Abstract
Background
Coronavirus disease 2019 (COVID-19)-associated acute kidney injury (AKI) frequency, severity and characterization in critically ill patients has not been reported.
Methods
Single-centre cohort performed from 3 March 2020 to 14 April 2020 in four intensive care units in Bordeaux University Hospital, France. All patients with COVID-19 and pulmonary severity criteria were included. AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. A systematic urinary analysis was performed. The incidence, severity, clinical presentation, biological characterization (transient versus persistent AKI; proteinuria, haematuria and glycosuria) and short-term outcomes were evaluated.
Results
Seventy-one patients were included, with basal serum creatinine (SCr) of 69 ± 21 µmol/L. At admission, AKI was present in 8/71 (11%) patients. Median [interquartile range (IQR)] follow-up was 17 (12–23) days. AKI developed in a total of 57/71 (80%) patients, with 35% Stage 1, 35% Stage 2 and 30% Stage 3 AKI; 10/57 (18%) required renal replacement therapy (RRT). Transient AKI was present in only 4/55 (7%) patients and persistent AKI was observed in 51/55 (93%). Patients with persistent AKI developed a median (IQR) urine protein/creatinine of 82 (54–140) (mg/mmol) with an albuminuria/proteinuria ratio of 0.23 ± 20, indicating predominant tubulointerstitial injury. Only two (4%) patients had glycosuria. At Day 7 after onset of AKI, six (11%) patients remained dependent on RRT, nine (16%) had SCr >200 µmol/L and four (7%) had died. Day 7 and Day 14 renal recovery occurred in 28% and 52%, respectively.
Conclusion
Severe COVID-19-associated AKI is frequent, persistent, severe and characterized by an almost exclusive tubulointerstitial injury without glycosuria.
Keywords: acute interstitial nephritis, acute kidney injury, acute tubular injury, COVID-19, critically ill patients, renal replacement therapy
INTRODUCTION
Since December 2019, coronavirus disease 2019 (COVID-19) has infected nearly 5 million people worldwide, leading to more than 300 000 deaths. Starting in China, it rapidly expanded to Europe, affecting first Italy, then Spain and France. Globally, it has resulted in the confinement of nearly 3 billion people [1, 2]. The severe respiratory damages requiring prolonged intubation have already been described, which are the determinant of intensive care unit (ICU) admission and have resulted in a consecutive risk of ICU saturation [3–6].
Acute kidney injury (AKI) is the second most common organ damage in COVID-19 patients previously reported in China. It is observed in up to 15% of patients, whatever their need for ICU admission [4]. However, very few data are available in patients admitted to ICUs, where this incidence should be significantly higher. According to the first US report of ICU patients (n = 24) [5], it is observed in 20% of subjects; this does not reflect the first Western European findings, where >50% of patients could experience AKI (unpublished data). The reason for this is not clearly established. AKI severity and potential recovery have not been reported. The need for renal replacement therapy (RRT) may considerably prolong the length of ICU hospitalization, leading to significant organizational and economic challenges for health-care systems. In addition, a high frequency of AKI could result in a very significant increase in the number of patients suffering from long-term chronic kidney disease (CKD) [7].
The hypothesis was that COVID-19-associated AKI is more severe and common than previously reported, and may be represented by different clinical presentations including transient AKI, acute tubular necrosis (ATN) or other types of injury. Each of these clinical presentations potentially requires a specific approach. The objective of the study was to describe the incidence and severity and to characterize COVID-19-associated AKI.
MATERIALS AND METHODS
Study design
This cohort study was carried out in the four ICUs dedicated to severe COVID-19 patients at the University Hospital of Bordeaux, France from 3 March 2020 to 14 April 2020. Patients data were routinely collected in dedicated electronic health records during their entire hospital stay. According to French law and the French Data Protection Authority, the handling of these data for research purposes was declared to the Data Protection Officer of the University Hospital of Bordeaux. The study obtained the approval of the Institutional Review Board of the University Hospital of Bordeaux (declaration number 2020-14). Patients (or their relatives, if any) were notified about the anonymized use of their health care data via the department’s booklet.
Participants
Patients were enrolled if they were aged ≥18 years, had a positive polymerase chain reaction for COVID-19 or typical computed tomography findings in patients with a high clinical pre-test probability of COVID-19 [8], and had severity criteria for admission to one of the four ICUs. These severity criteria were defined by the need for ≥4 L/min oxygen therapy (O2) to obtain an arterial oxygen saturation (SaO2) ≥94% or arterial partial pressure of oxygen/fraction of inspired oxygen ratio (PaO2/FiO2) ≤300 mmHg in patients receiving high-flow nasal cannula oxygen. The University Hospital of Bordeaux was confronted with a far less aggressive epidemic context than those reported in other French regions or other countries. Therefore, all patients presenting with this severity were systematically admitted to ICU except those who had withdrawal orders. No specific withdrawal policy was applied during this epidemic period. ICU patients were excluded from this study only if they had pre-existing end-stage kidney disease defined by the need for RRT.
Patient management
Patients were treated according to the standard of care. In three units, hydroxychloroquine (HCL) was administered because of physicians’ personal decision according to physiopathological studies suggesting an in vitro efficacy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The dosage schedule was per os 400 mg twice a day. at Day 1 and then 200 mg twice a day for 5–10 days. One unit added tocilizumab to HCL with a single 8 mg/kg infusion. Two units administered lopinavir/ritonavir (400 mg twice a day in syrup form) at first and then stopped because of drug shortage. All treatments were started on the day of admission to ICU. Importantly, no other modification in COVID-19 critically ill patients’ care occurred, and treatment administration was dependent on the unit and not on patients’ characteristics. All patients had Foley catheterization.
AKI classification
The Kidney Disease: Improving Global Outcomes (KDIGO) definition was used to define AKI Stages 1, 2 and 3 [9]. Serum creatinine (SCr) and urine output were both taken into account. Stage 1: SCr 1.5–1.9 times baseline or diuresis <0.5 mL/kg/h for 6–12 h; Stage 2: SCr 2.0–2.9 times baseline or diuresis <0.5 mL/kg/h for ≥12 h; Stage 3: SCr ≥3 times baseline, initiation of RRT, anuria ≥12 h or diuresis <0.3 mL/kg/h for ≥24 h. AKI stage was classified using the worst SCr or diuresis during the entire ICU stay.
Baseline SCr values corresponded to SCr values at admission in the case of normal renal function or SCr values from within 6 months in the case of abnormal SCr at admission. All SCr baseline values could be collected from previous medical records for patients with abnormal SCr at ICU admission.
Acute kidney disease (AKD) staging was performed using the consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup [9] and was defined as a condition wherein criteria for AKI Stage 1 or greater persists ≥7 days after an exposure. AKD Stage 1: SCr 1.5–1.9 times baseline; Stage 2: SCr 2.0–2.9 times baseline, Stage 3: SCr ≥3 times baseline or ongoing need for RRT.
Exposure variables
Information relative to drugs taken before admission, date and type of the first COVID-19 symptoms, and a medical history of hypertension, diabetes, chronic heart failure (chronic ischaemic, rhythmic, valvular heart disease or heart failure with preserved ejection fraction or reduced ejection fraction), stroke, chronic obstructive pulmonary disease, asthma and peripheral arterial disease was collected using prospectively recorded data, and patient or relative questioning. Pre-admission exposure to non-steroidal anti-inflammatory drugs and renin–angiotensin system inhibitors was considered if the patient had taken the drug the week prior to admission. Weight and height were measured at ICU admission. All other healthcare data were collected using Metavision ICU (iMDsoft).
The four ICUs of the University Hospital of Bordeaux received patients (n = 26) transferred from the ICUs of the Metz Haguenau, Aulnay Sous Bois, Corbeil Essonnes, Mulhouse or Strasbourg Hospitals in France. They were included whenever they met the inclusion criteria of the ICU admission cohort. Health-care data were collected using medical records from the previous hospital.
Patients chronically exposed to immunosuppressive drugs or suffering from haematologic malignancies were considered immunosuppressed.
CKD was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2, using the Chronic Kidney Disease Epidemiology Collaboration corresponding to CKD Stage 3 or more according to the KDIGO classification [9].
Fluid balance was the addition of fluid infusion volume, including enteral or parenteral nutrition minus diuresis and gastrointestinal losses.
Minimum diuresis was defined by the minimum diuresis/kg/h used for the KDIGO classification.
All blood or urine biological assessments were usually standardized in ICUs participating in the study and systematically collected. All biochemical parameters were determined on Abbot Architect analysers with enzymatic method for creatinine and colorimetric bromocresol purple method for albumin. SCr was measured at least once a day. A urinary analysis was performed at ICU admission or whenever AKI occurred or deteriorated during the ICU stay. Fractional excretion of sodium (FeNa+) and fractional excretion of urea (FeUrea) were calculated as follows: FeNa+ (%) = ([U/P sodium]/[U/P creatinine]) × 100; FeUrea (%) = ([U/P urea]/[U/P creatinine]) × 100. U/P is urinary/plasma ratio, and urinary osmolarity was measured.
The AKI day was the first day during which the patient was eligible for AKI using KDIGO classification.
Outcomes definitions
Since 2017, RRT initiation criteria have been standardized in the ICUs participating in the study, according to the delayed strategy of the Artificial Kidney Initiation in Kidney Injury (AKIKI) trial [10]. Patients were eligible for RRT whenever AKI Stage 3 occurred with clinical indications (anuria ≥72 h, [K+] >6 mmol/L or >5.5 mmol/L after medical correction, acute pulmonary oedema, pH <7.15 in the absence of other causes, or blood urea >40 mmol/L).
The ICU length of stay was only calculated for patients who were discharged from the ICU; most of them were transferred to medical wards or a continuing care unit.
The intubation length was only calculated for the subgroup of patients who were successfully extubated. If a patient ever needed to be re-intubated after having been weaned from the ventilator, the intubation length was calculated as the sum of the different periods during which invasive mechanical ventilation was required.
Transient AKI was defined by a ≥50% decrease in SCr or a return of SCr to its baseline value 72 h after AKI [11].
Recovery from AKI was defined as a return of SCr to ≤125% above baseline SCr for alive and non-dependent RRT patients during the ICU stay [12].
Major adverse kidney events (MAKEs) was a composite outcome defined by an SCr >200% of baseline, need for RRT or death [13].
Statistical analysis
Statistical analysis was carried out using JMP® Version 14 (SAS Institute Inc., Cary, NC, USA, 1989–2007). Descriptive statistics included mean ± standard deviation (SD) or median [interquartile range (IQR)] if the variable did not fit a normal distribution. Quantitative variables were compared using a t-test, and qualitative variables were compared using Fisher’s exact test when only two variables were studied; Pearson’s Chi-squared test was used for more variables. AKI incidence during ICU hospitalization and renal recovery during hospitalization was plotted using a Kaplan–Meier curve with a 95% confidence interval, and was censored with death. A value of double-sided P < 0.05 was considered statistically significant.
RESULTS
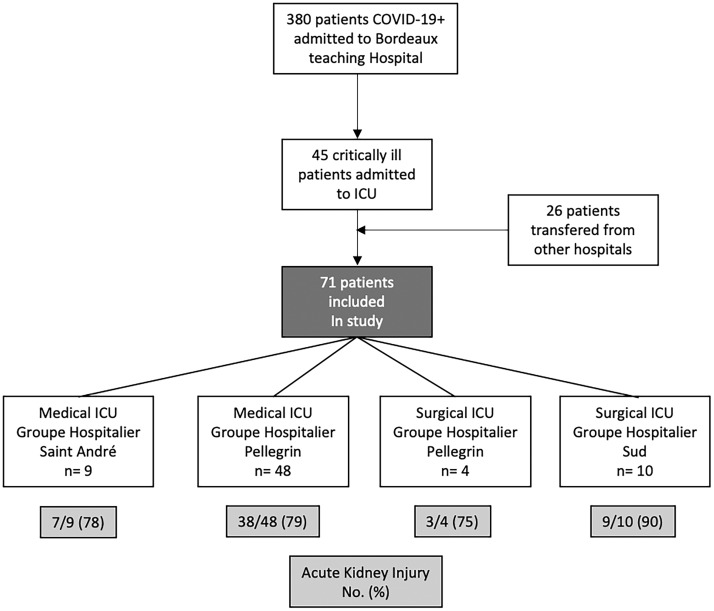
From 3 March 2020, 380 patients were admitted in the University Hospital of Bordeaux for COVID-19. Among them, 45/380 (12%) patients with severe COVID-19 were admitted to the ICU in addition to 26 critically ill patients admitted to ICU from other regions of France, for a total of 71 patients included (Figure 1). The reason for admission was acute respiratory failure for 70/71 (99%), with a majority of males—55/71 (77%)—and a mean age of 61 ± 12 years. Time from the onset of first viral symptoms to ICU admission was 8 (6–10) days. Details on all baseline characteristics, including blood test results at admission, are presented in Table 1.
FIGURE 1.
Flow diagram. Patients admitted from 3 March 2020 to 14 April 2020 were taken into consideration.
Table 1.
Baseline characteristics of patients at ICU admission
| Characteristics of Patients | n = 71 |
|---|---|
| Male, n (%) | 55/71 (77) |
| Age, mean ± SD | 61.2 ± 12.2 |
| BMI, mean ± SD | 31.0 ± 5.8 |
| Hypertension, n (%) | 43/71 (61) |
| Diabetes, n (%) | 21/71 (30) |
| Heart disease, n (%) | 17/71 (24) |
| OSA, n (%) | 9/71 (13) |
| COPD, n (%) | 8/71 (11) |
| Immunosuppression, n (%) | 7/71 (10) |
| Stroke, n (%) | 4/71 (6) |
| Asthma, n (%) | 3/71 (4) |
| PAD, n (%) | 0/71 (0) |
| NSAID exposure, n (%) | 7/71 (10) |
| RASi exposure, n (%) | 23/71 (32) |
| ACEi exposure, n (%) | 15/71 (21) |
| ARB exposure, n (%) | 8/71 (11) |
| Positive PCR COVID-19, n (%) | 68/71 (96) |
| COVID-19 typical CT scan feature, n (%) | 57/71 (80) |
| Onset of first symptoms to ICU admission (day), median (IQR) | 8 (6–10) |
| Temperature at ICU admission (°C), mean ± SD | 38.02 ± 1.25 |
| Admission for respiratory failure, n (%) | 70/71 (99) |
| PaO2/FiO2, median (IQR) | 156 (100–233) |
| MAP (mmHg), mean ± SD | 94 ± 19 |
| SAPS II, mean ± SD | 41.2 ± 17.1 |
| SOFA score, mean ± SD | 6.4 ± 3.9 |
| Respiratory | 2.7 ± 1.0 |
| Coagulation | 0.2 ± 0.5 |
| Liver | 0.1 ± 0.5 |
| Cardiovascular | 1.4 ± 1.5 |
| Neurologic | 1.4 ± 1.8 |
| Renal | 0.6 ± 1.0 |
| Na+ (mmol/L), mean ± SD | 139.8 ± 4.6 |
| K+ (mmol/L), mean ± SD | 4.07 ± 0.52 |
| Bicarbonate (mmol/L), mean ± SD | 24.7 ± 3.8 |
| D-dimers (ng/mL), mean ± SD | 6284 ± 13000 |
| Lymphocytes count (/mm3), mean ± SD | 0.91 ± 0.55 |
| Ferritin (ng/ml), mean ± SD | 1914 ± 1751 |
| Albumin (g/L), mean ± SD | 20.7 ± 5.2 |
| Total protein (g/L), mean ± SD | 65.6 ± 7.3 |
| Renal baseline | |
| CKD, n (%) | 4/71 (6) |
| Basal SCr (µmol/L)a, mean ± SD | 68.8 ± 20.9 |
| SCr at admission (µmol/L), mean ± SD | 115.6 ± 107.7 |
| BUN (mmol/L),vmean ± SD | 10.1 ± 7.4 |
| AKI, n (%) | 8 (11) |
| Defined using SCr criteria | 6 (75) |
| Defined using diuresis criteria | 2 (25) |
Basal SCr was SCr at admission in the case of normal renal function or SCr within 6 months in the case of abnormal SCr at admission.
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FiO2: fraction of inspired oxygen; K+, potassium; MAP, mean arterial pressure; Na+, sodium; NSAID, non-steroidal anti-inflammatory drug; OSA, obstructive sleep apnoea; PAD, peripheric arterial disease; PaO2, arterial partial pressure of oxygen; PCR, polymerase chain reaction; RASi, renin–angiotensin system inhibitor; SAPS, simplified acute physiology score.
Median follow-up was 17 (12–23) days, and length of ICU stay was 10 (7–14) days. Intubation was required for 55/71 (77%) patients for a median duration of 12 (9–17) days. The worst PaO2/FiO2 was 95 (79–125). Septic shock was rare since norepinephrine infusion rate [maximum 0.16 (0.10–0.37) µg/kg/min] was low and no lactate level exceeded 2 mmol/L except in two patients [14]. Four patients died (11%). Table 2 shows the main follow-up outcomes.
Table 2.
Main follow-up outcomes
| Outcomes | n = 71 |
|---|---|
| Follow-up (days), median (IQR) | 17 (12–23) |
| ICU length of stay (n = 38)a, median (IQR) | 10 (6.7–14.2) |
| Intubation, n (%) | 55/71 (77) |
| Length of intubation (n = 29)b, median (IQR) | 12 (9–17) |
| Worst PaO2/FiO2, median (IQR) | 95 (79–125) |
| Need for NE infusion, n (%) | 52/71 (73) |
| Maximal dose of NE infusion (µg/kg/min), median (IQR) | 0.16 (0.10–0.37) |
| Day of NE maximal dose from ICU admission (days), median (IQR) | 4 (1–12) |
| Maximal lactate level (mmol/L), mean ± SD | 1.5 ± 0.7 |
| ICU deaths, n (%) | 4/71 (6) |
| Onset of death from ICU admission (days), median (IQR) | 9 (5–19) |
The ICU length of stay was only calculated for patients who were discharged from the ICU, n = 38.
The length of intubation was only calculated for patients who were successfully weaned from the ventilator, n = 29.
FiO2, fraction of inspired oxygen; ICU, intensive care unit; NE, norepinephrine; PaO2, arterial partial pressure of oxygen.
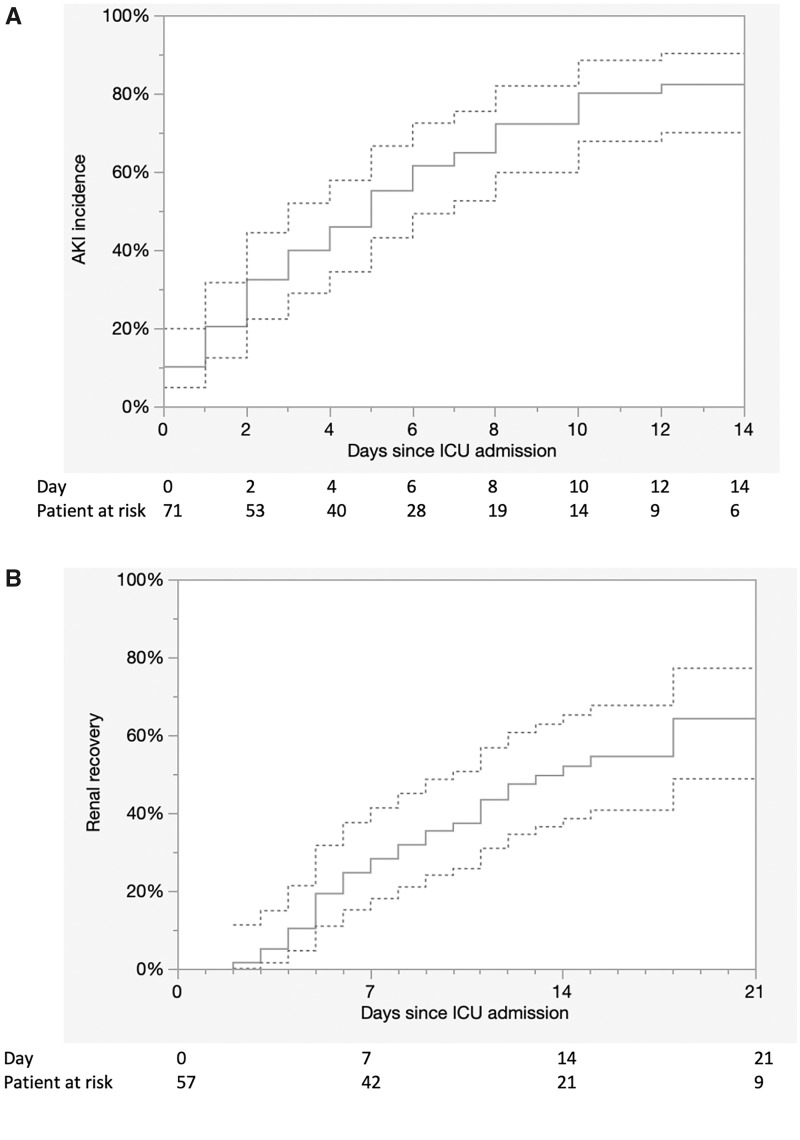
AKI
At admission, AKI was present in 8/71 (11%) patients. During their ICU stay, AKI developed in 49 other patients, reaching a total of 57/71 (80%) patients. The timing of AKI development is shown in Figure 2A. Stage 1 AKI was observed in 20/57 (35%) patients, Stage 2 in 20/57 (35%) and Stage 3 in 17/57 (30%); 10/57 (18%) required RRT. Patients with AKI had a higher body mass index (32 ± 6 versus 28 ± 5 kg/m2; P = 0.04); higher total sequential organ failure assessment (SOFA) (7 ± 3.8 versus 3.9 ± 3.3; P = 0.006), renal SOFA (0.7 ± 1.1 versus 0 ± 0; P < 0.001) and cardiovascular SOFA (1.6 ± 1.5 versus 0.4 ± 0.9, P = 0.004) scores; and lower PaO2/FiO2 ratios [93 (76–120) versus 109 (90–227); P = 0.04]. They had similar maximal norepinephrine infusion [0.16 (0.10–0.39) µg/kg/min versus 0.23 (0.12–0.30) µg/kg/min; P = 0.76], maximal lactate level (1.4 ± 0.7 mmol/L versus 1.5 ± 0.3 mmol/L; P = 0.76), contrast agent infusion [12/57 (21%) versus 3/14 (21%); P = 0.98] and crystalloid infusion during the first 24 h after admission (3074 ± 1577 mL versus 2761 ± 905 mL; P = 0.37). Comparisons between patients with or without AKI are presented in Table 3.
FIGURE 2.
(A) Incidence of AKI during the ICU stay censored with death with 95% confidence interval. (B) Renal recovery during hospitalization censored with death with 95% confidence interval.
Table 3.
Comparison of patients with and without AKI
| Characteristics of Patients | AKI (n = 57) | non-AKI (n = 14) | P-value |
|---|---|---|---|
| Male, n (%) | 46 (81) | 9 (64) | 0.28 |
| Age (years), mean ± SD | 61.7 ± 11.4 | 59.1 ± 15.0 | 0.54 |
| BMI, mean ± SD | 31.7 ± 5.9 | 28.4 ± 4.9 | 0.04 |
| Basal SCr (µmol/L), mean ± SD | 70.4 ± 21.4 | 62.4 ± 17.6 | 0.16 |
| MAP (mmHg), mean ± SD | 92.8 ± 19.9 | 98.6 ± 11.6 | 0.17 |
| Maximal SCr (µmol/L), mean ± SD | 214.1 ± 183.0 | 76.8 ± 19.3 | <0.001 |
| Minimum diuresis (mL/kg/h), mean ± SD | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.03 |
| PaO2/FiO2 at admission, median (IQR) | 150 (94–222) | 220 (172–249) | 0.07 |
| SAPSII, mean ± SD | 42.8 ± 16.5 | 34.4 ± 18.3 | 0.13 |
| SOFA score, mean ± SD | 7 ± 3.8 | 3.9 ±3.2 | 0.006 |
| Respiratory | 2.8 ± 0.9 | 2.3 ± 0.9 | 0.06 |
| Coagulation | 0.2 ± 0.5 | 0.2 ± 0.6 | 0.90 |
| Liver | 0.1 ± 0.5 | 0.1 ± 0.5 | 0.99 |
| Cardiovascular | 1.6 ± 1.5 | 0.4 ± 0.8 | 0.004 |
| Neurologic | 1.5 ± 1.8 | 0.8 ± 1.7 | 0.22 |
| Renal | 0.7 ± 1.1 | 0 ± 00 | <0.001 |
| Specific therapy, n (%) | |||
| Hydroxychloroquine | 31/57 (54) | 9/14 (64) | 0.56 |
| Lopinavir–Ritonavir | 21/57 (37) | 4/14 (29) | 0.76 |
| Tocilizumab | 5/57 (9) | 0/14 (0) | 0.57 |
| Crystalloid infusion during the first 24 h (mL), mean ± SD | 3074 ± 1577 | 2761 ± 905 | 0.37 |
| Contrast agent, n (%) | 12/57 (21) | 3/57 (21) | 0.98 |
| Worst PaO2/FiO2, median (IQR) | 93 (76–120) | 108 (90–227) | 0.04 |
| Maximal NE infusion (µg/kg/min), median (IQR) | 0.16 (0.10–0.39) | 0.23 (0.12–0.30) | 0.76 |
| Lactate level at maximal NE infusion (mmol/L), mean ± SD | 1.5 ± 0.7 | 1.5 ± 0.3 | 0.76 |
| C-reactive protein (mg/L) at admission, mean ± SD | 164 ± 111 | 125 ± 72 | 0.12 |
| Ferritin (ng/mL) at admission, mean ± SD | 1873 ± 1778 | 2103 ± 1686 | 0.68 |
| Lymphocytes at admission (/mm3), mean ± SD | 939 ± 590 | 782 ± 342 | 0.20 |
| AKI Stage 1, n (%) | 20/57 (35) | ||
| AKI Stage 2, n (%) | 20/57 (35) | ||
| AKI Stage 3, n (%) | 17/57 (30) | ||
| AKD Stage 1, n (%) | 2/57 (4) | ||
| AKD Stage 2, n (%) | 7/57 (12) | ||
| AKD Stage 3, n (%) | 9/57 (16) | ||
| Total AKD | 18/57 (32) | ||
| MAKEs, n (%) | 19 (33) | 0 | |
| SCr >200 µmol/L | 9/19 (47) | ||
| Dialysis at Day 7 | 6/19 (32) | ||
| Death | 4/19 (21) | ||
BMI, body mass index; FiO2, fraction of inspired oxygen; MAP, mean arterial pressure; NE, norepinephrine; PaO2, arterial partial pressure of oxygen; SAPS, simplified acute physiology score.
Characterization of AKI
Because two deaths occurred in the first 72 h after AKI, only 55 patients could be assessed to differentiate transient from persistent AKI. Transient AKI was present in only 4/55 (7%) patients, and persistent AKI was present in 51/55 (93%). Compared with patients with persistent AKI, patients with transient AKI had similar basal SCr (69 ± 21 µmol/L versus 76 ± 23 µmom/L; P = 0.62), natriuresis (53 ± 40 mmol/L versus 81 ± 75 mmol/L; P = 0.52), urinary osmolarity (515 ± 182 mmol/L versus 637 ± 330 mmol/L; P = 0.52), FeNa+ (0.6 ± 0.9% versus 0.6 ± 0.7%; P = 0.96), FeUrea (33 ± 12% versus 39 ± 11%; P = 0.35) and fluid balance at 72 h after AKI diagnosis (12805 ± 6378 mL versus 8070 ± 3732 mL; P = 0.29), respectively (Table 4).
Table 4.
Comparison between patients with transient versus persistent AKI
| Characteristics of Patients | Transient AKI (n = 4) | Persistent AKI (n = 51) | P-value |
|---|---|---|---|
| SCr (µmol/L) at admission, mean ± SD) | 87 ± 27 | 116 ± 106 | 0.16 |
| SCr (µmol/L) at AKI, mean ± SD) | 81 ± 36 | 147 ± 109 | 0.02 |
| SCr (µmol/L) 48h after AKI, mean ± SD) | 69 ± 29 | 196 ± 226 | <0.001 |
|
|
|
0.14 |
| AKI Stage 1, n (%) | 1/4 (25) | 20/51 (39) | 0.18 |
| AKI Stage 2, n (%) | 3/4 (75) | 16/51 (31) | |
| AKI Stage 3, n (%) | 0 | 15/51 (30) | |
| Fluid balance 48 h after AKI (mL), mean ± SD | 6146 ± 2519 | 8767 ± 4429 | 0.36 |
| Fluid balance 72 h after AKI (mL), mean ± SD | 8070 ± 3732 | 12 805 ± 6378 | 0.29 |
| Minimum diuresis (mL/kg/h), mean ± SD | 0.6 ± 0.2 | 0.5 ± 0.3 | 0.67 |
| Urinary Na+ (mmol/L), mean ± SD | 81 ± 75 | 53 ± 40 | 0.52 |
| Urinary K+ (mmol/L), mean ± SD | 34 ± 22 | 44 ± 21 | 0.46 |
| Urinary urea (mmol/L), mean ± SD | 291 ± 80 | 327 ± 164 | 0.69 |
| Urinary osmolarity (mmol/L), mean ± SD | 637 ± 330 | 515 ± 182 | 0.52 |
| FeNa+ (%), mean ± SD | 0.6 ± 0.7 | 0.6 ± 0.9 | 0.96 |
| FeUrea (%), mean ± SD | 39 ± 11 | 33 ± 12 | 0.35 |
| UNa+/UK+ ratio, mean ± SD | 2.3 ± 1.6 | 1.7 ± 2 | 0.54 |
| Proteinuria/creatininuria (mg/mmol), median (IQR) | 124 (38–203) | 82 (54–140) | 0.62 |
| Albuminuria/creatininuria (mg/mmol), median (IQR) | 6 (6–8) | 9 (5–32) | 0.31 |
| Albuminuria/proteinuria (%), mean ± SD | 8 ± 6 | 23 ± 20 | 0.03 |
| Haematuria, n (%) | 3/4 (75) | 35/51 (69) | 1 |
| Leucocyturia, n (%) | 1/4 (25) | 24/51 (47) | 0.62 |
| Glycosuria, n (%) | 0 (0) | 2/51 (4) | 1 |
| RRT, n (%) | NR | 10/51 (20) | NA |
| Date of RRT initiation, median (IQR) | NR | 8 (4–10) | NA |
| Indication of RRT initiation, n (%) | NR | N = 10 | NA |
| Anuria ≥72h | 7/10 (70) | ||
| [K+] >6 mmol/L | 1/10 (10) | ||
| pH <7.15 | 2/10 (20) | ||
| Acute pulmonary oedema | 0 (0) | ||
| Death, n (%) | 0 (0) | 2/51 (4) | 1 |
Fe, fractional excretion; K+, potassium; Na+, sodium; NA, not available; NR, not relevant; UNa+/K+ ratio, urinary sodium/potassium ratio.
Patients with persistent AKI developed a median proteinuria/creatininuria of 82 (54–140) mg/mmol with an albuminuria/proteinuria ratio of 0.13 (0.07–0.32). Haematuria was present in 35/51 (69%) patients; leucocyturia was present in 24/51 (47%) patients. Glycosuria was found in 2/51 (4%) patients.
Renal outcomes
At Day 7 after AKI, 6/57 (11%) patients remained dependent on RRT, 9/57 (16%) had SCr >200 µmol/L and 4/57 (7%) died, accounting for 19/57 (33%) patients reaching MAKE criteria. Renal recovery occurred in 28% of patients at Day 7, 52% at Day 14 and 64% at Day 21 (Figure 2B).
DISCUSSION
In this study, the incidence of COVID-19-associated AKI was higher (80%) than reported previously (3–20%) [4, 6, 15]. Cases were severe, with 30% Stage 3 and 18% RRT and not transient; >90% of cases were persistent AKI. The value of urinary indices to differentiate transient versus persistent AKI in this context might be questioned. Both high proteinuria and low albuminuria were not in favour of a prevalent glomerular injury and suggest acute tubular injury (ATI), interstitial nephritis (IN) or both. The very low incidence of glycosuria was not in favour of Fanconi syndrome. Haematuria and leucocyturia could not be interpreted by the presence of foley catheter for all patients.
To the best of our knowledge, this is the first study to systematically explore COVID-19-associated AKI, particularly in more severe patients. The higher incidence of AKI observed in this study can be explained in different ways. First, it could be related to the severity of the disease by an increase in the initial renal viral charge and/or severe systemic inflammation. Moreover, the exhaustive collection of basal SCr of ICU patients could contribute to this higher incidence, providing a more realistic spectrum of COVID-19-associated renal injury.
Whether the cause of AKI is ATI, IN or both is debatable. ATN, the most common form of ATI, is mainly influenced by haemodynamic parameters and iodinated contrast agents [16]. In this study, the use of these agents was limited (20%). The haemodynamic instability of patients with AKI was slight due to high sedation infusion rate [17]. No septic shock was observed and no differences in inflammatory states (C-reactive protein and ferritin) were observed between AKI and non-AKI patients. This suggests the possibility of a viral-specific ATI. SARS-CoV-2 (the virus causing COVID-19) penetrates the cells via two receptors [Angiotensin-converting enzyme 2 (ACE2) and Transmembrane protease, serine 2 (TMPRSS2)] [18]. While ACE2 is highly expressed in proximal tubular epithelial cells and in podocytes, TMPRSS2 is only detectable in the proximal tubule S3 segment. By infiltrating the renal tubular cell, SARS-CoV-2 might induce ATI. This could explain why 9 out of 26 autopsied patients in China with COVID-19-associated AKI had primarily diffused proximal tubular injury, with some frank necrosis and no glomerular injury [19]. Another post mortem study showed severe ATI with IN (CD68+ macrophage infiltration of the tubulointerstitium) [20]. However, in these post mortem studies, patients had less severe renal involvement and may not be representative of more severe patients. Pulmonary damages have been reported as an inflammatory pattern integrated into a cytokine storm syndrome [21]. The frequent association we report between renal and pulmonary injuries might have several explanations. First, co-occurrence does not necessarily mean a common pathway. However, one is left to wonder if infection with SARS-CoV-2 may result in inflammation or direct viral injury of both organs. Renal biopsies in patients with severe COVID-19-associated AKI might thus be considered and lead to specific anti-inflammatory therapies for IN-predominant AKI.
While FeUrea and FeNa+ have been previously reported as relevant for the differentiation of transient AKI from persistent AKI [11, 22], this was not shown in the current study.
Limitations of our work include the small number of patients (n = 71) and a limited median follow-up period (2 weeks). These results will have to be confirmed by larger studies with longer follow-up period.
In conclusion, this study should make physicians aware of the likely existence of a frequent, severe and specific COVID-19-associated AKI. The study provides additional insights into the characterization of AKI: a tubulointerstitial injury without glycosuria. Further work should be carried out promptly in order to identify and assess specific therapeutic options.
ACKNOWLEDGEMENTS
We would like to thank the subjects and the clinical and clerical teams involved in this study. We also thank Philippe Real (MD), Surgical Intensive Care Unit, Mulhouse Hospital; Khaldoun Kuteifan (MD) and Philippe Guiot (MD), Medical Intensive Care Unit, Mulhouse Hospital; Ferhat Meziani (MD), Medical Intensive Care Unit, CHU Strasbourg; and Pierre Diemunsch (MD) Surgical Intensive Care Unit, CHU Strasbourg.
FUNDING
No funding disclosures.
CONFLICT OF INTEREST STATEMENT
S.R. discloses support by Sanofi. A.B. discloses support by Gilead and Basilea. O.J.-B. is consultant for Baxter and BBraun. All other authors disclose no conflict of interest.
REFERENCES
- 1. Guan W-J, Ni Z-Y, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Pesenti A, Cecconi M.. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545. [DOI] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatraju PK, Ghassemieh BJ, Nichols M. et al. Covid-19 in critically ill patients in the Seattle Region—case series. N Engl J Med 2020; 500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grasselli G, Zangrillo A, Zanella A. et al. ; for the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323: 1574–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin S, Orieux A, Clouzeau B. et al. The incidence of chronic kidney disease three years after non-severe acute kidney injury in critically ill patients: a single-center cohort study. J Clin Med 2019; 8: 2215–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ai T, Yang Z, Hou H. et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 200642; doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chawla LS, Bellomo R, Bihorac A. et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 10. Gaudry S, Hajage D, Schortgen F. et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375: 122–133 [DOI] [PubMed] [Google Scholar]
- 11. Darmon M, Vincent F, Dellamonica J. et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care 2011; 15: R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pannu N, James M, Hemmelgarn B, Klarenbach S; for the Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 2013; 8: 194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Billings FI, Shaw AD.. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 2014; 127: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer M, Deutschman CS, Seymour CW. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arentz M, Yim E, Klaff L. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323: 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darmon M, Ostermann M, Cerdá J. et al. Diagnostic work-up and specific causes of acute kidney injury. Intensive Care Med 2017; 43: 829–840 [DOI] [PubMed] [Google Scholar]
- 17. Helms J, Kremer S, Merdji H. et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wrapp D, Wang N, Corbett KS. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367: 1260–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020: 1–25; doi: 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diao B, Feng Z, Wang C. et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv.org; doi: 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mehta P, McAuley DF, Brown M. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dewitte A, Biais M, Petit L. et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care 2012; 27: 505–510 [DOI] [PubMed] [Google Scholar]