Abstract
Background
Coronavirus disease-2019 (COVID-19) is an infectious disease appeared in China in December 2019 and, since then, has spread worldwide at a rapid pace.
Case summary
A patient with COVID-19 was hospitalized in our institution for a diabetic foot ulcer and presented afterwards a pulmonary oedema and concomitant anterior ST-segment elevation myocardial infarction. We report here on the initial presentation, coronary care and intervention, and clinical course of this patient.
Discussion
Emergent percutaneous coronary intervention is feasible and safe in COVID-19 patients but requires a multidisciplinary effort involving caregivers from infectious disease, intensive care, and cardiology teams.
Keywords: Acute coronary syndrome, Coronavirus, Percutaneous coronary intervention, Case report
Learning points
Coronavirus disease-2019 (COVID-19) screening by naso-pharyngeal swab was not systematically performed at admission and should not delay emergent cardiological care in ST-elevation myocardial infarction or high-risk non-ST-elevation myocardial infarction.
The occurrence of acute coronary syndrome will increase in COVID-19 pandemic, due to several parameters such as the profound inflammatory response and cytokine storm syndrome associated with severe acute respiratory syndrome coronavirus 2 infection.
A standardized protocol for emergent percutaneous coronary intervention should be established in each cathlab including protective measures in order to reduce the time to myocardial reperfusion in COVID-19 patients, without putting the caregivers at excessive risk of contamination.
Introduction
Coronavirus disease-2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The outbreak of COVID-19 appeared in China in December 2019 and, since then, has spread worldwide at a rapid pace and has been declared a global pandemic by the World Health Organization as of 11 March 2020.1
Patients with hypertension, diabetes, or cardiovascular disease are at high risk of being affected by COVID-192 and have more severe outcomes.3 Cardiovascular complications of COVID-19 include myocardial injury, myocarditis, type 1 or type 2 myocardial infarction, heart failure, cardiogenic shock, severe arrhythmias, and venous thrombo-embolic disease.4 Specifically, the occurrence of acute coronary syndromes (ACS) in COVID-19 patients may have significant implications both for patient care and caregiver safety.5
While the general management of ACS has been well-defined in international guidelines, reports of ACS in COVID-19 patients are scarce, and there is no clear recommendation as regard to optimal treatment in such patients.4
Timeline
| Time | Events |
|---|---|
| 13 March | First symptoms: mild fever (≤38.5°C) without dyspnoea |
| 15 March | Introduction of a combination of amoxicillin 1000 mg and clavulanic acid 125 mg t.i.d. |
| 18 March 19:00 | The patient was hospitalized for a neuro-ischaemic diabetic foot ulcer in the diabetology department |
| 18 March 22:00 | The patient became severely breathless and hypoxic (Sp02 85%) and was transferred to the intensive care unit (ICU) with strict respiratory isolation. A diagnosis of acute pulmonary oedema was made, and the patient improved on continuous positive airway pressure non-invasive ventilation, nitrates, and diuretics. An electrocardiogram (ECG) showed no significant repolarization abnormality. |
| 19 March 02:00 | Naso-pharyngeal swab resulted positive for severe acute respiratory syndrome coronavirus 2. |
| 19 March 09:30 | The respiratory state of the patient deteriorated with persistent hypoxaemia despite ventilation with a high oxygen concentration mask (6 L/min). Although the patient had no chest pain, an ECG showed transient anterior ST-segment elevation. |
| 19 March 12:00 | Emergent coronary angiography showed a tight stenosis of the mid-portion of the left anterior descending artery (LAD) with normal (TIMI 3) flow. In addition to this culprit LAD stenosis, a tight ostial stenosis of the obtuse marginal branch and an intermediate stenosis of the dominant mid-circumflex artery were observed but were not considered as related to the ACS. A biodegradable polymer, everolimus-eluting stent was deployed at the culprit site with an optimal result. |
| 19 March 13:00 | The patient was sent back to the ICU after percutaneous coronary intervention. The ECG normalized and the patient’s respiratory condition significantly improved without recurrent pulmonary oedema or need for non-invasive ventilation. |
| 20 March | High-sensitivity troponin I peaked at 8.8 µg/L (99th upper reference limit 0.045 µg/L). Echocardiography depicted a limited apical akinesia and mild anterior hypokinesia of the left ventricle with a left ventricular ejection fraction of 45%. |
| 21 March | Patient was transferred in another institution. |
Case presentation
A 63-year-old male patient was hospitalized for a neuro-ischaemic diabetic foot ulcer in the diabetology department of our institution on 18 March 2020. He had several cardiovascular risk factors, including type-2 diabetes mellitus, hypertension, dyslipidaemia, and was an active smoker. He had no history of heart disease, but had undergone percutaneous transluminal angioplasty of the right femoral artery 5 years earlier.
The patient reported no respiratory symptoms upon admission, but had complained of mild fever (38.5°C) for the last 5 days. Three days before hospitalization, he was prescribed a combination of amoxicillin 1000 mg and clavulanic acid 125 mg t.i.d. with no effect on fever.
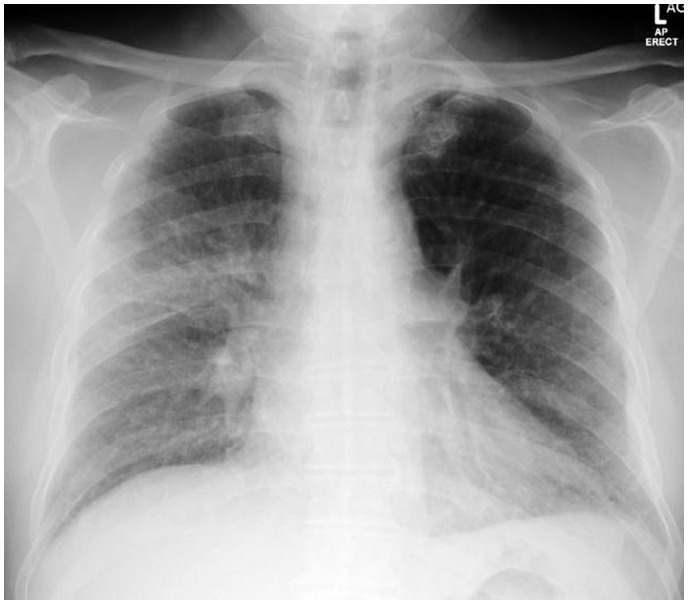
Shortly after admission, the patient became severely breathless and hypoxic (Sp02 85%) with a severe increase in blood pressure (226/118 mmHg). Physical examination showed normal cardiac murmurs, tachypnoea, and crackles in both lung fields without any other signs of congestion. No significant repolarization abnormality was observed on electrocardiogram (ECG). An echocardiography showed normal left ventricular ejection fraction (LVEF), high left ventricular filling pressure (E/E′ ratio at 23), and systolic pulmonary artery pressure (45 mmHg). The chest X-ray showed bilateral perihilar airspace opacity with dilatation of the main pulmonary artery (Figure 1). Based on the clinical presentation, a diagnosis of acute pulmonary oedema was made and the patient was transferred to the intensive care unit (ICU) with strict respiratory isolation. The patient recovered within an hour on continuous positive airway pressure non-invasive ventilation, nitrates, and diuretics, allowing for complete oxygen weaning. Because of systematic suspicion of COVID-19 in patients with respiratory failure and fever, polymerase chain reaction was performed on a naso-pharyngeal swab and returned positive for -CoV-2. In addition, blood tests showed a biological inflammatory syndrome [leucocytes 18 G/L, C-reactive protein 143 mg/L (N < 6 mg/L)], with negative cardiac troponin <0.015 µg/L (99th upper reference limit 0.045 µg/L) and elevated N-terminal-pro-B-type natriuretic peptide (2733 ng/L).
Figure 1.
Chest X-ray recorded during the first episode of acute respiratory failure, showing dilated upper pulmonary veins and bilateral perihilar airspace opacity with dilatation of the main pulmonary artery.
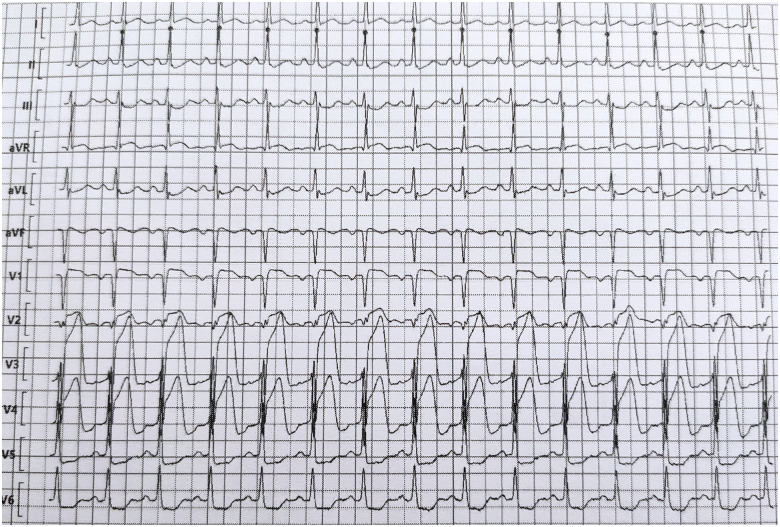
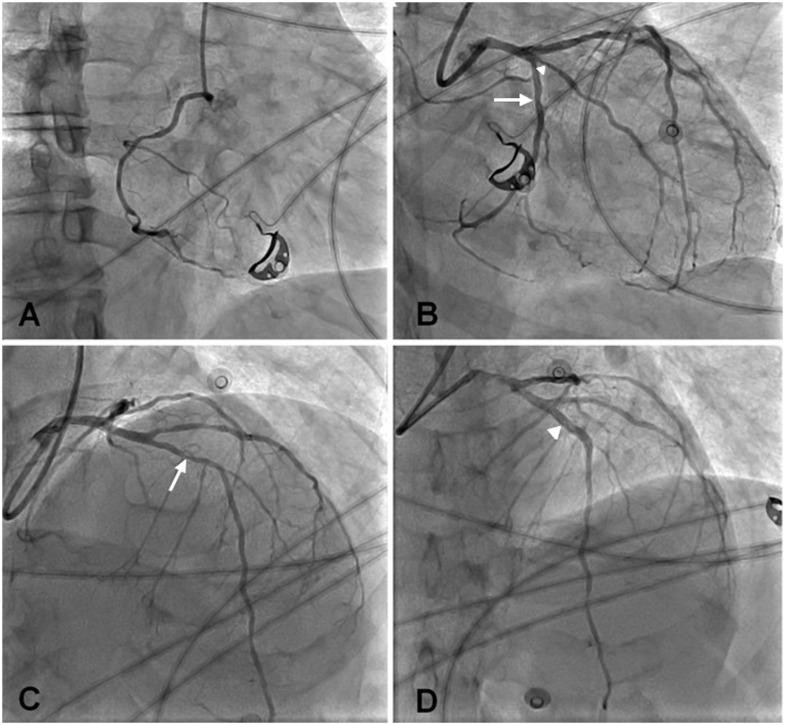
Twelve hours later, the respiratory state of the patient deteriorated with persistent hypoxaemia (PaO2 57 mmHg) despite ventilation with a high oxygen concentration mask (6 L/min). Although the patient had no chest pain, an ECG showed anterior ST-segment elevation. The patient received 4000 IU of intravenous unfractionated heparin, 250 mg of intravenous aspirin, and 180 mg of oral ticagrelor, and the patient was taken to the cathlab 150 min after the first abnormal ECG for emergent coronary angiography. The ECG recorded upon arrival in the cathlab showed persistent ST-segment elevation in the anterior leads with reciprocal changes in the inferior and lateral leads (Figure 2). The patient wore a surgical mask during the coronary angiography, while the interventional cardiologist and cathlab nurses wore protective equipments—including N95 mask, mobcap, gloves, plastic glasses, overshoes, and sterile gowns above the lead aprons—as advised by the local infection control unit, which was present during the procedure. A tight stenosis of the mid-portion of the left anterior descending artery (LAD) was found, with normal (TIMI coronary grade flow 3) flow. In addition to the culprit LAD stenosis, a tight ostial stenosis of the obtuse marginal branch and an intermediate stenosis of the dominant mid-circumflex artery were observed. A biodegradable polymer, everolimus-eluting stent (Synergy, Boston Scientific) was deployed at the culprit site 37 min after the arrival in the cathlab with an optimal result (Figure 3). No attempt was made to stent non-culprit stenosis.
Figure 2.
Electrocardiogram recorded during the second episode of acute respiratory failure. ST-segment elevation is present in leads V1 to V4 with reciprocal ST changes in leads II, II, V5, and V6.
Figure 3.
Coronary angiography. (A) Right coronary artery (left anterior oblique view). (B) Left coronary artery (right anterior oblique caudal view). Intermediate stenosis of the dominant mid-circumflex artery (white arrow) and tight ostial stenosis of the obtuse marginal branch (with arrowhead). (C) Left coronary artery (right anterior oblique cranial view). Tight stenosis of the mid-left anterior descending artery (white arrow). (D) Same view as in C after stent implantation in the left anterior descending artery (white arrowheads).
The ECG normalized within an hour after percutaneous coronary intervention (PCI) and the patient’s respiratory condition significantly improved, without recurrent pulmonary oedema or need for non-invasive ventilation. High-sensitivity troponin I peaked at 8.8 µg/L (99th upper reference limit 0.045 µg/L). Echocardiography depicted a limited apical akinesia and mild anterior hypokinesia with a LVEF of 45%. Given the favourable clinical course, no specific treatment of COVID-19 was introduced and the patient was transferred to another institution 2 days after PCI. Unfortunately, respiratory condition of the patient deteriorated 8 days after diagnosis of COVID-19 and he required sedation, intubation for mechanical ventilation and died 6 days later of refractory hypoxaemia.
Discussion
In this case report, the occurrence of an acute respiratory distress in a patient with infected diabetic foot ulcer, led to the simultaneous diagnosis of COVID-19 and high-risk ACS. The medical teams involved with the care of the patient had to deal with several challenges.
First, establish a formal diagnosis of COVID-19 and prevent COVID-19 transmission to the caregivers. Of note, COVID-19 screening was not performed upon initial admission since the patient’s fever was attributed to his foot infection and he had no respiratory symptoms. However, it was systematically performed when his respiratory condition deteriorated, leading to implement the protective measures, pending the results of the naso-pharyngeal swab.
Second, understand the aetiology of the two episodes of respiratory failure, which were not related to COVID-19, as suggested by the favourable clinical course on diuretics and oxygenation after the first episode, and the efficacy of PCI on symptoms after the second.
Third, to perform emergent PCI in a safe environment in a patient with COVID-19, which has been recognized as a medical challenge recently.6 In the present case, the transfer of the patient from the ICU to the cathlab took 150 min, an unusually long time for an inpatient presenting with ST-elevation ACS in a tertiary hospital with round-the-clock cathlab availability. This long delay was attributed to the extremely tensed medical activity at the start of COVID-19 epidemics, especially in the ICU. However, the cathlab team took advantage of this long transfer to receive proper training by the infection control unit, such that appropriate protective measures had been implemented and PCI could be rapidly performed. In order to prevent time delay for emergent PCI, our current policy is to refer all patients with very-high risk ACS and suspected COVID-19 directly to the cathlab and to transfer them immediately afterward to the ICU.
Fourth, even though it is unclear whether the ACS could be classified as a type 1 or type 2 myocardial infarction, the persistence of ST-segment elevation before PCI despite a patent LAD suggests that a profound imbalance between oxygen supply and demand was the key determinant of this ACS. Alternatively, a vasospasm cannot be ruled out. Fifth, due to potential infectious hazards related to the close exposure to the COVID-19 patient, it was decided not to perform further imaging of the culprit atherosclerotic plaque with intravascular ultrasound or optical coherence tomography. Hence, it was impossible to assess whether atherosclerotic plaque rupture or erosion was present at the culprit site. The profound inflammatory response and cytokine storm syndrome associated with SARS-CoV-2 infection may confer a greater risk for atherosclerotic plaque rupture in susceptible patients,7 and high prothrombotic state leading to transient occlusive arterial thrombosis.8
Finally, there are reports of individual centres developing alternate strategies in the setting of the COVID-19 crisis, such as preferential use of fibrinolytic therapy in patients with ST-elevation myocardial infarction.9 However, interventional approaches are more likely to improve the patient outcomes and should be preferred, provided they can be applied timely and without putting the caregivers at excessive risk of contamination.
Conclusion
Emergent PCI for ACS is feasible and safe in COVID-19 patients but requires a multidisciplinary effort involving caregivers from infectious disease, intensive care, and cardiology teams.
Lead author biography

Quentin Fischer is a 31-year-old interventional cardiologist working in Bichat Hospital, Paris, France. He performed a fellowship in the Quebec Heart & Lung Institute, Quebec city, Canada. His research interests include optimizing myocardial revascularization in ST-segment elevation myocardial infarction and transcatheter valve technologies. Member of the Young Community of the European Society of Cardiology, he is also very involved in teaching students at the Medicine University.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/whodirector-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-March-2020 (12 March 2020).
- 2. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y.. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 4. Driggin E, Madhavan M V, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown T S, Der Nigoghossian C, Zidar D A, Haythe J, Brodie D, Beckman J A, Kirtane A J, Stone G W, Krumholz H M, Parikh S A.. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020;395:1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng J, Huang J, Pan L.. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People’s Hospital. Intensive Care Med 2020;doi: 10.1007/s00134-020-05993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.