Abstract
Among patients hospitalized for novel coronavirus disease (COVID-19), between 10 and 14% develop an acute kidney injury and around half display marked proteinuria and haematuria. Post-mortem analyses of COVID-19 kidney tissue suggest that renal tubular cells and podocytes are affected. Here we report two cases of collapsing glomerulopathy and tubulointerstitial lesions in living COVID-19 patients. Despite our use of sensitive reverse transcription polymerase chain reaction techniques in this study, we failed to detect the virus in blood, urine and kidney tissues. Our observations suggest that these kidney lesions are probably not due to direct infection of the kidney by severe acute respiratory syndrome coronavirus 2.
Keywords: APOL1, collapsing glomerulopathy, COVID-19, kidney disease, SARS-CoV-2
INTRODUCTION
Novel coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) appeared in December 2019 and is currently responsible for a worldwide pandemic. Common symptoms of COVID-19 include fever, cough, dyspnoea and hypoxemia. The most severe form of COVID-19 features SARS [1, 2]. Renal manifestations have also been described, including acute kidney injury (AKI), proteinuria and haematuria in >10, 40 and 26% of patients, respectively [3, 4]. Interestingly, SARS coronaviruses use angiotensin-converting enzyme 2 (ACE2) as a host receptor for cell surface binding and internalization. Given that ACE2 is expressed by proximal tubules and podocytes, the kidney is conceivably a target for SARS-CoV-2. The results of post-mortem histologic assessments suggest that AKI and proteinuria are related to SARS-CoV-2’s renal tropism [5].
Collapsing glomerulopathy is a peculiar form of focal segmental glomerulosclerosis (FSGS) [6] that has been well characterized in patients infected by human immunodeficiency virus (HIV) type 1 [7]. Here we describe the clinical course and pathology findings for two COVID-19 patients with collapsing glomerulopathy and severe tubulointerstitial lesions. These kidney lesions appear to be related to a viral-induced inflammatory response against a peculiar genetic background, since we failed to detect the virus in blood, urine and kidney tissues.
CASE REPORT 1
A 53-year-old man of African origin was admitted to the emergency department after the onset of asthenia, fever and cough 4 days previously. He had a history of chronic hypertension and (in 2013) acute cardiac failure. His hypertension was well controlled by multitarget therapy, including angiotensin II receptor blockers.
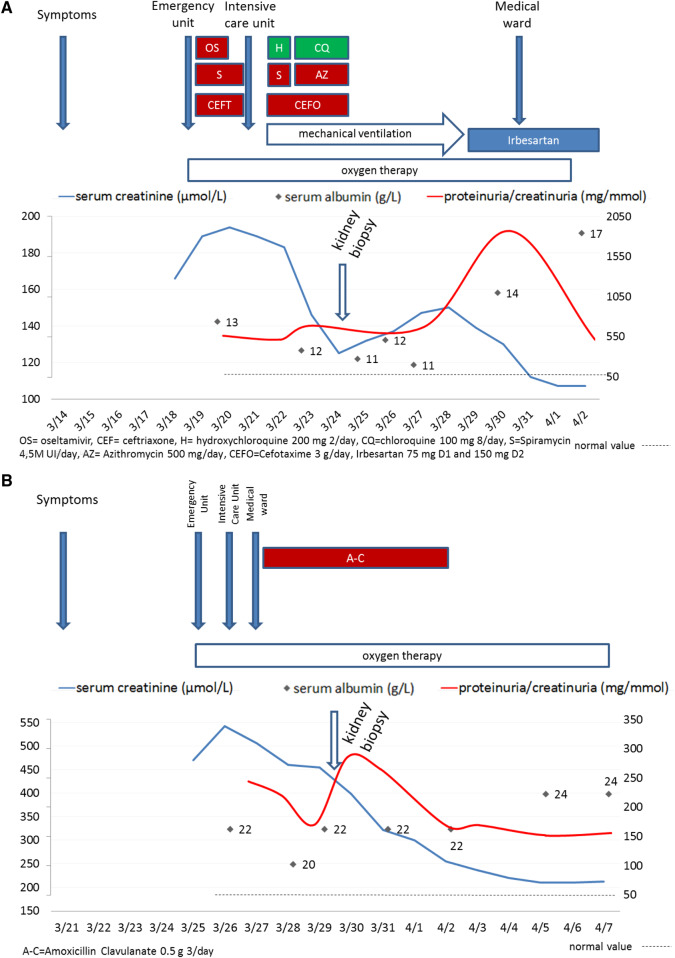
On admission, the patient’s blood pressure was 116/82 mmHg, with a heart rate of 80 bpm and a body temperature of 36.6°C. His body mass index was 24.9 kg/m2. The haemoglobin oxygen saturation was 80% in ambient air (with an arterial partial pressure of oxygen of 49 mmHg) and increased to 92% with 3 L/min oxygen. A computerized tomography (CT) scan of the chest evidenced peripheral ground-glass opacities and alveolar consolidation. Laboratory workup showed a platelet count of 138 G/L, a neutrophil count of 8.4 G/L, a lymphocyte count of 900/mm3, a serum C-reactive protein (CRP) level of 106 mg/L and a serum creatine phosphokinase (CPK) level of 923 U/L. The serum creatinine level was 166 µmol/L, whereas his baseline value had been 90 µmol/L in 2019 [estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation for African Americans: 98 mL/min/1.73 m2]. Proteinuria/creatinuria was only performed on intensive care unit (ICU) admission, revealing significant leucocyturia but no haematuria; urine sodium and potassium concentrations were <20 and 22 mEq/L and urine protein:creatinine ratio was 564 mg/mmol (normal range <50 mg/mmol) (Figure 1A). The only urinalysis test performed before was around 2010 by an occupational physician, and no proteinuria was found at that point. An HIV test was negative. Our diagnosis of COVID-19 pneumonia was further confirmed by a positive reverse transcription polymerase chain reaction (RT-PCR) assay with a nasopharyngeal swab. Treatment with oseltamivir, ceftriaxone and spiramycin was initiated. The patient’s clinical condition worsened, prompting his transfer to the ICU on Day 3 for invasive mechanical ventilation.
FIGURE 1.
Schematic descriptions of (A) Patient 1 and (B) Patient 2.
On admission to the ICU, the patient’s serum creatinine level was 194 µmol/L and his serum albumin level was 13 g/L (normal range 38–52 g/L), with a urine protein:creatinine ratio of 564 mg/mmol (Figure 1A). The patient received intravenous fluids, cefotaxime, azithromycin and hydroxychloroquine and then chloroquine from Days 3 to 7.
Although the patient’s renal function progressively improved, high-grade proteinuria (peaking at 1870 mg/mmol) persisted. A kidney biopsy showed eight glomeruli, of which two displayed global sclerosis suggestive of chronicity and three showed segmental or global lesions. One glomerulus displayed glomerular capillary collapse and a large number of swollen, proliferating podocytes that contained many cytoplasmic vacuoles and periodic acid–Schiff (PAS)-positive cytoplasmic droplets, filling Bowman’s space (Figure 2A and B, respectively). No microcystic tubular dilatation was observed. The proximal tubules contained PAS-positive protein resorption droplets. The pathology assessment also showed moderately severe vascular lesions, mild interstitial fibrosis and tubular atrophy with a dispersed mononuclear interstitial inflammatory infiltrate composed of circular dichroism (CD)68+ macrophages and CD3+ T cells with a CD4:CD8 ratio of 2 (Figure 3). The proportion of CD20+ B cells was weaker (Figure 3). No CD56+ natural killer cells were observed. An immunofluorescence analysis revealed segmental glomerular deposits of immunoglobulin M (IgM) and C3 only. Taken as a whole, the pathology findings were consistent with a collapsing variant of FSGS and non-cystic tubulointerstitial lesions.
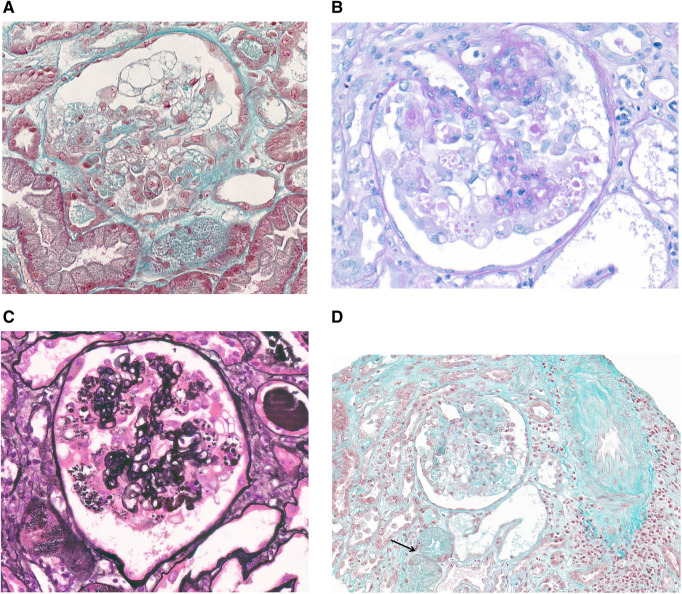
FIGURE 2.
(A) Patient 1 (trichrome stain, ×400): a glomerulus with global collapse of the capillaries, associated with marked hyperplasia and swelling of overlying podocytes that contain cytoplasmic large vacuoles. (B) Patient 1 (PAS stain, ×400): swollen podocytes showing numerous PAS-positive cytoplasmic droplets. (C) Patient 2 (Jones methenamine silver stain, ×400): pronounced collapsing features, with vacuolated podocytes and capillary obliteration. (D) Patient 2 (trichrome stain, ×200): a glomerulus with a FSGS collapsing variant, together with acute tubular injury and a few tubules with cytoplasmic protein droplets (arrow).
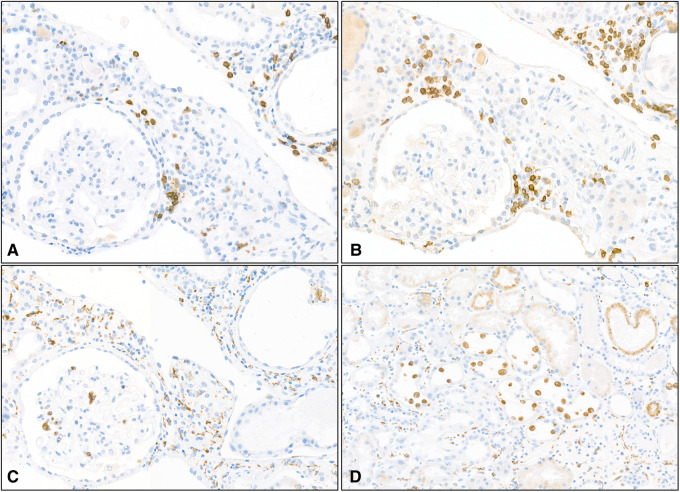
FIGURE 3.
Patient 1: immunohistochemistry of infiltrating mononuclear cells. Inflammation consisted of B lymphocytes labelled with anti-CD20 antibody (A, magnification ×200), T lymphocytes labelled with anti-CD3 antibody (B, magnification ×200) and numerous macrophages within the interstitium, more rarely in the glomerulus (C, magnification ×200) and in the lumen of tubules (D, magnification ×100) labelled with anti-CD68 antibody.
Mechanical ventilation and oxygen therapy were discontinued on Days 11 and 14, respectively. The serum creatinine level decreased and the serum albumin level increased, although the proteinuria level remained high (510 mg/mmol). Treatment with an angiotensin II receptor blocker was resumed and the patient was discharged on Day 20.
CASE REPORT 2
A 53-year-old man of African origin was admitted to the emergency department after the onset of a dry cough (in the absence of fever) 4 days previously. His medical history notably included chronic hypertension and untreated chronic hepatitis B.
On admission, the patient’s blood pressure was 149/90 mmHg, with a heart rate of 95 bpm and a temperature of 36.5°C. His body mass index was 24.7 kg/m2. The haemoglobin oxygen saturation was 86% in ambient air, with an arterial partial pressure of oxygen of 56 mmHg. A CT scan of the chest revealed bilateral ground-glass opacities and parenchymal band condensation suggestive of COVID-19 infection. A nasopharyngeal swab was positive for SARS-CoV-2 in an RT-PCR assay.
Laboratory workup revealed a platelet count of 98 G/L, a neutrophil count of 9.5 G/L and a lymphocyte count of 0.8 G/L. The serum creatinine level was 470 µmol/L; a value of 119 µmol/L had been recorded in 2017 (eGFR 72 mL/min/1.73 m2). The serum CRP level was 284 mg/L, the serum albumin level was 22 g/L and the serum CPK level was 313 U/L. The urine sodium and potassium concentrations were <20 and 10 mEq/L, respectively. A urinalysis revealed significant leucocyturia but no haematuria. Proteinuria was 1.55 g/day on ICU admission, with an albumin:creatinine ratio of 154.7 mg/mmol. An HIV test was negative. The renal ultrasound results were normal.
The patient was given intravenous fluids and 2 L/min oxygen. Nevertheless, the serum creatinine level increased to 529 µmol/L (Figure 1B). The oxygen delivery rate was increased to 4 L/min. Amoxicillin–clavulanate was introduced for 7 days. In view of persistent high-grade proteinuria (2.65 g/day), a kidney biopsy was performed. A pathology assessment showed 11 glomeruli, including 5 with global sclerosis suggestive of chronicity. Three were characterized by global capillary collapse and a large number of swollen, proliferating podocytes (Figure 2C) containing cytoplasmic vacuoles and PAS-positive cytoplasmic droplets, filling Bowman’s space. Interstitial fibrosis was mild and featured a widely dispersed mononuclear inflammatory infiltrate. Acute tubular necrosis was observed and the proximal tubules contained PAS-positive protein resorption droplets consistent with severe proteinuria (Figure 2D). No microcystic dilatation was noted. The interlobular arteries displayed marked intimal fibrosis and the walls of some arterioles had been thickened by hyaline deposits. An immunofluorescence assessment showed sparse areas of segmental granular mesangial staining for C3. Staining for other immunoglobulins and complement components was negative. Taken as a whole, these findings were consistent with a diagnosis of collapsing FSGS.
The maintenance of intravenous rehydration led to a prompt improvement in renal function. The serum albumin level increased to 24 g/L and proteinuria decreased to 1.5 g/day. The patient was weaned off oxygen therapy but was still in the ICU.
MATERIALS AND METHODS
SARS-CoV-2 detection was performed after total nucleic acid extraction by using Nuclisens reagent (Biomérieux, Marcy L’étoile, France). RT-PCR targeting E and ribonucleic acid (RNA)-dependent RNA polymerase (RDRP) genes [8] were performed by using the superscript III one-step RT-PCR system (Invitrogen) on a VIIA7 instrument (Applied Biosystems, Waltham, MA, USA).
To confirm a diagnosis of COVID-19, we ran an RT-PCR assay (targeting the SARS-CoV-2 E and RDRP gene) on nasopharyngeal mucus, blood, urine and/or kidney biopsy samples. Renal biopsy samples were fixed in formalin, acetic acid and alcohol, embedded in paraffin and prepared as 2.5-µm-thick sections for routine light microscopy. Slides were stained with haematoxylin–eosin–saffron, Masson’s trichrome, periodic acid–Schiff and Jones methenamine silver reagents. Immunostaining for CD20, CD3, CD4, CD8, CD68 and CD56 was performed using an automated immunostainer (Leica Biosystems Newcastle, Newcastle-upon-Tyne, UK). For the immunofluorescence analyses, 3-µm cryostat sections were incubated with polyclonal fluorescein–isothiocyanate-conjugated antibodies against human IgG, IgA, IgM, kappa, lambda, C1q, C3 and fibrinogen (Dako France SAS, Les Ulis, France).
COMPLEMENTARY INVESTIGATIONS
In order to better characterize the relationship between SARS-CoV-2 infection and collapsing FSGS, we performed an RT-PCR assay on the renal tissue specimen from Patient 1. Molecular expression of SARS-CoV-2 could not be detected in the whole kidney biopsy extract. A specific SARS-CoV-2 RT-PCR assay performed on a urine sample from Patients 1 and 2 (on Days 12 and 13 from admission, respectively) was also negative. Similarly, an RT-PCR assay performed on a whole blood sample from Patient 1 on Day 12 was negative.
Given that both patients were of African origin, we genotyped the apolipoprotein L1 (APOL1) locus in order to determine the potential relationship between APOL1 G1 and G2 risk variants and the collapsing glomerulopathy. Patient 1 was homozygous for the G1 polymorphism, whereas Patient 2 was heterozygous (G1/G2). Thus both patients had an at-risk combination of APOL1 variants.
DISCUSSION
Here we report two cases of collapsing FSGS and tubulointerstitial lesions in two African patients with COVID-19 and APOL1 polymorphism. Despite our use of sensitive RT-PCR assays, we did not detect SARS-CoV-2 expression in the kidney, blood and urine specimens. Our findings suggest that the virus had been completely cleared by the time of kidney biopsy and SARS-CoV-2 infection only has an indirect effect on glomerular and tubular cells. We also highlighted a potentially crucial role of the APOL1 G1 and G2 risk alleles in the genesis of SARS-CoV-2-associated collapsing FSGS.
Recent reports have emphasized the significant prevalence of AKI and high-grade proteinuria in the setting of COVID-19, as observed for other coronaviruses [9, 10]. In two Chinese series of patients with COVID-19, the incidence of AKI varied from 10 to 14% [3, 4]. The remarkably high incidence of proteinuria (in 44% and 59% of the patients in the respective studies) and haematuria (in 27% and 44%, respectively) [3, 4] highlights the risk of underdiagnosing glomerular involvement in COVID-19 [11]. Our current understanding of COVID-19 renal lesions is solely based on post-mortem studies. Our results are in line with a recent in-press report that describes the presence of collapsing glomerulopathy and tubulointerstitial lesions in living COVID-19 patients of African origin, homozygous for APOL1 risk allele G1 and evidence of chronicity on kidney biopsy [12].
The physiopathological mechanisms that underlie viral renal lesions have not been identified. A direct viral cytopathogenic effect (as shown in HIV and parvovirus B19 infections) has been discussed in the literature; HIV-1 may persist in the kidney after otherwise effective antiretroviral therapy [13], whereas parvovirus B19 may infect podocytes and tubular cells and thus induce collapsing FSGS [14]. Interestingly, Yeung et al. [15] showed that Middle East respiratory syndrome-related coronavirus (MERS-CoV) nucleoprotein was expressed in renal tubular cells and podocytes and appeared to induce apoptosis. A similar hypothesis has been put forward for SARS-CoV-2. In this respect, proximal tubule cells and podocytes are known to express ACE2, a receptor for the SARS-CoV [2]. Immunostaining of post-mortem kidney tissues from patients with AKI revealed the expression of SARS-CoV-2 nucleoprotein in both tubules and glomeruli [5, 11]. Virus particles were also identified by electron microscopy in proximal tubules, podocytes and (to a lesser extent) distal tubules [5]. These findings suggest that SARS-CoV-2 infects podocytes and tubular epithelial cells, resulting in renal injury. Importantly, not all the patient samples in these reports stained positive, and the presence of virus particles in kidney tissue may not be a marker of viral replication. Despite our use of sensitive RT-PCR techniques in this study, we failed to detect the virus in kidney tissues. This might have been due to complete viral clearance at the time of sampling and suggests rather that SARS-CoV-2 injures the kidneys indirectly. Severe cases of COVID-19 are associated with a cytokine storm that can lead to severe acute respiratory distress syndrome. This type of inflammatory response may also harm the kidney and glomerular function. Many studies have emphasized the potential involvement of interferon pathways [16, 17] and a viral trigger [18–21] in glomerular injury. Podocyte dysregulation might be due to an infection-driven inflammatory response that releases cytokines or viral products; in turn, these products circulate and interact with receptors on podocytes [22]. This hypothesis is supported by the fact that renal injury can appear several weeks after the general symptoms of parvovirus B19 infection [14].
Strikingly, not all COVID-19 patients with AKI and proteinuria develop collapsing FSGS. Biopsies from living Caucasian COVID-19 patients present tubulointerstitial lesions but not glomerular lesions (personal and unpublished data). Similarly, not all HIV-infected patients develop HIV-associated nephropathy, which is mainly observed in people of African American origin [23]. Interestingly, collapsing FSGS in some settings (including HIV and lupus) is closely related to the genetic expression of APOL1 G1 and/or G2 risk variants [24] and those variants are known to be a risk factor of CKD [25]. Both our patients were of African origin and both harboured a risk variant combination (i.e. homozygosity for G1 and G1/G2 compound heterozygosity) and had histologic evidence of chronicity compatible with an subclinical nephroangiosclerosis. Similar to HIV infection, SARS-CoV-2 infection might unmask APOL1-conferred genetic susceptibility to podocyte injury, and thus collapsing FSGS. SARS-CoV-2 likely acts as a ‘second hit’ on a subclinical nephropathy related to high BP and APOL1 risk variants and cytokine storm exacerbated this prior injury, leading to collapsing FSGS. We therefore expect that COVID-19 patients with high-grade proteinuria but without at-risk APOL1 variants will not display collapsing FSGS, explaining why this pattern was not reported in Chinese patients.
This study has a few limitations, including the lack of electron microscopy or immunohistochemistry for viral particles. However, we performed SARS-CoV-2 RT-PCR assay on blood, urine and renal tissues, with negative results, which favours indirect effects.
CONCLUSION
AKI and high-grade proteinuria are severe complications of COVID-19. Glomerular and tubulointerstitial lesions represent a peculiar renal injury pattern. Although our findings do not definitely rule out a direct infection of kidney cells by SARS-CoV-2, COVID-19-related collapsing FSGS appears to be related to a viral-induced inflammatory response against a peculiar genetic background. SARS-CoV-2 likely acts as a ‘second hit’ that, when combined with APOL1 at-risk variants, results in collapsing FSGS. Given that SARS-CoV-2 has now spread to almost all regions worldwide (notably including the USA and sub-Saharan Africa), our results suggest that kidney disease is, along with respiratory distress, a major risk in the COVID-19 pandemic.
ACKNOWLEDGEMENTS
We thank Dr Nicolas Pallet for APOL1 genotyping.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Chen T, Wu D, Chen H. et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu D, Zhang H, Zhou W. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med2020; http://link.springer.com/10.1007/s00134-020-06026-1 (2 April 2020, date last accessed) [DOI] [PMC free article] [PubMed]
- 3. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int2020; https://linkinghub.elsevier.com/retrieve/pii/S0085253820302556 (2 April 2020, date last accessed) [DOI] [PMC free article] [PubMed]
- 4. Zhen L. Caution on kidney dysfunctions of COVID-19 Patients. medRxiv 2020; https://www.medrxiv.org/content/10.1101/2020.02.08.20021212v2
- 5. Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int2020; https://linkinghub.elsevier.com/retrieve/pii/S0085253820303690 (13 April 2020, date last accessed) [DOI] [PMC free article] [PubMed]
- 6. D’Agati VD, Alster JM, Jennette JC. et al. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 2013; 8: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rao TKS, Filippone EJ, Nicastri AD. et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 1984; 310: 669–673 [DOI] [PubMed] [Google Scholar]
- 8. Corman VM, Landt O, Kaiser M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020; https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.3.2000045 (20 April 2020, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu KH, Tsang WK, Tang CS. et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67: 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cha R, Joh J-S, Jeong I. et al. Renal complications and their prognosis in korean patients with middle east respiratory syndrome-coronavirus from the central MERS-CoV designated hospital. J Korean Med Sci 2015; 30: 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bo D, Wang C, Wang R et al Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv 2020; 10.1101/2020.03.04.20031120 [DOI]
- 12. Larsen CP, Bourne TD, Wilson JD. et al. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep2020; https://linkinghub.elsevier.com/retrieve/pii/S2468024920311724 (16 April 2020, date last accessed) [DOI] [PMC free article] [PubMed]
- 13. Bruggeman LA, Ross MD, Tanji N. et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 2000; 11: 2079–2087 [DOI] [PubMed] [Google Scholar]
- 14. Moudgil A, Nast CC, Bagga A. et al. Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int 2001; 59: 2126–2133 [DOI] [PubMed] [Google Scholar]
- 15. Yeung M-L, Yao Y, Jia L. et al. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat Microbiol. 2016; 1:16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abid Q, Best Rocha A, Larsen CP. et al. APOL1-associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-associated vasculopathy with onset in infancy (SAVI). Am J Kidney Dis 2020; 75: 287–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Markowitz GS, Nasr SH, Stokes MB. et al. Treatment with IFN-α, -β, or -γ is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2010; 5: 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kopp JB. Expanding the spectrum of APOL1-related renal disease: de novo collapsing glomerulopathy following kidney transplant. Kidney Int 2018; 94: 1048–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNicholas BA, Nelson PJ.. Immunity unmasks APOL1 in collapsing glomerulopathy. Kidney Int 2015; 87: 270–272 [DOI] [PubMed] [Google Scholar]
- 20. Besse W, Mansour S, Jatwani K. et al. Collapsing glomerulopathy in a young woman with APOL1 risk alleles following acute parvovirus B19 infection: a case report investigation. BMC Nephrol 2016; 17: 125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah PB, Cooper JE, Lucia MS. et al. APOL1 polymorphisms in a deceased donor and early presentation of collapsing glomerulopathy and focal segmental glomerulosclerosis in two recipients. Am J Transplant 2016; 16: 1923–1927 [DOI] [PubMed] [Google Scholar]
- 22. D'Agati VD, Kaskel FJ, Falk RJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–2411 [DOI] [PubMed] [Google Scholar]
- 23. Rosenberg AZ, Naicker S, Winkler CA. et al. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol 2015; 11: 150–160 [DOI] [PubMed] [Google Scholar]
- 24. Salvatore SP, Barisoni LMC, Herzenberg AM. et al. Collapsing glomerulopathy in 19 patients with systemic lupus erythematosus or lupus-like disease. Clin J Am Soc Nephrol 2012; 7: 914–925 [DOI] [PubMed] [Google Scholar]
- 25. Foster MC, Coresh J, Fornage M. et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 2013; 24: 1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]