Pharmacy, Henry Ford Hospital, Detroit, Michigan, USA, 4Internal Medicine, Henry Ford Hospital, Detroit, Michigan, USA, 5Emergency Medicine, Henry Ford Hospital, Detroit, Michigan, USA, 6Surgical Critical Care, Henry Ford Hospital, Detroit, Michigan, USA, 7Pathology and Microbiology, Henry Ford Hospital, Detroit, Michigan, USA.
Potential conflicts of interest. S. H. received speakers’ bureau honoraria from Bayer. I. B. received speakers’ bureau honoraria from Gilead, ViiV, and Jansssen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Henry Ford COVID-19 Management Task Force. Varidhi Nauriyal, MD,1,2 Jayanth Lakshmikanth, MD,2 Asif Abdul Hamed, MD,2 Owais Nadeem, MD,2 Kristin Griebe, PharmD,3 Joseph M. Johnson, PharmD,3 Patrick Bradley, MD,2 Junior Uduman, MD,2 Sara Hegab, MD,2 Jennifer Swiderek, MD,2 Amanda Godfrey, MD,2 Jeffrey Jennings, MD,2 Jayna Gardner-Gray, MD,5 Adam Ackerman, MD,6 Jonathan Lezotte, MD,6 Joseph Ruhala, MD,6 Linoj Samuel, PhD, D(ABMM),7 Robert J. Tibbetts, PhD, D(ABMM), F(CCM),7 Indira Brar, MD,1 John McKinnon, MD,1 Geehan Suleyman, MD,1 Nicholas Yared, MD,1 Erica Herc, MD,1 Jonathan Williams, MD,1 Odaliz Abreu Lanfranco, MD,1 Anne Chen, MD,1 Marcus Zervos, MD.1
Contributor Information
Henry Ford COVID-19 Management Task Force:
Varidhi Nauriyal, Jayanth Lakshmikanth, Asif Abdul Hamed, Owais Nadeem, Kristin Griebe, Joseph M Johnson, Patrick Bradley, Junior Uduman, Sara Hegab, Jennifer Swiderek, Amanda Godfrey, Jeffrey Jennings, Jayna Gardner-Gray, Adam Ackerman, Jonathan Lezotte, Joseph Ruhala, Linoj Samuel, Robert J Tibbetts, Indira Brar, John McKinnon, Geehan Suleyman, Nicholas Yared, Erica Herc, Jonathan Williams, Odaliz Abreu Lanfranco, Anne Chen, Marcus Zervos, and Eric Scher
References
- 1. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases and latest updates. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Accessed 7 April 2020
- 2. Centers for Disease Control and Prevention. COVID-NET: COVID-19 associated hospitalization surveillance network. Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html. Accessed 8 April 2020.
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; Feb 28. doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020; 39:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol 2020; 25:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian S, Hu W, Niu L, et al. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 2020; pii:S1556-0864(20)30132–5. doi: 10.1016/j.jtho.2020.02.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Y, Fu B, Zhang X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in Severe COVID-19 patients. Natl Sci Rev nwaa041. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCreary EK, Pogue JM. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect Dis 2020; 7:ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; pii:S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020. doi: 10.1097/CCM.0000000000004363. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020. doi: 10.1056/NEJMoa2001282. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020; 395:683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 2013; 84:465–70. [DOI] [PubMed] [Google Scholar]
- 19. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ranieri V, Rubenfeld GD, Thompson B, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 21. Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2:1–38. [Google Scholar]
- 22. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA 2020. doi: 10.1001/jama.2020.5046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23. Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med 2020; 382:e41. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed 3 April 2020).
- 25. Arabi YM, Mandourah Y, Al-Hameed F, et al. ; Saudi Critical Care Trial Group Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 2018; 197:757–67. [DOI] [PubMed] [Google Scholar]
- 26. Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006; 129:1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu K, Chen Y, Yhan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis 2020. ciaa351. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.