Abstract
Background
Washington State served as the initial epicenter of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in the United States. An understanding of the risk factors and clinical outcomes of hospitalized patients with coronavirus disease 2019 (COVID-19) may provide guidance for management.
Methods
All laboratory-confirmed COVID-19 cases in adults admitted to an academic medical center in Seattle, Washington, between 2 March and 26 March 2020 were included. We evaluated individuals with and without severe disease, defined as admission to the intensive care unit or death.
Results
One hundred five COVID-19 patients were hospitalized. Thirty-five percent were admitted from a senior home or skilled nursing facility. The median age was 69 years, and half were women. Three or more comorbidities were present in 55% of patients, with hypertension (59%), obesity (47%), cardiovascular disease (38%), and diabetes (33%) being the most prevalent. Most (63%) had symptoms for ≥5 days prior to admission. Only 39% had fever in the first 24 hours, whereas 41% had hypoxia at admission. Seventy-three percent of patients had lymphopenia. Of 50 samples available for additional testing, no viral coinfections were identified. Severe disease occurred in 49%. Eighteen percent of patients were placed on mechanical ventilation, and the overall mortality rate was 33%.
Conclusions
During the early days of the COVID-19 epidemic in Washington State, the disease had its greatest impact on elderly patients with medical comorbidities. We observed high rates of severe disease and mortality in our hospitalized patients.
Keywords: COVID-19, SARS-CoV-2, outcomes, hospitalized, comorbidities
In this case series of 105 consecutively hospitalized COVID-19 patients, the median age was 69 years, and 55% had 3 or more comorbidities. Severe disease occurred in 49% of patients, and overall mortality was 33%.
Infections with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first occurred in late 2019 in Hubei Province, China, and has since disseminated worldwide [1, 2]. The first confirmed case in the United States was a returning traveler from Wuhan, China, who presented to medical attention on 19 January 2020, in Snohomish County, Washington [3]. In February, a cluster of coronavirus disease 2019 (COVID-19) cases was identified at a nursing home in Kirkland, Washington, resulting in the first reported death in the United States on 29 February [4, 5]. Since that time, the disease has occurred widely in the greater Seattle area, and cases have been reported in all 50 states [6].
The epidemiology and clinical features of hospitalized COVID-19 patients have been described in China, South Korea, and Singapore [1, 7–10]. The prominent clinical manifestations have included fever, cough, dyspnea, fatigue, myalgia, and radiographic evidence of pneumonia. Shock, acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury (AKI), and deaths were reported in severe cases [1, 5, 9, 11]. Early reports indicated that the infection occurs in clusters within groups in close contact, and that severe disease was more common in the elderly, men, and those with comorbidities [9]. The epidemiologic and clinical features of COVID-19 disease in the United States are beginning to be elucidated, specifically in critically ill patients [5]. Additional information is needed to help clinicians understand the characteristics of this disease. In this case series, we examine the epidemiology, clinical features, and outcomes of 105 hospitalized patients from 3 university-affiliated hospitals in Seattle, Washington. The characteristics associated with severe versus nonsevere clinical courses are described.
METHODS
Study Design and Participants
We conducted a retrospective chart review examining the clinical characteristics of adult patients with laboratory-confirmed SARS-CoV-2 infection. Patients were included if they were admitted to 1 of 3 University of Washington (UW) affiliated hospitals in Seattle between 2 March and 26 March 2020.
The hospitals included UW Medical Center (composed of 2 campuses: Montlake, a 570-bed tertiary/quaternary care facility and Northwest, a 281-bed community-based care facility), and Harborview Medical Center (HMC), a 413-bed level 1 trauma center.
Data Sources and Collection
Demographic data, encounter dates, and laboratory measurements were extracted electronically from the UW clinical data warehouse for confirmed cases. The medications, vital signs, radiology studies, and clinical events were reviewed by physicians in the Division of Infectious Diseases and entered into a standardized data collection instrument using REDCap (Research Electronic Data Capture) [12].
Study Outcomes
The primary outcome was severe COVID-19, defined as a composite endpoint of admission to an intensive care unit (ICU) or death [13]. Each death was reviewed by 2 physicians independently to determine whether it was related to COVID-19. Secondary outcomes included shock and acute respiratory distress syndrome (ARDS), which were also adjudicated by 2 physicians independently. These outcomes were evaluated until 8 May 2020, 43 days from the date the last patient was admitted.
Study Definitions
Fever was defined as a recorded temperature of 38.0°C or higher. Hypoxia was defined as an oxygen saturation <90% on ambient air, or requirement of supplemental O2 [14]. AKI was defined as an increase in creatinine of 0.3 mg/dL from baseline, or 1.5 times the patient’s baseline creatinine [15]. Leukopenia was defined as white blood cell count <4 × 109/L, and lymphopenia was defined as a lymphocyte count <1.5 × 109/L. Cardiac injury was defined as a maximum troponin value of 0.1 ng/mL or higher during hospitalization. Patients were denoted as having ARDS if the physician indicated this in their clinical notes or, in the absence of this documentation, if the patient had new respiratory failure, bilateral lung opacities on chest imaging, and a PaO2:FIO2 ratio ≤ 300 [16]. Shock was defined as hypotension requiring vasopressors. Patients were considered to have received antibiotics during their admission if these were given for more than 48 hours. Patients were considered to have received corticosteroids during their admission if they were newly started on systemic corticosteroids.
Laboratory Testing
Nasopharyngeal samples were tested for SARS-CoV-2 by real-time reverse-transcription polymerase chain reaction (RT-PCR) developed by the University of Washington Virology laboratory [17]. Indeterminate results were confirmed at the Washington State Department of Health (WA DOH) laboratory or the Centers for Disease Control and Prevention (CDC). If a patient had an indeterminate result from the UW Virology Lab and a positive result from the WA DOH or CDC laboratory, this subject was considered positive. If available, residual clinical samples were subsequently tested by quantitative real-time RT-PCR for 12 additional respiratory viruses, including influenza A and B, respiratory syncytial virus, adenovirus, human metapneumovirus, parainfluenza viruses 1–4, rhinovirus, bocavirus, and seasonal coronaviruses [18]. Semiquantitative viral load was defined as the cycle threshold (Ct) of the initial sample tested.
Statistical Analysis
Continuous variables were displayed as median values with simple or interquartile ranges as appropriate. Categorical variables were summarized as counts of all patients or a subset of evaluated patients with percentages. All analyses were conducted using Stata 14.2 (StataCorp).
Study Oversight
This study was approved by the University of Washington Institutional Review Board (STUDY00006181). Informed consent was waived for retrospective review of patient charts.
RESULTS
One hundred five patients with confirmed SARS-CoV-2 infection were hospitalized between 2 March and 26 March 2020, with a median follow-up of 9 days (range, 1–53). The demographic and clinical characteristics are shown in Table 1. The median age was 69 (range, 23–97), half were women, and 71% were white. Thirty-five percent of patients lived in a skilled nursing facility or senior living. Most patients (93%) had at least 1 comorbidity; 55% had 3 or more chronic medical problems. The most common comorbidities were hypertension (59%), obesity (47%), cardiovascular disease (38%), and diabetes (33%).
Table 1.
Demographics and Baseline Characteristics of Patients Infected With SARS-CoV2
| All Patients (N = 105) | Not Severe (n = 54) | Severe (n = 51) | |
|---|---|---|---|
| Age, years | 69 (23–97) | 67 (25–96) | 70 (23–97) |
| >60 years | 70 (67) | 38 (70) | 32 (63) |
| Sex | |||
| Male | 53 (50) | 23 (43) | 30 (59) |
| Female | 52 (50) | 31 (57) | 21 (41) |
| Race | |||
| White | 75 (71) | 41 (76) | 34 (67) |
| Black | 6 (6) | 4 (7) | 2 (4) |
| Asian/Pacific Islander | 18 (17) | 7 (13) | 11 (21) |
| Unknown or other | 6 (6) | 2 (4) | 4 (8) |
| Hispanic ethnicity | 18 (17) | 10 (19) | 8 (16) |
| Smoking (ever) | 22/86 (26) | 12/45 (27) | 10/41 (24) |
| Alcohol (any) | 26/87 (30) | 14/45 (31) | 12/42 (29) |
| Comorbidity | |||
| Hypertension | 62 (59) | 32 (59) | 30 (59) |
| Obesity, BMI >30 kg/m2 | 44/93 (47) | 21/50 (42) | 23/43 (53) |
| Cardiovasculara disease | 40 (38) | 22 (42) | 18 (35) |
| Diabetes | 35 (33) | 17 (31) | 18 (35) |
| Chronic kidney disease | 27 (26) | 12 (22) | 15 (29) |
| Heart failure | 20 (19) | 13 (24) | 7 (14) |
| Cancer | 16 (15) | 5 (9) | 11 (22) |
| Asthma | 10 (10) | 7 (13) | 3 (6) |
| Obstructive sleep apnea | 12 (11) | 5 (9) | 7 (14) |
| COPD | 11 (10) | 4 (7) | 7 (14) |
| Autoimmune disease | 9 (9) | 5 (10) | 4 (8) |
| Transplant (solid organ) | 4 (4) | 3 (6) | 1 (2) |
| HIV infection | 1 (1) | 0 | 1 (2) |
| Pregnancy | 2 (2) | 2 (4) | 0 |
| Comorbidity burden | |||
| None | 7 (7) | 3 (5) | 4 (8) |
| 1–2 | 40 (38) | 23 (43) | 17 (33) |
| 3 or more | 58 (55) | 28 (52) | 30 (59) |
| Residence | |||
| Homeless | 1 (1) | 1 (2) | 0 |
| Senior living | 10 (10) | 7 (13) | 3 (6) |
| Skilled nursing facility | 26 (25) | 10 (19) | 16 (31) |
| Home | 67 (64) | 35 (66) | 32 (63) |
| Epidemiologic risk factor | |||
| None | 50 (48) | 27 (50) | 23 (45) |
| Travel | 2 (2) | 0 | 2 (4) |
| Known contact | 17 (16) | 10 (19) | 7 (16) |
| Nursing/care facility | 35 (33) | 17 (31) | 18 (35) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus.
aIncludes cerebrovascular disease. Values are noted as median (range), n of all patients (%) or a proportion of subset, n/N (%).
The majority of patients (63%) presented with symptoms lasting more than 5 days (Table 2). The most common presenting symptoms were cough (72%), dyspnea (53%), and fatigue (49%). Twenty-three percent of patients reported diarrhea, and 22% either nausea or vomiting. Nasal symptoms (congestion/rhinorrhea) and sore throat occurred in 14% and 7%, respectively. The initial chest x-ray was abnormal in 86% of cases. A minority of patients (39%) had a fever on admission or within the first 24 hours. Hypoxia was observed in 41% at the time of admission. Blood laboratories at admission showed leukopenia in 15% and lymphopenia in 73% of patients (Table 3).
Table 2.
Clinical Signs and Symptoms on Presentation
| All Patients (N = 105) | Not Severe (n = 54) | Severe (n = 51) | |
|---|---|---|---|
| Vitals | |||
| First temperature | 36.9 (35.3–39.8) | 36.8 (36.1–39.3) | 37 (35.3–39.8) |
| T >38.0 C × 1st 24 hours | 37 (39) | 13 (25) | 24 (55) |
| Heart rate >100 bpm | 39 (37) | 19 (35) | 20 (39) |
| Systolic pressure, mmHg | 129 (70–188) | 130 (93–188) | 125 (70–173) |
| Respiratory rate | 20 (14–48) | 19 (14–32) | 22 (14–48) |
| Hypoxiaa | 43 (41) | 10 (19) | 33 (65) |
| Symptoms | |||
| Symptoms × >5 days | 54/86 (63) | 31/43 (72) | 23/43 (53) |
| Cough | 76 (72) | 41 (76) | 35 (69) |
| Dyspnea | 56 (53) | 24 (44) | 32 (63) |
| Fatigue | 51 (49) | 29 (54) | 22 (43) |
| Fever/chills | 49 (47) | 25 (46) | 24 (47) |
| Anorexia | 33 (31) | 18 (33) | 15 (29) |
| Sputum production | 19 (18) | 8 (15) | 11 (22) |
| Diarrhea | 24 (23) | 12 (22) | 12 (24) |
| Myalgia/arthralgia | 25 (24) | 15 (28) | 10 (20) |
| Nausea/vomiting | 23 (22) | 14 (26) | 9 (18) |
| Chest pain | 15 (14) | 9 (17) | 6 (12) |
| Congestion/rhinorrhea | 15 (14) | 9 (17) | 6 (12) |
| Headache | 8 (8) | 4 (7) | 4 (8) |
| Sore throat | 7 (7) | 5 (9) | 2 (4) |
| Imaging | |||
| Abnormal CXR | 78/91 (86) | 36/45 (80) | 42/46 (91) |
| Abnormal chest CT | 18/19 (95) | 7/8 (88) | 11/11 (100) |
Values are noted as median (range), n of all patients (%) or a proportion of subset, n/N (%).
Abbreviations: CT, computed tomography; CXR, chest x-ray.
aDefined as O2 saturation of <90% on ambient air or requiring supplemental O2.
Table 3.
Laboratory Measurements on Presentation
| All patients (n = 105) | Not Severe (n = 54) | Severe (n = 51) | |
|---|---|---|---|
| White blood cell count, x 109/L | 6.4 (4.6–8.8) | 5.3 (4.3–7.3) | 7.3 (5.8–13.3) |
| <4 | 15 (15) | 10 (18) | 5 (10) |
| 4–10 | 68 (66) | 41 (76) | 27 (55) |
| ≥10 | 20 (19) | 3 (6) | 17 (35) |
| Lymphocyte count, x 109/L | 1.1 (0.6–1.5) | 1.2 (0.8–1.6) | 0.9 (0.5–1.4) |
| <1.5 | 74 (73) | 36 (69) | 38 (78) |
| Neutrophil count, × 109/L | 4.3 (3.0–6.3) | 3.8 (2.8–5.1) | 5.4 (3.3–8.4) |
| Hemoglobin, g/dL | 12 (10.8–13.2) | 12.1 (11.3–13.4) | 11.7 (10.2–12.6) |
| Platelet count, × 109/L | 210 (147–260) | 207 (158–237) | 211 (128–279) |
| <150 | 27 (26) | 11 (20) | 16 (33) |
| Prothrombin time, s (n = 70) | 14.1 (13.4–15.8) | 14 (13.2–16.3) | 14.2 (13.5–15.6) |
| Partial thromboplastin time, s (n = 53) | 35 (30–40) | 36 (31–40) | 33.5 (30–41) |
| Alanine aminotransferase, U/L (n = 94) | 25 (17–39) | 22 (13–31) | 30 (19–55) |
| >40 | 21/94 (22) | 6/47 (13) | 15/47 (32) |
| Aspartate aminotransferase, U/L (n = 94) | 32 (22–48) | 27 (20–37) | 39 (25–60) |
| >40 | 31/94 (33) | 9/47 (19) | 22/47 (47) |
| Total bilirubin, mg/dL (n = 94) | 0.5 (0.4–0.8) | 0.5 (0.4–0.7) | 0.5 (0.3–0.9) |
| Blood urea nitrogen, mg/dL | 21 (13–32) | 16 (12–23) | 26 (21–37) |
| Creatinine (serum), mg/dL | 0.84 (0.66–1.32) | 0.83 (0.61–1.06) | 0.88 (0.69–1.45) |
All values are noted as median (interquartile range [IQR]), n of all patients (%) or a proportion of subset, n/N (%).
Of 39 patients who had SARS-CoV-2 samples available for semi-quantitative testing, the median Ct was 26.5 (range, 12.4–35.4) with no difference between severe and nonsevere cases. Fifty samples were available for viral coinfection testing. No coinfections with influenza A or B, respiratory syncytial virus, adenovirus, human metapneumovirus, parainfluenza viruses, rhinovirus, or bocavirus were identified. There were no coinfections with coronaviruses 229E, NL63, OC43, or HKU1.
Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) were used in 33% of patients (Table 4), whereas aspirin or nonsteroidal anti-inflammatory drugs were used in 35%. The proportion of all patients on corticosteroids was 10% or other immunosuppressants was 11%.
Table 4.
Medications, Interventions, and Complications
| Total (n = 105) | |
|---|---|
| Medications on admission | |
| Statin | 44 (42) |
| Aspirin/NSAID | 37 (35) |
| ARB | 16 (15) |
| ACE inhibitor | 19 (18) |
| Immunosuppressant(s) | 12 (11) |
| Corticosteroid | 10 (10) |
| Medications during hospital course | |
| Antibiotics | 51 (50) |
| Hydroxychloroquine | 44 (42) |
| Remdesivir vs placebo (trial) | 21 (20) |
| Remdesivir (compassionate use) | 4 (4) |
| Corticosteroids | 3 (3) |
| Interventions | |
| Highest level of oxygen support | |
| None | 22 (21) |
| O2 by nasal cannula | 39 (37) |
| High-flow O2 or nonrebreather | 24 (23) |
| Noninvasive (BiPAP) | 1 (1) |
| Mechanical ventilation | 17 (16) |
| ECMO | 2 (2) |
| Hemodialysis/SLED/CRRT (newly initiated) | 3 (3) |
| Complications | |
| Shock | 17 (16) |
| ARDS | 28 (27) |
| Cardiac injury | 13/67 (19) |
| AKI | 31 (30) |
| Death | 35 (33) |
Values represent all patients, N (%) or a proportion of subset, n/N (%).
Abbreviations: ACE, angiotensin converting enzyme; AKI, acute kidney injury; ARB, angiotensin II receptor blockers; ARDS, acute respiratory distress syndrome; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; NSAID, nonsteroidal anti-inflammatory drug; SLED, sustained low efficiency dialysis.
During hospitalization, 42% of patients were treated with hydroxychloroquine (Table 4). In nearly all cases it was dosed at 400 mg PO BID × 1 day, then 200 mg PO BID × 4 days, according to a published study [10]. Remdesivir was provided to 4 patients on a compassionate use protocol. Subsequently, a clinical trial was initiated, and 21 patients were randomized to receive remdesivir or placebo. No other antiviral drugs were administered to treat COVID-19 infection. Half of patients received antibiotics for at least 48 hours during their hospitalization. Only a small fraction (3%) of all patients received corticosteroids at doses that were greater than baseline doses.
With respect to nonpharmacological interventions, 76% of all patients required supplemental oxygen either by nasal cannula (37%), high-flow (23%), or mechanical ventilation (18%). Two were placed on extracorporeal membrane oxygenation (ECMO). Three patients (3%) were newly started on renal replacement therapy.
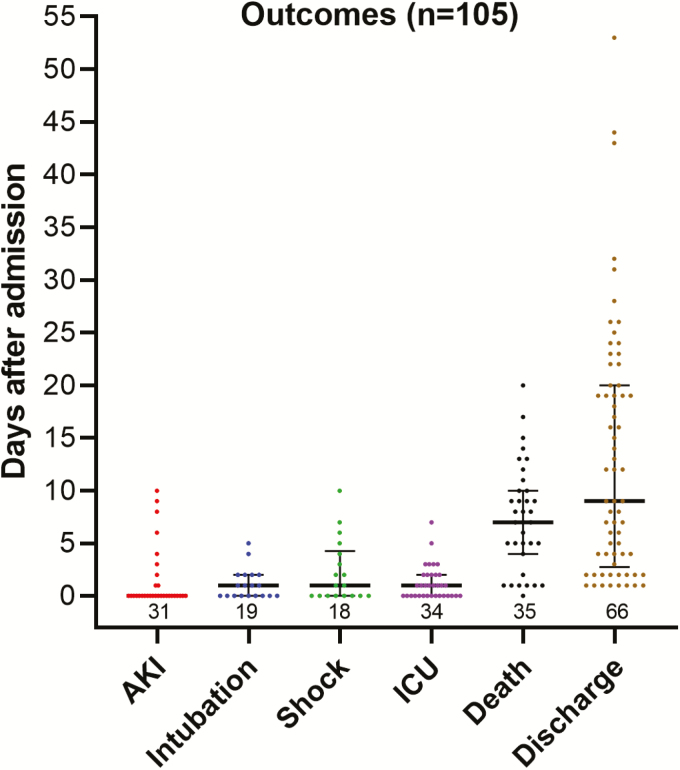
Thirty-one (30%) patients developed AKI, which tended to be present on admission (Figure 1). Shock occurred in 17 (16%) patients and occurred on median hospital day 1 (range, 0–10). Acute respiratory distress syndrome was seen in 28 (27%), and 19 (18%) underwent mechanical ventilation, with intubation occurring on median hospital day 1 (range, 0–5). Nineteen percent of patients who had a troponin sent (13 of 67) had evidence of cardiac injury. Fifty-one patients (49%) had severe illness, and 35 patients (33%) died. Among those who died, all deaths were confirmed to be COVID-19-related, and death occurred on median hospital day 7 (range, 0–20). At the end of our chart review, 66 patients survived to discharge (63%), with discharge occurring on median hospital day 9 (range, 1–53), and 4 patients remained hospitalized.
Figure 1.
Time to onset of COVID-19 complications. Points represent individual patients, and bars represent the median with interquartile ranges; number of observations is noted above the x-axis. Abbreviations: AKI, acute kidney injury; COVID-19, coronavirus disease 2019; ICU, intensive care unit admission.
DISCUSSION
This is among the first case series of COVID-19 patients hospitalized in the United States. At 3 academic hospitals in Seattle, 105 patients with SARS-CoV-2 infection were admitted between 2 March and 26 March 2020. Fifty-one patients (49%) had severe illness, and 35 patients (33%) died. This is a relatively high mortality rate compared to reports from China and Singapore, where mortality rates in hospitalized patients were 1.4% [9], 4.3% [7], and 0% [8]. This is likely due to the more advanced age (median 69 years) of our cohort compared with these previous case series, which reported average ages of 47 [9], 56 [7], and 47 [8]. Additionally, the burden of comorbidities in our population was extremely high, with 93% of patients having at least 1 comorbidity, also higher than the previously reported rate of comorbidity of 46.4% [7] and 32% [1].
Patients living in nursing or senior living homes composed 35% of the patients, reflecting the increased risk of living in congregated settings. Aside from 1 homeless patient, the remaining 65% of patients were domiciled at private residences prior to hospitalization, indicating that the infection was already distributed throughout the community. Travel was not an important risk, indicating that infections were unlikely to be imported. Only 16% of participants reported a known contact, indicating ongoing community transmission in the several weeks between the first case and this time period [19].
With respect to comorbidities, hypertension (59%), obesity (47%), cardiovascular disease (38%), diabetes (33%), and chronic kidney disease (26%) were the most notable and tended to be more prevalent in cases with severe clinical outcomes. It is striking that 55% of the patients had 3 or more comorbidities, underscoring the vulnerability of chronically ill individuals to COVID-19. Similar associations have been observed in influenza: diabetes and cardiovascular disease are known to increase the risk of developing complications from influenza [20], and previous work has shown an association between obesity and poor outcomes in influenza infection [21, 22]. Furthermore, the association between the presence of hypertension, cardiovascular disease, and diabetes with poor outcomes was documented among patients with Middle East respiratory syndrome coronavirus infection [23]. Chronic lung disease including chronic obstructive pulmonary disease (10%) and asthma (10%) were surprisingly uncommon. Only 2 pregnant patients were observed in this cohort, and they recovered without complications. The frequency of obesity, hypertension, and diabetes associated with our hospitalized COVID-19 patients is concerning given the high prevalence of these conditions in the US population.
Of preadmission medications, statins were the most common (42%) followed by ACE inhibitors or ARBs (33% combined). There is conflicting evidence on the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on angiotensin-converting enzyme 2 (ACE2) [24]. In animal models, ACE inhibitors and ARBs upregulate surface expressed ACE2, a binding target of SARS-CoV2 [25], which could worsen disease. However, animal studies have shown that these medications decrease severe lung injury in some viral pneumonias [26]. Our study could not determine if these medications were a risk factor for severe disease.
For most patients, COVID-19 was not an abrupt illness leading to sudden hospitalization; the majority (63%) of patients reported symptoms for least 5 days prior to admission. Only a minority of patients (39%) had documented fever in the first 24 hours of hospitalization. Absence of fever in some patients has been previously reported [9]. The absence of fever in the majority of this largely geriatric cohort is an important finding. Clinicians evaluating older patients will need to have a high index of suspicion for COVID-19 in those who present without fever. Cough (72%), dyspnea (53%), and fatigue (49%) were the most common symptoms reported by patients, consistent with other studies [1, 7]. Gastrointestinal complaints were noted in a minority. Hypoxia and elevated temperatures in the first 24 hours of hospitalization were more frequent in severe COVID-19, but notably, there were few presenting clinical signs or symptoms that distinguished those who would go on to severe disease.
Objective data on the patients demonstrated findings previously reported. Chest imaging was abnormal in the vast majority of patients, consistent with previous reports [7]. A majority of patients (73%) had lymphopenia, which was more commonly observed in severe COVID-19. Transaminases were mildly elevated in 22–33% of patients but rarely more than twice the upper limit of normal. Viral coinfection was not identified, concordant with previous published studies describing influenza coinfection only in case reports.
Few patients (3%) received corticosteroids, consistent with recent published recommendations [27]. A subset of patients was treated with hydroxychloroquine (40%) or were enrolled in a trial with remdesivir (20%) based on preliminary data suggesting antiviral activity against SARS-CoV2 [28, 29]; whether these provide clinical benefit remains unknown.
A large fraction (76%) of these patients required oxygen supplementation, including 18% who received mechanical ventilation. Hemodynamic shock, which occurred in 16% of patients in this group, has been reported previously [5, 9]. The frequency of AKI (30%) appears higher than the reports from China of 0.5% to 7% [1, 9] and likely attributable to the advanced age and high comorbidity burden of our cohort, although severe illness was not limited to the elderly patients in this group.
Our study has some limitations. First, as with any observational study derived from routinely collected clinical data, there is the potential for incomplete capture or misclassification of baseline characteristics, particularly for patients who did not receive primary care in our system. Next, this study was performed early in the COVID-19 outbreak when testing was not widespread, and attention was heavily focused on nursing homes. In addition, 4 of these patients remained hospitalized at the time of manuscript submission with partial follow-up. A larger clinical cohort may reveal additional features not readily apparent in our series.
In conclusion, COVID-19 has spread rapidly throughout the United States and most of the world. The early experience in Seattle indicated that patients requiring hospitalization for COVID-19 were generally of advanced age with multiple comorbidities. The high morbidity and mortality we observed underscores the urgency of widespread preventive measures to protect this vulnerable population.
Notes
Author contributions. H. N. K. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: F. S. B., H.Y. C., D. J. M., and H. N. K. Acquisition, analysis, or interpretation of data: V. A., F. S. B., M. B., D. J. M., S. A. M., A. K. N., and M. L. H. Drafting of the manuscript: F. S. B., D. J. M., H. Y. C., and H. N. K. Critical Revisions of manuscript for important intellectual content: K. R. J., A. L. G., M. L. G., S. N., and S. A. C.
Acknowledgments. The authors thank the clinical staff at University of Washington affiliated hospitals for their excellent and tireless care of patients with and without COVID-19. We are grateful to Kristine Lan and Ayushi Gupta for their assistance with data extraction.
Financial support. This work for supported by National Institute of Allergy and Infectious Diseases (grant number 5T32AI007044).
Potential conflicts of interest. H. Y. C. reports consulting fees from Glaxo Smith Kline and Merck, receives research support from Sanofi Pasteur, Genentech, Cepheid, and Ellume unrelated to this work. H. Y. C. also reports grants from Gates Ventures during the conduct of the study. A. L. G. reports personal fees from Abbott Molecular, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus disease 2019 (COVID-19) situation report - 56, 2020. [Google Scholar]
- 3. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC COVID-19 Response Team. Severe outcomes among patients with Coronavirus disease 2019 (COVID-19) - United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:343–6. doi:10.15585/mmwr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA Published online March 19, 2020. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) cases in U.S. Updated April 13, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-in-us.html#2019coronavirus-summary. Accessed 13 April 2020. [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA Published online March 3, 2020. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Covid-19 National Emergency Response Center E, Case Management Team KCfDC, Prevention. Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect 2020; 11:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med Published online March 13, 2020. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013; 368:2277–85. [DOI] [PubMed] [Google Scholar]
- 14. Charles PG, Wolfe R, Whitby M, et al. ; Australian Community-Acquired Pneumonia Study Collaboration SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008; 47:375–84. [DOI] [PubMed] [Google Scholar]
- 15. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–84. [DOI] [PubMed] [Google Scholar]
- 16. Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 17. Nalla AK, Casto AM, Huang MW, et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J Clin Microbiol 2020; 58:e00557–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bedford T, Greninger AL, Roychoudhury P, et al. Cryptic transmission of SARS-CoV-2 in Washington State. medRxiv Published April 6, 2020. Available at: https://www.medrxiv.org/content/10.1101/2020.04.02.20051417v1. Accessed 10 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Center for Disease Control and Prevention. People at high risk for flu complications. Updated August 27, 2018. Available at: https://www.cdc.gov/flu/highrisk/index.htm. Accessed 27 March 2020. [Google Scholar]
- 21. Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One 2010; 5:e9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011; 52:301–12. [DOI] [PubMed] [Google Scholar]
- 23. Alanazi KH, Abedi GR, Midgley CM, et al. Diabetes mellitus, hypertension, and death among 32 patients with MERS-CoV infection, Saudi Arabia. Emerg Infect Dis 2020; 26:166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with COVID-19. N Engl J Med 2020; Published online March 30, 2020. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagiwara S, Iwasaka H, Hidaka S, Hasegawa A, Koga H, Noguchi T. Antagonist of the type-1 ANG II receptor prevents against LPS-induced septic shock in rats. Intensive Care Med 2009; 35:1471–8. [DOI] [PubMed] [Google Scholar]
- 27. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020; 395:683–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; Published online March 9, 2020. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ko WC, Rolain JM, Lee NY, et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents 2020; Published online March 6, 2020. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]