Abstract
Coronaviruses are a major pathogen for adults, causing up to one-third of community-acquired respiratory tract infections in adults during epidemics. Although the pandemic outbreak of coronavirus disease-2019 (COVID-19) targets preferentially patient’s lungs, recent data have documented that COVID-19 causes myocarditis, acute myocardial infarction, exacerbation of heart failure and acute kidney injury. Studies show that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), similar to its predecessor SARS-CoV, engages angiotensin-converting enzyme 2 (ACE2) as the entry receptor. ACE2 is also expressed in the heart, providing a link between coronaviruses and the cardiovascular system.
Keywords: ACE2, cardiorenal syndrome, CKD, COVID-19, myocardial injury
Coronaviruses are a major pathogen for adults, children and animals, causing up to one-third of community-acquired respiratory tract infections in adults during epidemics. In December 2019, a novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] was identified from a cluster of pneumonia cases in Wuhan, China. At the time of this report, the virus is spreading globally and, in March 2020, the World Health Organization declared COVID-19 a global pandemic.
Cardiorenal syndrome (CRS) comprises a number of conditions involving both the heart and kidneys, in which acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ [1]. In addition, both heart and kidney function can be impaired by an acute or chronic systemic disorder [2]. SARS-CoV-2 enters the body using an evolutionarily evolved arm that binds human angiotensin-converting enzyme 2 (ACE2) on human pneumocytes [3]. ACE2 also serves a role in lung protection and therefore viral binding to this receptor deregulates a lung-protective pathway. Furthermore, ACE2 is also expressed in the heart and kidney, providing a link between coronavirus infection and the cardiovascular and renal system. While infection progresses, ACE2 is downregulated, resulting in increased angiotensin II action and/or loss of the cardioprotective effects of angiotensins 1–7.
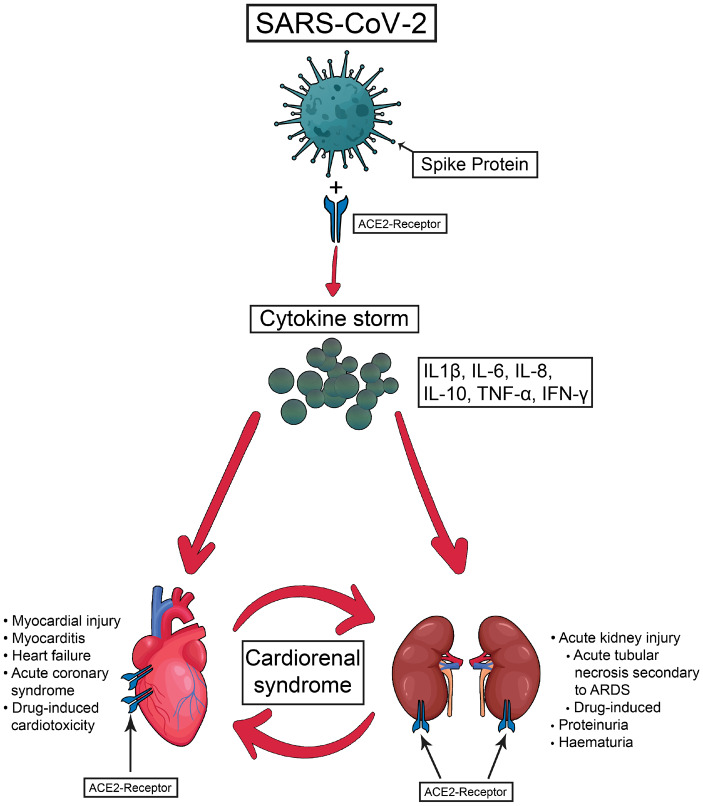
The characteristic initial symptoms, consistent with the port of entry, are a dry cough and, with time, dyspnoea, with a relatively high rate of progression to ventilator dependence. However, consistent with the cardiovascular repercussions of a renin–angiotensin system–destructive infection, a rapidly growing body of evidence suggests a significant contribution from extrapulmonary cardiorenal manifestations, including arrhythmias, acute cardiac injury and acute kidney injury; therefore this could be regarded as a Type 5 (secondary) CRS (see Figure 1).
FIGURE 1.
The main physiopathological pathways of cardiorenal syndrome associated with SARS-CoV-2 infection.
CARDIAC INVOLVEMENT IN COVID-19
It has been documented that COVID-19 causes myocarditis [4], acute myocardial infarction [5] and exacerbation of heart failure [6, 7]. This is actually not unique to COVID-19 and is a documented source of mortality in prior epidemics of severe acute respiratory syndrome (SARS) [8, 9] and Middle East respiratory syndrome (MERS) coronavirus [10]. Interestingly, in COVID, the pulmonary risk is greater in those with pre-existing cardiac disease [4]. The SARS-CoV-2 infection causes acute myocardial injury, shock and arrhythmia in 7.2, 8.7 and 16.7% of patients, respectively, according to a recent epidemiological study [11].
Myocardial injury in patients with COVID-19
In patients with SARS-CoV-2 infection, the most important features that suggest myocardial injury are electrocardiogram changes and troponin elevation coupled with echocardiography showing signs of subclinical left ventricular diastolic impairment or even reduced ejection fraction (EF) in severe cases [11], with a higher likelihood of the need for mechanical ventilation in those with reduced EF, as was seen during previous coronavirus outbreaks [9].
Myocardial injury can occur due to myocardial ischaemia or non-ischaemic myocardial processes, including myocarditis. Up to one in five COVID-19 patients will get acute myocardial injury (12–17% of cases) [12, 13]. Zhou et al. [13] observed a progressive increase (reaching the upper reference limit at Day 11) of high-sensitivity cardiac troponin I (hs-cTnI) levels in non-survivors, while hs-cTnI remained low in survivors. In a meta-analysis of four studies including a total of 341 patients, the standardized mean difference of cTnI levels was significantly higher in those with severe COVID-19 compared with milder disease [14]. The inflammatory response and haemodynamic changes associated with severe disease may confer a risk for atherosclerotic plaque rupture in susceptible patients. It is important to note a potential overlapping symptomatology between acute coronary syndrome and COVID-19. Interestingly, cardiac dysfunction overlapped with renal dysfunction, both occurring 2–4 days after the beginning of sepsis and acute respiratory distress syndrome (ARDS). The increase in cTnI may relate to endotoxin production as is known to occur in sepsis [15], an overall pro-inflammatory state, or direct cardiac attack via ACE2 receptors in heart tissue.
A more aggressive form of cardiac involvement cited in the literature among patients with COVID-19 is fulminant myocarditis [16–18]. Chen et al. [18] observed higher interleukin-6 (IL-6) levels in patients with fulminant myocarditis in their series of 120 COVID patients. Zhou et al. [13] also observed a worse prognosis in IL-6-elevated patients and this group had a higher likelihood of acute myocardial injury; the increase in IL-6 parallels the increase in hs-cTnI, raising the possibility that this reflects viral myocarditis. Ruan et al. [17] reported on myocardial damage as a cause of death by fulminant myocarditis in five patients (7%) and a cause of respiratory failure in 22 patients. In one patient with fulminant COVID-19 myocarditis, early glucocorticoid and immunoglobulin initiation resulted in complete remission of symptoms and echocardiographic changes after 1 week and fully normalized myocardial injury markers at 3 weeks [16]. However, even after a successful recovery from fulminant myocarditis, there is a lasting cardiac risk, with higher rates of cardiac death and heart transplantation in patients who developed fulminant forms of myocarditis compared with non-fulminant forms (47.7% of patients with fulminant versus 10.4% of patients with non-fulminant forms at the 7-year follow-up—data from a retrospective, international registry comprising a total of 220 patients) [19]; this will remain to be determined in long-term follow-up studies of patients with COVID-19 myocarditis.
Case reports have noted the development of arterial pulmonary thromboembolism (PTE) in patients with COVID-19 [20], vascular inflammation being considered the main culprit of the hypercoagulable state and endothelial dysfunction. However, it is not clear if the risk of PTE is higher in patients with COVID-19 than other critically ill patients. Elevated D-dimer levels (>1 g/L) are strongly associated with in-hospital death in these patients [21]. In the setting of critically ill COVID-19 patients, although non-specific, an elevated D-dimer level coupled with respiratory deterioration evidenced by hypoxia or haemodynamic instability should raise suspicion for pulmonary embolism. In the absence of a clear indication for full anticoagulation, all hospitalized patients with COVID-19 should receive pharmacologic thromboprophylaxis with low molecular weight heparin [22].
Additional risk factors for Type 1 cardiorenal syndrome are related to sepsis-induced pulmonary disease—well recognized to precipitate acute heart failure (due to cardiometabolic stress, i.e. increased cardiac demand in the face of limited cardiac reserve [23–27], especially if there is pre-existing HF)—and pre-existing coronary artery disease, additionally predisposed to plaque rupture (due to the systemic inflammatory state).
Overall, existing data from China suggest that between one-quarter and one-third of the COVID-19 patients have significant heart failure: Zhou et al. [13] reported a 23% incidence of heart failure in their 191 patient series of SARS-CoV-2, while Chen et al. [18] reported elevated NT-pro-BNP in 27.5% of their patients.
Arterial hypertension and the use of ACE inhibitors or angiotensin II receptor blockers in patients with COVID-19
Arterial hypertension is among the most frequent comorbidities associated in patients infected with SARS-CoV-2, with an estimated prevalence of 15–30% in most of the studies from China, with a higher value in patients who ultimately had severe COVID-19 infection [12, 13, 28–30]. However, these values might be higher due to the likely underreporting of pre-existing conditions in China [31]. Importantly, the presence of underlying hypertension may be a risk factor for severe patients compared with non-severe patients, according to a recent meta-analysis of eight studies including 46 248 COVID-19 patients [32].
Given the fact that SARS-CoV-2 invades cells through the ACE2 receptor, the use of ACE inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) has become controversial. The unproven hypothesis is that ACE2 is overexpressed in these patients, giving more ports of entry for the virus [33].
There are conflicting data from studies demonstrating whether these drugs increase or have a minimal effect on ACE2 levels. Ferrario Carlos et al. [34] noted increased ACE2 expression in rat myocardial tissue after 12 days of lisinopril and losartan, similar to the findings of Ocaranza et al. [35] after enalapril and Ishiyama et al. [36] after losartan or olmesartan. However, other groups failed to show a difference in ACE2 expression with ACEis/ARBs versus controls in animals [37, 38] or in humans [39, 40]. Thus studies on myocardial ACE2 expression are not demonstrating a concordant signal. Nevertheless, pulmonary ACE2 expression remains understudied.
Kuba et al. [41] studied the molecular basis of lung injury in mice exposed to the preceding SARS-CoV (2003 epidemic). SARS downregulated ACE2 by binding the SARS-CoV Spike protein to ACE2. Interestingly, when SARS-CoV Spike protein was injected into the bloodstream, renin–angiotensin blockade prevented further worsening of acute lung failure. In COVID-19 patients, it was noted that viral load, lung injury (via the partial pressure of oxygen:fraction of inspired oxygen ratio) and higher levels of angiotensin II were all positively correlated [42]. As a result, recombinant ACE2 protein to provide ‘false targets’ for viral binding, and to modulate the RAS, might be used to protect individuals with SARS by potentially lowering both the viral load and the deleterious effects of angiotensin II activity. Notably, a recombinant human ACE2 (rhACE2; APN01, GSK2586881) has been found to be safe, with no negative haemodynamic effects in a small cohort of non-COVID-19 patients with ARDS [43].
At the time of this report, the data do not support a COVID-19-related shift away from ACEis/ARBs in patients with heart failure, hypertension or myocardial infarction, irrespective of SARS-CoV-2 according to the latest Position Statement of the European Society of Cardiology Council on Hypertension [44] and a joint statement from the American College of Cardiology, American Heart Association and Heart Failure Society of America posted online on 17 March [45]. This makes sense, as the upregulation of ACE2 (protein transcription) likely occurs over a protracted period of time, and halting these mediations will worsen cardiac status and overall COVID outcomes, thus measures to prevent COVID infection (social distancing and hand washing) remain more useful than ACEi/ARB medication cessation based on the available evidence.
RENAL COMPLICATIONS OF COVID-19
Once SARS-CoV-2 enters the bloodstream, the postulated mechanisms for kidney damage include sepsis, cytokine storm and direct cellular injury [46]. Although in a post-mortem analysis of SARS patients with acute kidney injury (AKI) using electron transmission microscopy, SARS-CoV was not detectable in any of the renal specimens [47], thus the presence of the SARS-CoV-2 in renal tissue is still under debate. Using polymerase chain reaction, Wang et al. [48] did not identify the virus in the urine in any of the 72 urine specimens tested. However, using immunohistochemistry, in situ SARS-CoV nucleocapsid protein was detected in six post-mortem samples from patients with AKI [49]. Moreover, recent human tissue RNA sequencing data have demonstrated that ACE2 expression in the kidney tubules is nearly 100-fold higher than in the lungs [50]. Corroborating this with the increased binding affinity to ACE2 of the SARS-CoV-2, as compared with the SARS-CoV [51], could support and explain the observed differences between the two infections in regard to a direct viral attack on the kidney. Other factors that may negatively interfere with kidney function are the presence of haemodynamic instability and concomitant medication, which may cause direct tubular toxicity or immunoallergic effects.
Previous studies of SARS and MERS-CoV infections reported a prevalence of AKI of 5–15% associated with a high mortality rate of up to 90% [47]. Although early reports suggested a lower incidence (3–9%) of AKI in COVID-19 patients (in the Chinese population) [11], more recent data have shown a higher frequency of renal abnormalities. The most prominent findings were albuminuria or haematuria, found by dipstick assessment in almost one-third of patients on the first day of admission, and elevated serum creatinine and blood urea nitrogen, found in 15.5% and 14.1% of patients, respectively [52]. Importantly, the presence of any of these markers of kidney damage in these patients was associated with a significantly higher in-hospital mortality [52].
MANAGEMENT OF CRS IN COVID-19 PATIENTS
At the time of this report there is no direct antiviral therapy for COVID-19 and the treatment regimens prescribed for patients are the main drugs that have previously been effective in SARS-CoV and MERS-CoV. A number of investigational agents are being explored for antiviral treatment of COVID-19, most of them being used based on in vitro or extrapolated evidence. However, therapeutics for COVID-19 have the potential for adverse cardiovascular effects. Therapeutic use of corticosteroids, especially for the management of severely ill patients with ARDS and/or myocarditis [12], might further increase the possibility of adverse cardiovascular events, including fluid retention and arrhythmias [53].
Overall critical care treatment goals include avoidance of volume overload, since overhydration may precipitate or exacerbate ARDS, maintenance of blood pressure (mean arterial pressure >65 mmHg) and good oxygenation. Renal treatment goals include supportive care and renal replacement therapy (RRT) [47]. The question about starting RRT early versus late in these patients is still a matter of debate. We could extrapolate the results from the non-COVID-19 population, where there is no difference in outcomes for early versus late starts [3]. Therefore the decision of starting dialysis may be taken after putting in balance the traditional clinical indicators (with the prioritization of volume control) for RRT versus the risk of exposing healthcare workers to procedures that maybe are not needed in some of these patients and require additional manpower and machines. The type of RRT in COVID-19 CRS patients is another unanswered question. Once again, data from the non-COVID-19 population with AKI failed to find significant differences between several types of continuous therapies (continuous veno-venous haemofiltration, continuous veno-venous haemodialysis or continuous veno-venous haemodiafiltration) versus prolonged intermittent RRT [54]. If none of the above is available, intermittent haemodialysis is acceptable.
Although, for the moment, clinicians are dealing only with the acute cardiorenal complications of COVID-19, we can speculate that systemic inflammatory and pro-coagulant activity can persist in survivors of hospitalization. Since these acute complications might represent risk factors for chronic cardiac and kidney disease, a close follow-up of these patients is mandatory.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Rangaswami J, Bhalla V, Blair JEA. et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019; 139: e840–e878 [DOI] [PubMed] [Google Scholar]
- 2. Ronco C, Haapio M, House AA. et al. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52: 1527–1539 [DOI] [PubMed] [Google Scholar]
- 3. Sun ML, Yang JM, Sun YP. et al. [ Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020; 43: 219–222. [DOI] [PubMed] [Google Scholar]
- 4. Driggin E, Madhavan MV, Bikdeli B. et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 2020; doi: 10.1016/j.jacc.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tam CF, Cheung KS, Lam S. et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes2020: doi: 10.1161/circoutcomes.120.006631 [DOI] [PMC free article] [PubMed]
- 6. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet2020; doi: 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed]
- 7. Arentz M, Yim E, Klaff L. et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA2020; doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed]
- 8. Yu CM, Wong RS, Wu EB. et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 2006; 82: 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li SS, Cheng CW, Fu CL. et al. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 2003; 108: 1798–1803 [DOI] [PubMed] [Google Scholar]
- 10. Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med 2016; 36: 78–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed]
- 14. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis2020; doi: 10.1016/j.pcad.2020.03.001 [DOI] [PMC free article] [PubMed]
- 15. Thygesen K, Alpert JS, Jaffe AS. et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264 [DOI] [PubMed] [Google Scholar]
- 16. Hu H, Ma F, Wei X. et al. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020; doi: 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruan Q, Yang K, Wang W. et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med2020; doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed]
- 18. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz2020; doi: 10.1007/s00059-020-04909-z [DOI] [PMC free article] [PubMed]
- 19. Ammirati E, Veronese G, Brambatti M. et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2019; 74: 299–311 [DOI] [PubMed] [Google Scholar]
- 20. Xie Y, Wang X, Yang P. et al. COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging 2020; 2: e200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang N, Li D, Wang X. et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Society of Hematology. Statement on COVID-19 and VTE-anticoagulation. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation (accessed 28 March 2020)
- 23. Xiong T-Y, Redwood S, Prendergast B. et al. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 2020; doi: 10.1093/eurheartj/ehaa231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merx MW, Weber C.. Sepsis and the heart. Circulation 2007; 116: 793–802 [DOI] [PubMed] [Google Scholar]
- 25. Arrigo M, Tolppanen H, Sadoune M. et al. Effect of precipitating factors of acute heart failure on readmission and long-term mortality. ESC Heart Fail 2016; 3: 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corrales-Medina VF, Musher DM, Shachkina S. et al. Acute pneumonia and the cardiovascular system. Lancet 2013; 381: 496–505 [DOI] [PubMed] [Google Scholar]
- 27. Kakihana Y, Ito T, Nakahara M. et al. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 2016; 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen N, Zhou M, Dong X. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513 [DOI] [PMC free article] [PubMed]
- 29. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 30. Zhang JJ, Dong X, Cao YY. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. JAMA Intern Med. 2020; doi: 10.1001/jamainternmed.2020.0994 [DOI] [PubMed]
- 31. Li D, Lv J, Liu F. et al. Hypertension burden and control in mainland China: analysis of nationwide data 2003–2012. Int J Cardiol 2015; 184: 637–644 [DOI] [PubMed] [Google Scholar]
- 32. Yang J, Zheng Y, Gou X. et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis 2020; 94: 91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrario Carlos M, Jessup J, Chappell Mark C. et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111: 2605–2610 [DOI] [PubMed] [Google Scholar]
- 35. Ocaranza MP, Godoy I Fau-Jalil JE, Jalil Je Fau-Varas M. et al. Enalapril attenuates downregulation of angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 2006; 48: 572–578 [DOI] [PubMed]
- 36. Ishiyama Y, Gallagher Pe Fau-Averill DB, Averill Db Fau-Tallant EA. et al. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004; 43: 970–976 [DOI] [PubMed]
- 37. Burchill LJ, Velkoska E, Fau-Dean RG, Dean Rg Fau-Griggs K. et al. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond) 2012; 123: 649–658 [DOI] [PubMed]
- 38. Burrell LM, Risvanis J, Kubota E. et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 2005; 26: 369–375 [DOI] [PubMed] [Google Scholar]
- 39. Ramchand J, Patel SK, Srivastava PM. et al. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One 2018; 13: e0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walters TE, Kalman JM, Patel SK. et al. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. EP Europace 2016; 19: 1280–1287 [DOI] [PubMed] [Google Scholar]
- 41. Kuba K, Imai Y, Rao S. et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Yang Y, Zhang C. et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020; 63: 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan A, Benthin C, Zeno B. et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 2017; 21: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Society of Cardiology. Position statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang
- 45.American College of Cardiology. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19
- 46. Tai W, He L, Zhang X. et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol2020; doi: 10.1038/s41423-020-0400-4 [DOI] [PMC free article] [PubMed]
- 47. Chu KH, Tsang WK, Tang CS. et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang W, Xu Y, Gao R. et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; doi: 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diao B, Feng Z, Wang C. et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection MedRxiv 2020; doi: 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed]
- 50. Li ZW, Guo J. et al. Caution on kidney dysfunctions of 2019-nCoV patients. MedRxiv 2020; doi: 10.1101/2020.02.08.20021212 [DOI] [Google Scholar]
- 51. Wan Y, Shang J, Graham R. et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020; 94: e00127–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed]
- 53. Wei L, MacDonald TM, Walker BR.. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 2004; 141: 764–770 [DOI] [PubMed] [Google Scholar]
- 54. Zhao Y, Chen Y. Effect of renal replacement therapy modalities on renal recovery and mortality for acute kidney injury: a PRISMA-compliant systematic review and meta-analysis. Semin Dial 2020; 33: 127-132 [DOI] [PubMed]