Since the initial outbreak of the novel coronavirus disease [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/coronavirus disease 2019 (COVID-19)] in December 2019 (Wuhan, China),1 there have been 2 667 532 confirmed cases and 186 252 deaths worldwide as of 23 April 2020. Given the rapid spread of this virus with consequences on a global scale, COVID-19 was declared a pandemic by the World Health Organization on 11 March 2020. A worse prognosis and a more severe progression of COVID‐19 have been associated with cardiovascular risk factors, previous cardiac diseases and myocardial injury.2 SARS-CoV-2 is an enveloped virus with a single-stranded, positive-sense RNA genome possibly originating in bats (its genome is 96.2% identical to that of a bat coronavirus).2 SARS-CoV-2 uses the angiotensin-converting enzyme 2 (ACE2) membrane-bound protein for cell entry; ACE2 is highly expressed in lung alveolar cells, providing the main site of entry for the virus into human hosts.3 After ligand binding, SARS-CoV-2 enters cells via receptor-mediated endocytosis. Of note, ACE2 also serves a role in protection of the lung and therefore viral binding to this receptor disrupts this anti-inflammatory pathway, contributing to viral pathogenicity.3 ACE2 is also expressed in the human heart, mainly in cardiomyocytes and in pericytes, but also in endothelial cells and more in heart failure patients than in controls.4 Notably, COVID-19 patients exhibit a cytokine storm related to a hyperactivation of the immune system also promoting thrombogenicity.5 It is well recognized that the inflammatory outburst associated with infectious diseases, particularly intense in the case of COVID-19, contributes to both atherosclerotic plaque progression and plaque destabilization, leading to thrombus formation and acute coronary syndromes (ACS).6
Thus, theoretical considerations might predict an increase in the incidence of myocardial infarction (MI) during the COVID pandemic. First, acute respiratory syndromes are associated with type 1 MI due to plaque rupture related to inflammatory activation and expression of collagenase-digesting enzymes.6 Secondly, COVID-19 infection appears to increase blood thrombogenicity with higher risk of both venous and arterial thrombosis.7 Thirdly, social isolation inevitably leads to decreased daily activity, sedentary lifestyles, and dietary changes, all increasing the atherothrombotic risk, at least in the long run.8 Moreover, the COVID-19 pandemic has produced damaging economic effects and affected accessibility to drugs, including antithrombotic drugs, possibly affecting patients’ treatment.8 Finally, in COVID-19 patients, drug–drug interaction of prescribed antiviral drugs with other cardiac medications may affect the efficacy of such therapies with particular regard to antiplatelet, anticoagulant, and lipid-lowering drugs, thus increasing the risk of ACS.8
Surprisingly, and in contrast to these theoretical expectations, recent data suggest that the admission rate for ACS during the COVID-19 pandemic is much lower than expected rather than higher. Indeed, in Austria, hospital admissions for ACS decreased by 39% in the last calendar week in March 2020 as compared with the first week, mainly affecting patients with non-ST-segment elevation myocardial infarction (NSTEMI).9 Similarly, in Italy, a survey of the Italian Society of Cardiology (SIC) comparing a 1-week period during the COVID-19 outbreak vs. the equivalent week in 2019 showed a 48% reduction in admissions for acute MI (26% for STEMI and 65% for NSTEMI).10
These findings lead to an important question: why is the admission rate for ACS much lower than expected rather than higher, as predicted by theoretical considerations?
Undoubtedly, the psychological impact of the pandemic on patients is important. Thus, it is possible to speculate that the fear of contagion in hospitals has discouraged access to emergency medical services (EMS). This is supported by the fact that admissions for other acute cardiac conditions (heart failure and atrial fibrillation) seem to be similarly reduced during the pandemic. Additionally, during the pandemic, EMS and the healthcare systems, in general, are overwhelmed by COVID-19 patients and therefore less available for other patients. Taken together, these observations might have promoted deferral of less urgent cases, at both the patient and healthcare system levels.
On the other hand, we cannot exclude a true reduction of MI rate due to a paradoxical beneficial effect of social containment, which leads to a more relaxed lifestyle (‘Life on standstill’). Indeed, shear stress associated with sudden exercise, hectic daily activities, and even sports activities, among others, are known triggers for MI.11 In line with this interpretation is the fact that, although physical exercise is protective in the long run, it acutely increases the risk of ACS and sudden death.11 As such, a sedentary lifestyle may be acutely protective in those carrying vulnerable plaques. In contrast, extremely stressful acute events such as earthquakes are followed by an increased incidence of MI.12
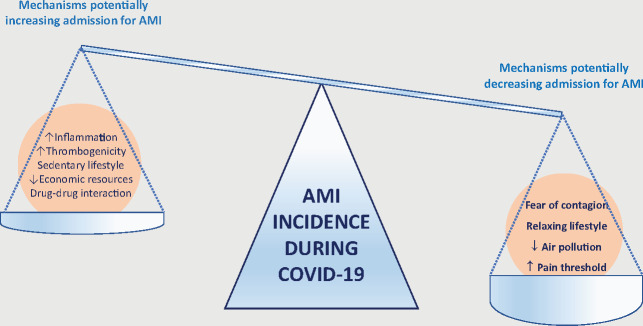
Figure 1.
Mechanism potentially increasing or decreasing admission to hospitals during the COVID-19 pandemic. Recent data suggest that admissions for acute myocardial infrarction (AMI) are reduced during the COVID-19 pandemic. The weight of mechanisms potentially decreasing admissions for AMI seem to overcome those increasing admissions for AMI.
Furthermore, patients with COVID-19 infections might have an increased pain threshold leading to a higher prevalence of silent or near silent myocardial infarctions. Of interest, loss of smell and taste is a typical symptom of COVID-19 and may reflect nerve involvement of the viral infection. Indeed, neurological complications have been reported in COVID-19 patients.13
Another important protective factor is the spectacular reduction of air pollution which we are enjoying during this period of social containment. Recent data show that major cities that suffer from the world’s worst air pollution have seen reductions of deadly particulate matter by up to 60% from the previous year, during a 3-week lockdown period. It is well recognized that one of the triggering factors of STEMI is the presence of air pollutants including sulfur dioxide, nitric dioxide, carbon monoxide, ozone, and particulate matter (PM) including PM under 2.5 μm (PM2.5) and PM under 10 μm (PM10).14 Air pollution can trigger STEMI with various mechanisms such as increasing inflammatory factors and changing the heart rate or blood viscosity.14 Furthermore, recent evidence strongly suggests a link between air pollution levels and the lethality of SARS-CoV-2.15
Of course, both the fear of contagion discouraging ACS patients to contact the EMS and a true reduction of ACS incidence can contribute to the global reduction of patients’ admissions during the COVID-19 pandemic. Epidemiological studies documenting mortality at home and in hospital during the COVID-19 epidemic compared with previous years during the same months will probably clarify in the future which of these two mechanisms is more relevant. Furthermore, a documentation of the symptom onset to arrival time of ACS patients and infarct size during the epidemic and control periods would be of interest. Indeed, a rebound of patient admissions with heart failure after the pandemic would probably suggest an undertreatment of ACS during the period of social containment due to late presentations and larger infarcts, while the lack of a rebound might suggest a true reduction of ACS incidence.
If the hypothesis of a true reduction of ACS during the COVID pandemic were to be confirmed by future epidemiological studies, it might become convenient to take advantage of the ‘COVID-19 model’ after the pandemic: a less frenetic life and less air pollution might become the best form of prevention of coronary artery disease.
Conflict of interest: none declared.
Authors

Biography: Dr Giampaolo Niccoli graduated in Medicine at the Sacred Heart Catholic University in 1997 and obtained his specialization in Cardiology under the supervision of Professor Maseri at the same university in 2001. He subsequently obtained a PhD in cellular and molecular cardiology at the Cardiology Institute directed by Professor Crea at the same university in 2005. During his PhD, he was in England at the John Radcliffe Hospital in Oxford where he did a fellowship in interventional cardiology under the supervision of Dr Banning. He has been assistant professor at the Cardiology Institute of the Sacred Heart Catholic University since December 2006.

Biography: Professor Lüscher studied medicine at the University of Zurich and obtained the board certification in internal medicine and cardiology. He trained in cardiovascular research and in cardiology, and specifically in echocardiography, at the Mayo Clinic in Rochester, MN, USA, and was later Professor of Pharmacotherapy at the University of Basel, then Professor of Cardiology at the University of Berne, before assuming a position as Professor and Chairman of Cardiology and Director of the University Heart Center at the University Hospital Zurich and Director of the Center for Molecular Cardiology at the University of Zurich, Switzerland. He is now Director of Research, Education & Development and Consulting Cardiologist at the Royal Brompton & Harefield Hospital Trust, and Professor of Cardiology at the National Heart and Lung Institute, Imperial College in London.

Biography: Professor Crea trained in Pisa Medical School in Cardiology and in Pulmonary Diseases. He has been Senior Lecturer in Cardiology at RPMS-Hammersmith Hospital in London. Since 2008, he is Professor of Cardiology, Director of the Department of Cardiovascular Sciences, Director of the Postgraduate School in Cardiology, and Coordinator of the PhD program in Cellular and Molecular Cardiology at the Catholic University in Rome.
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W.. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA.. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci 2020;doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray E, Tomaszewski M, Guzik TJ.. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: clinical implications. Cardiovasc Res 2020;doi: 10.1093/cvr/cvaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicin L, Tyler Abplanab WT, Tombor L.. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 2020;41:1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJHLH Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crea F, Libby P.. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 2017;136:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH.. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;doi: 10.1016/j.jacc.2020.04.031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Dreyfus I, Driggin E, Der Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH.. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 2020;75:2352–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ.. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J 2020;41:1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Rosa S, Spaccarotella C, Basso C, Calabro’ MP, Curcio A, Perrone Filardi P, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R, Sinagra G, Indolfi C, on behalf of SIC, Società Italiana di Cardiologia. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;doi.org/10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maron BJ. The paradox of exercise. N Engl J Med 2000;343:1409–1411. [DOI] [PubMed] [Google Scholar]
- 12. Kloner RA. Lessons learned about stress and the heart after major earthquakes. Am Heart J 2019;215:20–26. [DOI] [PubMed] [Google Scholar]
- 13. Li YC, Bai WZ, Hashikawa T.. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol 2020;doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lelieveld J, Klingmüller K, Pozzer A, Pöschl U, Fnais M, Daiber A, Münzel T.. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 2019;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Xie J, Huang F, Cao L.. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci Total Environ 2020;727:138704. [DOI] [PMC free article] [PubMed] [Google Scholar]