Abstract
The altered metabolic program of cancer cells facilitates their cell autonomous proliferation and survival. In normal cells, signal transduction pathways control core cellular functions, including metabolism, to couple signals from exogenous growth factors, cytokines, or hormones to adaptive changes in cell physiology. The ubiquitous, growth factor-regulated PI3K-AKT signaling network has diverse downstream effects on cellular metabolism through either direct regulation of nutrient transporters and metabolic enzymes or the control of transcription factors that regulate the expression of key components of metabolic pathways. Aberrant activation of this signaling network is one of the most frequent events in human cancer and serves to disconnect the control of cell growth, survival, and metabolism from exogenous growth stimuli. Here, we discuss our current understanding of the molecular events controlling cellular metabolism downstream of PI3K and AKT, and how it couples two major hallmarks of cancer, growth factor independence through oncogenic signaling and metabolic reprogramming to support cell survival and proliferation.
[H1] Introduction
The phosphoinositide 3-kinase (PI3K)-AKT pathway is the most commonly activated pathway in human cancers1. Under physiological conditions, this pathway is activated in response to insulin, growth factors, and cytokines and regulates key metabolic processes, including glucose metabolism, biosynthesis of macromolecules, and maintenance of redox balance to support both systemic metabolic homeostasis and the growth and metabolism of individual cells. Oncogenic activation of the PI3K-AKT pathway in cancer cells reprograms cellular metabolism by augmenting the activity of nutrient transporters and metabolic enzymes, thereby supporting the anabolic demands of aberrantly growing cells. Understanding how the PI3K-AKT pathway governs metabolic networks in normal cells and how this control is altered in cancer cells could reveal metabolic vulnerabilities to inform new therapeutic strategies, which is underscored by the “druggable” nature of metabolic enzymes. Here, we review the physiological functions of the PI3K-AKT network in controlling metabolic networks and the potential consequences from oncogenic PI3K-AKT signaling leading to dysregulation of these key metabolic control points in cancer cells and tumors.
Signal transduction networks are the cellular lines of communication, allowing cells to perceive and relay signals, including those from the extracellular environment, to downstream targets that suitably adapt cellular functions to maintain cell, tissue, and organismal homeostasis. The PI3K-AKT signaling network is activated downstream of receptor tyrosine kinases (RTKs), cytokine receptors, integrins, and G protein-coupled receptors (GPCRs) and plays a central role in promoting cell survival and growth2. Class Ia PI3K exists as heterodimers of a catalytic subunit (p110α, β, or δ) associated with a regulatory subunit (p85α or p85β, or shorter variants thereof), while class Ib is comprised of the catalytic subunit p110γ associated with the regulatory subunit p1013. Class Ia PI3K is activated upon engagement of SH2 domains within the regulatory subunits with phospho-tyrosine residues on activated receptors (e.g., RTKs or cytokine receptors) or adaptor proteins, whereas class 1b is activated by GPCRs. Activation of PI3K at the plasma membrane stimulates phosphorylation of its phospholipid substrate phosphatidylinositol 4,5-bisphosphate (PIP2) to produce the second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3) (Fig. 1a). PI3K signalling is attenuated by phosphatase and tensin homolog (PTEN), which dephosphorylates PIP3 to regenerate PIP2. PIP3 accumulation at the plasma membrane, and perhaps other intracellular membranes, creates docking sites to recruit downstream effector proteins that contain a subclass of pleckstrin homology (PH) domain that specifically engage this lipid species2. One such protein is the serine-threonine kinase AKT (also known as protein kinase B or PKB), which upon PIP3 binding is subsequently phosphorylated by phosphoinositide-dependent protein kinase 1 (PDK1) at T308, an event essential for kinase activation, and by mechanistic target of rapamycin (mTOR) complex 2 (mTORC2) at S473, which further increases the activity of AKT4–6 (Fig. 1a). There are three isoforms of AKT (AKT1, AKT2, and AKT3), which are all activated in this manner. AKT1 and AKT2 are broadly expressed, with AKT2 being particularly important in insulin-responsive metabolic tissues, while AKT3 is more restricted in its tissue distribution, being highest in the brain7,8. Active AKT phosphorylates a large and diverse array of downstream substrates, the majority of which appear to be redundantly regulated by the three AKT isoforms (hereon referred to as AKT unless an isoform-specific function is noted). AKT-mediated phosphorylation of these protein targets serves to influence a variety of cell biological functions, including cell growth, proliferation, survival and, as detailed here, metabolism8.
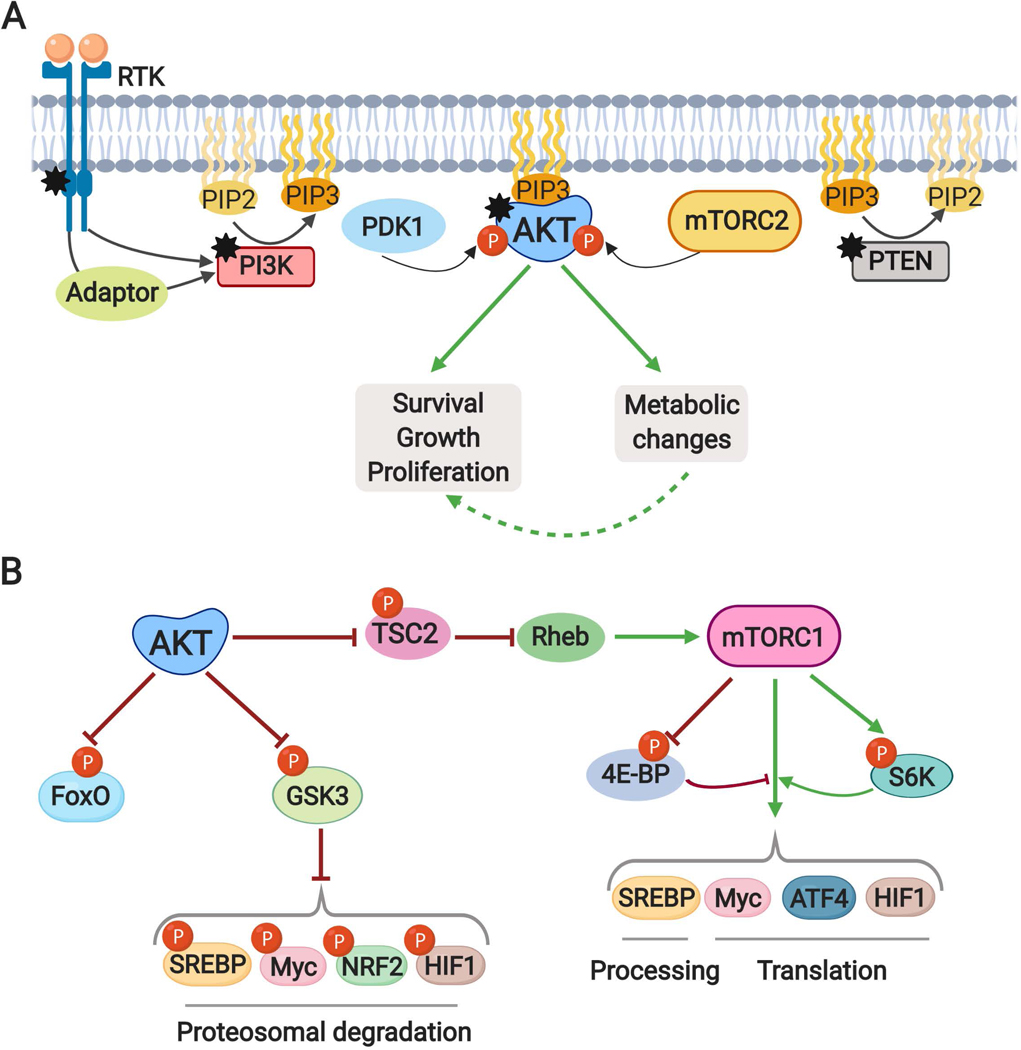
Fig. 1: The PI3K-AKT pathway and its major downstream effectors.
A. Mechanisms of AKT activation. Receptor tyrosine kinase (RTK) activation and tyrosine phosphorylation of its cytosolic domain or of scaffolding adaptors create binding sites that recruit the lipid kinase PI3K to the plasma membrane. PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to produce phosphatidylinositol 3,4,5-trisphosphate (PIP3), which can be dephosphorylated back to PIP2 by the lipid phosphatase PTEN. PIP3 acts as a second messenger to recruit the serine/threonine protein kinase AKT to the plasma membrane, where it is fully activated through phosphorylation at T308 and S473 by the PDK1 and mTORC2 protein kinases, respectively. AKT signaling serves to promote cell survival, growth, and proliferation, in part, by inducing various changes to cellular metabolism. Coloured hexagons denote common points of activation and inactivation by cancer-associated mutations. “P” indicates protein phosphorylation events.
B. AKT controls cellular metabolism, in part, through three key downstream substrates: TSC2, GSK3, and the FOXO transcription factors. AKT phosphorylates and inhibits TSC2, a component of the TSC complex, to activate mTORC1 by relieving TSC complex-mediated inhibition of Rheb. S6K1 and 4EBP1 are canonical downstream targets of mTORC1, which together with other targets, serve to stimulate the processing and activation of the SREBP family of transcription factors and the mRNA translation of the MYC, HIF1α, and ATF4 transcription factors. GSK3-mediated phosphorylation of the transcription factors SREBP, MYC, NRF2, and HIF1α targets them for ubiquitination and proteasomal degradation.
PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog; PDK1, phosphoinositide-dependent protein kinase 1; mTORC2, mechanistic target of rapamycin (mTOR) complex 2; mTORC1, mTOR complex 1; TSC2; tuberous sclerosis complex 2; GSK3, Glycogen synthase kinase 3; FOXO, forkhead box O; Rheb, Ras homolog enriched in brain; S6K1, Ribosomal protein S6 kinase 1; 4EBP1, eukaryotic translation initiation factor 4E binding protein, SREBP: Sterol regulatory element binding proteins; HIF1, Hypoxia-inducible factor 1, ATF4, activating transcription factor 4, NRF2, nuclear factor erythroid 2-related factor 2.
Genetic events leading to growth factor-independent activation of the PI3K-AKT pathway are amongst the most frequently occurring drivers of human cancer. Common alterations in cancer include 1) activating mutations in PIK3CA, the p110α catalytic subunit of PI3K, which is the most frequently mutated single oncogene found in analyses across cancer lineages1; 2) loss of function mutations and deletions in PTEN, the second most mutated tumor suppressor gene (following p53); 3) amplification and activation of specific PI3K-activating RTKs, including EGFR and HER2; 4) amplification and gain-of-function missense mutations in one of the three isoforms of AKT1,9–11 (Fig. 1a).
[H1] Key effectors controlling cell metabolism
While there are many downstream effectors of both PI3K and Akt that alter the function of both normal and cancer cells, we focus here on those that influence cellular metabolism. In a cell intrinsic manner, the metabolic functions of Akt serve to support its canonical functions in promoting cell survival, growth, and proliferation (Fig. 1a). AKT signaling alters metabolism either directly, through phosphorylation-mediated regulation of metabolic enzymes, or indirectly, through control of various transcription factors. Phosphorylation of metabolic enzymes allows for acute changes in the activity of metabolic pathways and the directionality of metabolic flux. Whereas, longer-term changes in cellular metabolism are often achieved through control of gene expression programs. While AKT directly phosphorylates several metabolic enzymes or regulators of nutrient transport, it also activates a few key downstream effectors that play a major role in cellular metabolic reprogramming, including mTOR complex 1 (mTORC1), glycogen synthase kinase 3 (GSK3), and members of the forkhead box O (FOXO) family of transcription factors8 (Fig. 1b).
mTOR is a serine/threonine kinase that functions as the catalytic subunit of two multi-protein complexes, mTORC1 and mTORC2, with distinct subunit composition, substrate specificity, and functions12. Both mTORC1 and mTORC2 can be activated by PI3K signaling, with mTORC2 being an upstream regulator of AKT and mTORC1 being a downstream effector. The primary mechanism by which AKT activates mTORC1 is through phosphorylation of the tuberous sclerosis complex 2 (TSC2) protein, which as a component of the TSC protein complex acts as a GTPase-activating protein (GAP) to inhibit the Ras-related small G protein RHEB13. The AKT-mediated phosphorylation of TSC2 disrupts colocalization of the TSC complex with RHEB, thereby allowing accumulation of RHEB-GTP, which binds to and activates mTORC114 (Fig. 1b). The AKT-mediated stimulation of mTORC1 acts in parallel to nutrient- and energy-sensing mechanisms that also control the activation state of mTORC1 and, as discussed below, serves as a key point of regulation for anabolic metabolism and cell growth13.
Glycogen synthase kinase 3 (GSK3) was the first identified AKT substrate and is a key regulator of cellular metabolism, established originally for its role in blocking glycogen synthesis via phosphorylation and inhibition of its namesake substrate glycogen synthase15,16. GSK3 is active under basal conditions and is inhibited in response to growth factors and insulin via AKT-mediated phosphorylation15,17 (Fig. 1b). GSK3 has many downstream substrates, the phosphorylation of which often exerts inhibitory control over these targets18. Among these are a several transcription factors that regulate the metabolism of both normal and cancer cells downstream of PI3K-AKT signaling. GSK3-mediated phosphorylation of these factors marks them for ubiquitination and proteasomal degradation (Fig. 1b).
Another canonical substrate of AKT is the FOXO family of transcription factors (FOXO1, FOXO3A, FOXO4), which upon phosphorylation are sequestered from the nucleus, thus preventing expression of their target genes19,20 (Fig. 1b). Given the established FOXO gene expression program, which includes numerous suppressors of growth, proliferation, and survival, together with specific metabolic enzymes, the AKT-mediated inhibition of FOXO has been implicated in various aspects of cancer development and progression21.
[H1] Control of glucose metabolism
Altered glucose metabolism is perhaps the most common metabolic change distinguishing cancer cells from their cell of origin. This metabolic feature, characterized by an increased rate of glucose uptake and its glycolytic conversion to lactate even under oxygen-rich conditions, was originally described nearly one-hundred years ago by Otto Warburg22 and is referred to as aerobic glycolysis or the Warburg effect23. Glycolysis, in addition to producing ATP, provides metabolic intermediates as substrates for metabolic pathways that branch off of glycolysis and support biosynthetic processes for production of protein, lipids, and nucleotides, required for cell growth and proliferation. Under aerobic glycolysis, the predominant diversion of pyruvate to lactate, rather than its entry into the mitochondria for oxidation, also serves a key role in redox homeostasis [G] by regenerating NAD+. How the genetic events leading to cellular transformation and cancer promote the Warburg effect is still being elucidated. However, the PI3K-AKT pathway can control several aspects of this metabolic program (Fig. 2).
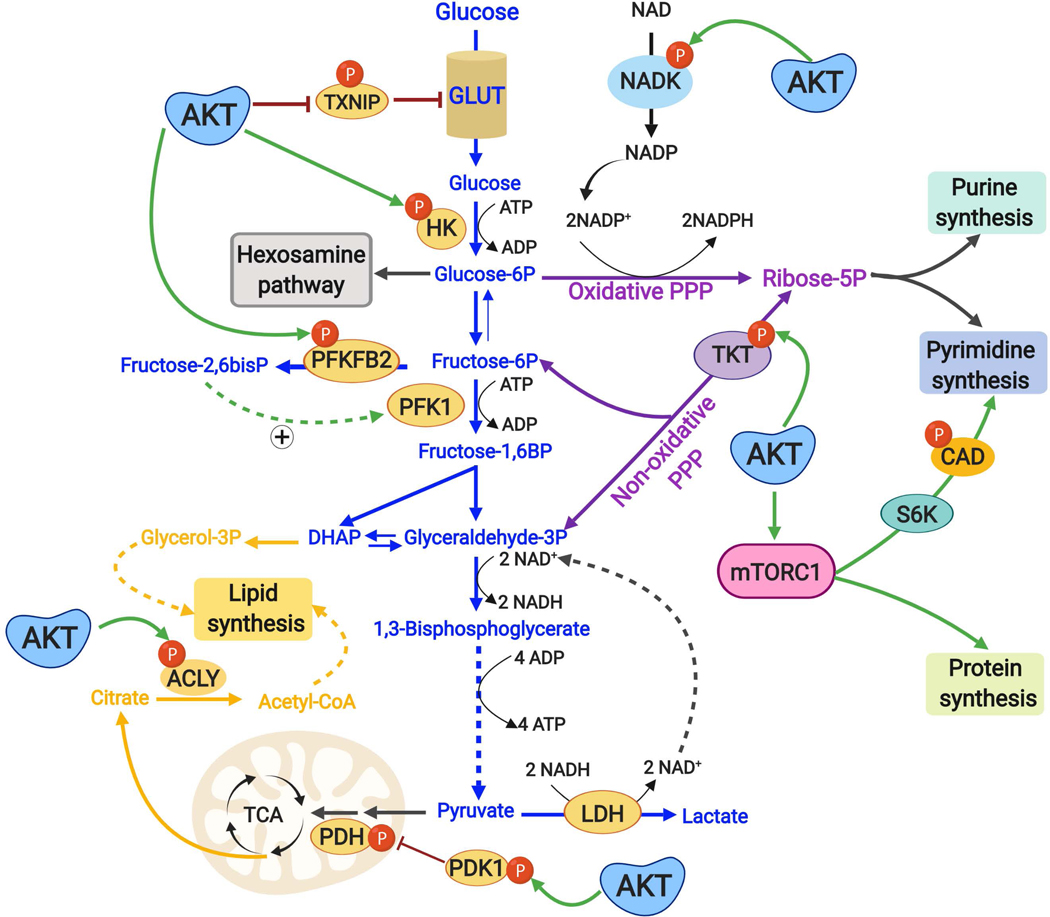
Fig. 2: Direct post-translational regulation of metabolic enzymes and processes downstream of the PI3K-AKT pathway.
AKT stimulates metabolic changes that contribute to anabolic metabolism by directly phosphorylating key metabolic enzymes. AKT promotes plasma membrane localization of the glucose transporter GLUT1 and increased glucose uptake by directly phosphorylating and inhibiting TXNIP, a protein that promotes endocytosis of GLUT1. AKT signaling also promotes retention and metabolic activation of the newly acquired glucose by activating HK2, which phosphorylates glucose to generate glucose 6-phosphate, which cannot be transported out of the cell by GLUT1 and is the entry metabolite for the hexosamine pathway, the oxidative pentose phosphate pathway (PPP), and glycolysis. AKT enhances flux into glycolysis through phosphorylation and activation of PFKFB2, which produces fructose-2,6-bisphosphate, an allosteric activator of the rate-limiting glycolytic enzyme PFK1, which commits the glucose-derived carbon to glycolysis. Both the oxidative and non-oxidative PPP, which branch off of glycolytic intermediates, generate ribose-5-phosphate that serves as the sugar moiety for purine and pyrimidine nucleotides. AKT phosphorylates and activates the non-oxidative PPP enzyme TKT, thereby contributing to ribose-5-phosphate production for nucleotides. AKT also phosphorylates and increases the activity of NADK, which catalyses the phosphorylation of NAD+ to generate NADP+, a limiting substrate for the oxidative PPP, which generates two molecules of the reducing cofactor NADPH through its oxidation of glucose 6-phosphate. Downstream of AKT, mTORC1 activation acutely stimulates de novo pyrimidine synthesis through an S6K-dependent phosphorylation of the pyrimidine synthesis enzyme CAD. Pyruvate, the end product of glycolysis, is either converted to lactate by LDH, which regenerates NAD+ needed for sustained glycolysis, or can enter the mitochondria and TCA cycle for oxidation initiated by the pyruvate dehydrogenase (PDH) complex, the activity of which can be inhibited by pyruvate dehydrogenase kinase (PDK). AKT phosphorylates PDK and promotes its inhibition of PDH, thus favouring the LDH reaction [Note: this phosphorylation is believed to occur within the mitochondria]. AKT directly regulates lipid synthesis through phosphorylation of ACLY, which generates acetyl-CoA in cytosol from the TCA cycle-derived citrate.
TXNIP, thioredoxin-interacting protein; GLUT1, glucose transporter 1; PPP, Pentose Phosphate Pathway; PFKFB2, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase; PFK1, phosphofructokinae 1; TKT, transketolase; NADK, NAD kinase; mTORC1, mTOR complex 1; CAD, carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase, S6K1, ribosomal protein S6 kinase 1; NAD+, nicotinamide adenine dinucleotide, NADP+, NAD+ phosphate, LDH, lactate dehydrogenase.
AKT activation has been shown to be sufficient to promote aerobic glycolysis24,25. Expression of constitutively-active AKT results in a growth factor-independent increase in glucose uptake and glycolytic rate24–29. This AKT-mediated induction of aerobic glycolysis can render cancer cells dependent on glucose for survival25,29. Multiple control points in glycolysis have been found to be regulated by PI3K-AKT signaling, and these include both acute, post-translational modifications and more prolonged transcriptional effects on glucose transporters and glycolytic enzymes (Fig. 2, 3).
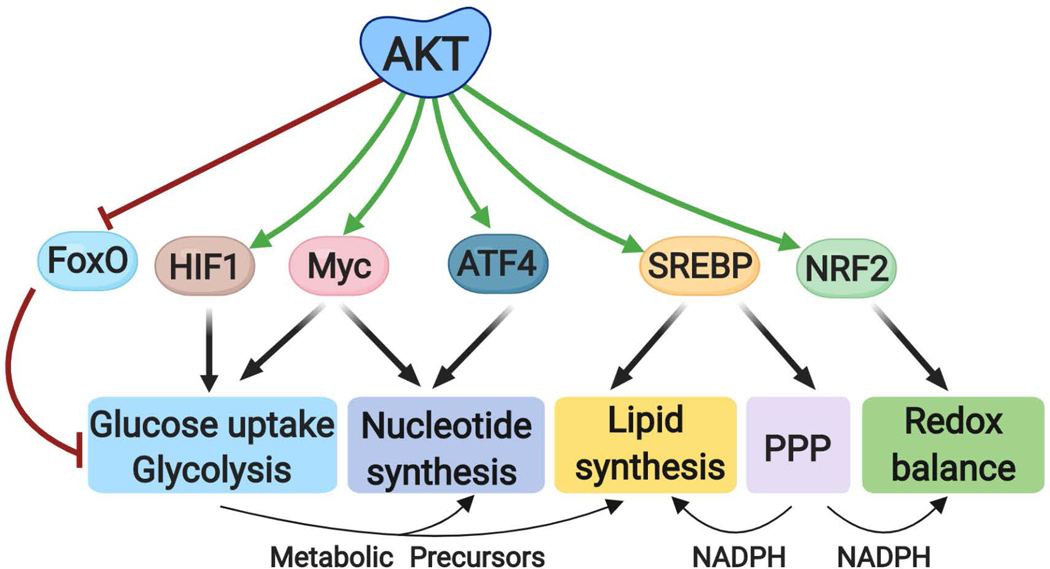
Fig. 3: Transcriptional control of metabolic processes downstream of AKT signaling.
AKT regulates metabolism through a number of downstream transcription factors that control the expression of genes encoding metabolic enzymes. FOXO, HIF1 and MYC regulate the expression of glucose transporters and glycolytic enzymes, and intermediates of glycolysis contribute to nucleotide and lipid synthesis. MYC and ATF4 induce the expression of enzymes contributing to nucleotide synthesis. SREBP isoforms globally induce the expression of lipogenic enzymes, as well as enzymes in the oxidative PPP, which can provide NADPH as reducing equivalents for lipid synthesis and redox. NRF2 regulates redox homeostasis. FOXO, forkhead box O; HIF1, Hypoxia-inducible factor 1; ATF4, activating transcription factor 4, SREBP: Sterol regulatory element binding proteins; NRF2, nuclear factor erythroid 2-related factor 2 and NADPH, nicotinamide adenine dinucleotide phosphate (reduced).
[H2] Direct regulation of glucose uptake and glycolysis
Glucose is a primary carbon and energy source in all organisms. The controlled uptake of glucose into cells is mediated by the glucose transporter family (GLUTs)30. Among these, GLUT1 is ubiquitously expressed and most frequently elevated in cancer31, while GLUT4 is mainly expressed in insulin-responsive muscle and adipose tissue to function in plasma glucose clearance after food intake32. AKT promotes glucose uptake through both GLUT1 and GLUT4. Studies of insulin-stimulated glucose uptake found that AKT2 associates with vesicles containing GLUT4, and AKT2 induces trafficking of GLUT4 from these vesicles to the plasma membrane32,33. A major mechanism underlying this regulation is the AKT -mediated phosphorylation and inhibition of TBC1D4 (also known as AS160), a GAP for Rab GTPases that promote GLUT4 trafficking34,35. However, this mechanism appears to be specific for GLUT4 and, thus, is unlikely to play a major role in glucose uptake into cancer cells, which predominantly use GLUT1. Studies in both normal and transformed hematopoietic cells have demonstrated that the cytokine-stimulated translocation of GLUT1 to the plasma membrane is mediated through AKT activation24,36,37. Recent studies have implicated thioredoxin-interacting protein (TXNIP) as a direct AKT substrate in the regulation of both GLUT1 and GLUT4 trafficking38 (Fig. 2). TXNIP promotes endocytosis of GLUT1 and inhibits glucose uptake39,40. AKT phosphorylates and inhibits TXNIP, resulting in a rapid increase in GLUT1 and GLUT4 at the plasma membrane and enhanced glucose uptake in various cell types (mouse embryonic fibroblasts and 3T3-L1 adipocytes) and mouse tissues (skeletal muscle, white adipose tissue, and liver)38. Regulation of TXNIP by oncogenic PI3K-AKT signaling has also been suggested to enhance aerobic glycolysis in non-small cell lung cancer cell lines41, but the significance of the AKT-TXNIP-GLUT1 axis in the widely observed increase in tumor glucose uptake in vivo remains to be determined42.
In addition to glucose uptake, AKT controls key steps in glycolysis through phosphorylation and activation of specific glycolytic enzymes (Fig. 2). Following its transport into cells, glucose becomes activated to enter metabolic pathways through the action of hexokinases that phosphorylate glucose to form glucose-6-phosphate, which cannot be transported out of the cell. AKT activation has been found to promote hexokinase 2 (HK2) activity, at least in part, by increasing its association with voltage-dependent anion channel (VDAC) at the outer mitochondrial membrane 27,43. This mechanism has been proposed to provide a rich source of mitochondria-derived ATP for rapid and sustained HK2-mediated glucose phosphorylation and has also been found to promote cell survival by preserving mitochondrial integrity44–46. HK2 expression is elevated in many human cancers, including ovarian, colorectal, pancreatic, glioblastoma, and liver cancer, and its activity has been found to be critical for tumorigenesis and metastasis in various mouse models47,48–50. HK2 has also been exploited as a metabolic liability in mouse tumor models displaying hyperactive AKT signaling, such PTEN-deficient prostate cancer, where HK2 deletion attenuates tumor growth50–53. HK2, and other hexokinases, can be competitively inhibited by 2-deoxy-D-glucose, and HK2 has attracted a lot of interest as a potential target for cancer treatment54, although more specific and potent inhibitors will likely be needed. The HK2 product, glucose-6-phosphate can then enter one of three metabolic pathways: the hexosamine pathway, the pentose phosphate pathway, or glycolysis.
AKT signaling indirectly stimulates the activity of phosphofructokinae 1 (PFK1), the first committed step of glycolysis. AKT phosphorylates and activates 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB2)55, a key enzyme that catalyses the interconversion of fructose-6-phosphate and fructose-2,6-bisphosphate, with the latter metabolite serving as a potent allosteric activator of PFK156. The AKT-mediated phosphorylation of PFKFB2 increases its synthesis of fructose 2,6-bisphosphate leading to enhanced PFK1-driven glycolytic flux (Fig. 2). Akt has also been found to activate the related isoform PFKFB3 through phosphorylation of a conserved residue that it also phosphorylates in PFKFB257, but it is worth noting that the AKT site on PFKFB3 lacks a critical sequence feature found in other established Akt substrates58. Fructose-2,6-bisphosphate levels have been reported to be markedly increased in mouse models of lung and mammary carcinomas compared to non-malignant cells, perhaps promoting high glycolytic flux 59,60. However, the role of oncogenic AKT-mediated phosphorylation of PFKFB2 or PFKFB3 in the induction of aerobic glycolysis in various cancers remains to be elucidated.
PI3K signaling has also been found to potentiate glycolytic flux in an AKT-independent manner. Growth-factor stimulation of PI3K induces a Rac-dependent release of the glycolytic enzyme aldolase A from an actin-bound, low-activity state to increase glycolytic flux61. High aldolase A expression in cancer is correlated with poor patient prognosis62,63. While the role of PI3K-stimulated aldolase A release from the actin cytoskeleton in tumorigenesis has yet to be established, studies have reported that targeting aldolase A in tumor xenograft models can attenuate tumor growth64,65.
[H2] Transcriptional regulation of glucose uptake and glycolysis
In addition to acute regulation of glycolytic enzymes, the PI3K-AKT pathway also promotes sustained aerobic glycolysis through an increase in protein levels of glucose transporters and glycolytic enzymes mediated by control of downstream transcription factors (Fig. 3). Hypoxia-inducible factor 1 (HIF1α), which is degraded in an oxygen-dependent manner and stabilized under conditions of hypoxia, induces the expression of GLUT1 and nearly all the enzymes of glycolysis66. As part of the adaption to hypoxia, HIF1α also activates expression of lactate dehydrogenase 1 (LDH1) and pyruvate dehydrogenase kinase 1 (PDK1), which together function to channel pyruvate to lactate and away from its oxidation into acetyl-CoA for entry into the mitochondrial tricarboxylic acid (TCA) cycle.
AKT has also been proposed to phosphorylate and increase the activity of PDK1 during hypoxia, thus further facilitating a switch towards glycolysis to support cancer cell proliferation under these conditions67 (Fig. 2). However, it is unknown how activated AKT in the cytosol gains access to PDK1 in the mitochondria and whether AKT exerts this direct regulation of PDK1 in response to oncogenic PI3K signaling. Importantly, several studies have found that the activation of mTORC1 downstream of AKT signaling in cell and tumor models, including prostate cancer, leads to an increase in HIF1α protein levels, expression of gene targets, and increased glucose uptake and glycolytic conversion to lactate, even under normal oxygen concentrations (i.e., normoxia)68–71. HIF1α remains unstable under such conditions, but its protein levels increase due to enhanced, mTORC1-regulated translation of the HIF1A mRNA 68,72,73. It is worth noting that hypoxia is a much stronger inducer of HIF1α, and the PI3K-mTOR pathway is not involved in this oxygen-mediated regulation of HIF1α protein stability. The importance of HIF1α as a downstream effector of AKT signaling in cancer development and progression is unclear, and one study found this transcription factor to be dispensable for AKT-driven tumor growth in a hepatoma xenograft model74. However, the normoxic upregulation of the HIF1α program downstream of PI3K-mTOR signaling is likely to contribute to the induction of aerobic glycolysis in cancer cells and may provide metabolic flexibility [G] in growing tumors that facilitates regional adaptation to nutrient and oxygen fluctuations.
The transcription factor MYC induces expression of numerous genes that promote cell growth and proliferation, and is one of the most frequently altered oncogenes in human cancers75. MYC induces expression of the major glucose transporters and most glycolytic enzymes and can drive aerobic glycolysis75. In contrast to HIF1α, MYC activation does not appear to lead to preferential directing of glycolysis-derived pyruvate toward lactate and away from mitochondrial respiration. Instead, many MYC gene targets function to enhance mitochondrial metabolism75. MYC activity is generally dictated by its abundance, and it is increased downstream of PI3K-AKT signalling through a combination of transcriptional, translational, and posttranslational mechanisms75. For example, mTORC1 signaling enhances the translation of MYC76,77, while AKT promotes MYC stabilization by inhibiting GSK3, which phosphorylates MYC and targets it for proteasomal degradation78–80. The FOXO transcription factors have been found to suppress the expression of glycolytic genes in some settings through an antagonistic effect on MYC function81,82–84. Activation of AKT relieves the inhibitory effects of FOXO transcription factors on glycolytic enzymes and MYC, thereby further enhancing glycolysis83. However, it is important to note that MYC activation downstream of the PI3K-AKT pathway is likely to be context dependent in cancer. For instance, PI3K inhibitors have failed to reduce MYC levels in various cancer models, including colorectal cancer, acute myeloid leukemia, and multiple myeloma, likely due to dominant regulatory inputs from the RAS-ERK pathway to MYC that are active in many cancer settings85–87. Thus, the vast metabolic program downstream of MYC should not be universally equated to PI3K-Akt signalling.
This collective work demonstrates the central role that the PI3K-AKT pathway plays in promoting glucose uptake and glycolysis under both physiological conditions and in cancer. In the setting of oncogenic PI3K-AKT signaling, these combined regulatory mechanisms are likely to drive the constitutive induction of aerobic glycolysis. This common feature of cancer cells facilitates metabolic flux into pathways that branch off of glycolysis and contribute to the synthesis of cellular macromolecules23, and these biosynthetic processes are also further controlled downstream of Akt. While mitochondrial metabolism through the TCA cycle has also emerged as a process supporting the energetic and biosynthetic demands of proliferating cancer cells88,89, defined functions of the PI3K-AKT pathway in direct control of the TCA cycle have not been established. It is possible that under conditions of aerobic glycolysis, the PI3K-AKT pathway might promote anaplerotic metabolism [G] to sustain TCA cycle flux, for instance, by promoting glutaminolysis [G] via MYC activation77,90.
[H1] Control of anabolic metabolism
Proliferating cells need to double their protein, lipid, and nucleotide content with each cell division. In order to meet this biosynthetic demand, cells stimulate anabolic processes [G] to drive the production of these macromolecules. Here, we discuss the role of AKT, mTORC1, and MYC in inducing a PI3K-driven anabolic program in cancer.
[H2] De novo lipid synthesis
Aberrant activation of lipid biosynthesis is a common feature of cancer cells91. While the majority of cells in our body rely on the uptake of fatty acids and lipoproteins from the bloodstream to fulfill their lipid requirements, cancer cells activate de novo lipid biosynthesis to facilitate the generation of cellular membranes and support their increased growth and proliferation92. Both sterols and fatty acids are synthesized from cytosolic acetyl-CoA, which is produced from the tricarboxylic acid (TCA) cycle intermediate citrate via ATP-citrate lyase (ACLY) or from acetate via acetyl-CoA synthetase. The PI3K-AKT pathway induces de novo lipid synthesis through both post-translational and transcriptional mechanisms.
AKT can initiate de novo lipid synthesis by directly phosphorylating ACLY93, thereby increasing its activity94 and boosting the production of cytosolic acetyl-CoA to be used for sterol and fatty acid synthesis, as well as protein acetylation reactions (Fig. 2). The AKT-ACLY axis has been reported to promote tumor growth and to globally influence histone acetylation95,96. In addition to its oncogenic regulation through the PI3K-AKT pathway, ACLY is frequently overexpressed in various human cancers, and inhibition of ACLY diminishes cancer cell proliferation both in vitro and in vivo, making this enzyme a potentially attractive target for cancer therapy97–99. Owing to its key role in lipid biogenesis, inhibitors of ACLY, originally developed for metabolic disorders such as hypercholesterolemia and type-2 diabetes100,101, are being considered as potential anti-cancer drugs102,103.
AKT signaling also promotes de novo lipid synthesis through activation of the SREBP family of transcription factors (SREBP1a, SREBP1c, and SREBP2), which induce the expression of nearly all enzymes of fatty acid and sterol synthesis, including ACLY104,105. The SREBPs exist as inactive endoplasmic reticulum (ER) transmembrane proteins that must traffic to the Golgi for proteolytic processing to be activated. SREBP processing releases the N-terminal portion of the protein that serves as the mature active form, which then translocates to the nucleus to initiate transcription at sterol response elements contained in the promoters of lipogenic genes and those involved in NADPH production required to support lipid synthesis. AKT activates SREBP through at least two downstream branches. mTORC1 has been shown to stimulate the processing and nuclear translocation of SREBPs through several different proposed mechanisms, leading to the induction of lipogenic gene expression and an increase in de novo lipid synthesis in the liver in response to insulin or in cancer cells downstream of oncogenic PI3K or Ras68,105–108. Once processed, the mature active form of SREBP is targeted for ubiquitin-dependent degradation by GSK3-mediated phosphorylation109,110. Thus, AKT signaling can stimulate the processing of SREBP through mTORC1 activation and promote stability of the processed active SREBP by inhibiting GSK3. Recently, MYC was also reported to cooperate with SREBP to induce lipogenesis and promote cancer growth111. Interestingly, many of the mRNAs induced by SREBP are regulated by the serine/arginine-rich (SR) protein family of splicing factors. The splicing of these mRNAs encoding lipogenic enzymes has been found to be stimulated by mTORC1 signaling through its downstream target S6K, which phosphorylates and activates SR protein kinase 2 (SRPK2) leading to subsequent phosphorylation and activation of the SR proteins and enhanced splicing112. Many factors and enzymes involved in de novo lipid synthesis, including the SREBPs, SRPK2, and the lipogenic enzymes induced by the SREBPs have been found to be elevated in diverse cancer lineages, and these remain potential targets of interest for cancer therapy91,108,113.
[H2] Nucleotide synthesis
Nucleotides, comprised of purines and pyrimidines, are essential building blocks for the synthesis of nucleic acids (RNA and DNA), among other cellular functions114. In contrast to normal, quiescent cells, cancer cells stimulate robust de novo synthesis of nucleotides to accommodate the nucleic acid synthesis required for cell growth and proliferation115,116. The de novo nucleotide synthesis pathways require coordinated input from multiple metabolic pathways, including the pentose phosphate pathway (PPP) and the serine and glycine synthesis pathway (both of which branch off of glycolysis), aspartate synthesis from the TCA cycle intermediate oxaloacetate, one carbon metabolism, and glutamine uptake, to supply the ribose sugar and necessary atoms to form the corresponding pyrimidine and purine bases (Fig. 4a). Thus, it is not surprising that AKT signaling appears to regulate nucleotide synthesis through multiple parallel mechanisms affecting these metabolic inputs.
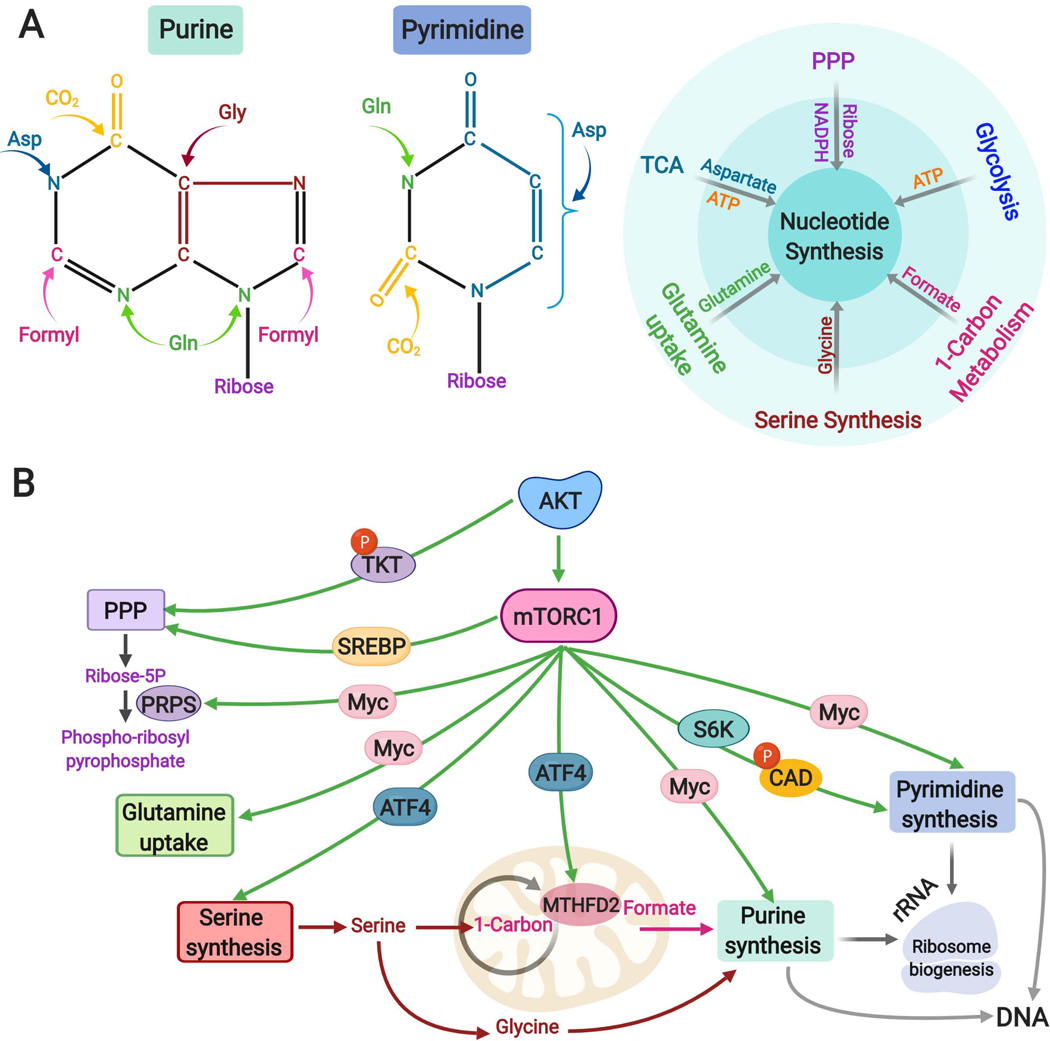
Fig. 4: Regulation of nucleotide metabolism downstream of the AKT-mTORC1 pathway.
A. Left to right: Schematic of purine and pyrimidine nucleotides indicating the donors of carbon and nitrogen atoms that form nucleotides. The small molecules are color coded according to the contributing metabolic pathways from which they are commonly derived, shown schematically to the right.
B. Transcriptional and post-translational mechanisms contributing to de novo nucleotide synthesis downstream of the AKT-mTORC1 pathway. Left to right: AKT-mediated phosphorylation of the non-oxidative PPP enzyme TKT and SREBP-mediated regulation of the oxidative PPP enhance the production of ribose-5-phosphate, which can then be used for nucleotide synthesis by conversion to phospho-ribosyl pyrophosphate through the enzyme PRPS, the levels of which are elevated upon MYC activation. MYC promotes glutamine uptake and stimulates expression of several genes encoding enzymes of both purine and pyrimidine synthesis pathways. In addition to being downstream of mTORC1, SREBP and MYC can also be stabilized via AKT-mediated inhibition of GSK3. ATF4 activation downstream of mTORC1 contributes to enhanced purine synthesis through the induction of serine biosynthesis enzymes and the mitochondrial tetrahydrofolate cycle enzyme MTHFD2, thus supplying both glycine and one-carbon formyl units. mTORC1 acutely stimulates pyrimidine synthesis through the S6K1-mediated phosphorylation of the pyrimidine synthesis enzyme CAD. The newly synthesized purine and pyrimidines are used for the synthesis of RNA, predominantly rRNA for ribosome biogenesis, and DNA in proliferating cells.
NADPH, nicotinamide adenine dinucleotide phosphate (reduced), PPP, Pentose Phosphate Pathway; TKT, transketolase, SREBP: Sterol regulatory element binding proteins; PRPS, phosphoribosyl pyrophosphate synthase; MTHFD2, methylenetetrahydrofolate dehydrogenase 2, ATF4, activating transcription factor 4; mTORC1, mTOR complex 1.
The PI3K-AKT-mTORC1 network promotes glucose carbon flux into both the oxidative and non-oxidative branches of the PPP, thus producing ribose for nucleotide synthesis. As an alternative to entering glycolysis, glucose 6-phosphate, can enter the oxidative PPP through the action of glucose-6-phosphate dehydrogenase (G6PD) to be irreversibly oxidized to produce ribose-5-phosphate. Downstream of AKT signaling, mTORC1 activation enhances oxidative PPP flux, at least in part, via activation of SREBP and its transcriptional induction of G6PD expression68. AKT has also been found to directly activate transketolase (TKT), a key enzyme in the non-oxidative PPP117 (Fig. 4b). Treatment of breast cancer cells with PI3K inhibitors was found to disproportionally reduce glucose flux through the non-oxidative PPP and result in nucleotide depletion and DNA damage118, suggesting a higher dependence on the non-oxidative PPP in this setting. Enzymes in both branches of the PPP, including G6PD and TKT, are overexpressed in cancer, and their inhibition attenuates cell proliferation in various settings, including colorectal, breast, lung, and liver cancer cells119–121. More research is required to develop potent and selective inhibitors of key PPP enzymes and to identify the cancer contexts in which PPP inhibition is a vulnerability122.
AKT exerts transcriptional control of nucleotide synthesis, in part, through its regulation of MYC. MYC drives expression of an array of metabolic genes involved in supplying metabolite precursors for nucleotide synthesis, including glutamine, and directly induces the expression of many of the enzymes of the pyrimidine and purine synthesis pathways123,124 (Fig. 4b). MYC controls both the transcription and translation of phosphoribosyl pyrophosphate (PRPP) synthase 2 (PRPS2), which catalyzes the reaction that commits ribose-5-phosphate to nucleotide synthesis by producing PRPP, and PRPS2 has been found to be a critical effector of MYC-mediated tumorigenesis125,126. Glutamine serves as the major nitrogen source for the synthesis of both pyrimidine and purine bases, and MYC promotes glutamine uptake by inducing the expression of the glutamine transporters SLC1A5 and large amino acid transporter 1 (LAT1), which is a heterodimer composed of SLC7A5 and SLC3A290,127 (Fig. 4b).
As a downstream effector of PI3K-AKT signaling, mTORC1 has also emerged as a major driver of de novo nucleotide synthesis, which it regulates through both posttranslational and transcriptional mechanisms (Fig. 2, 4b). Growth factor signaling to mTORC1 acutely stimulates pyrimidine synthesis through the S6K1-mediated phosphorylation and activation of the first and rate-limiting enzyme in this pathway, carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (CAD), which catalyses the first three steps of pyrimidine biosynthesis128,129 (Fig. 4b). While this phosphorylation is not required for basal CAD activity, it is required for PI3K-AKT signaling to increase metabolic flux through this pathway and increase pyrimidine synthesis. With more delayed kinetics, mTORC1 signaling also induces de novo purine synthesis through transcriptional mechanisms involving MYC, SREBP and ATF4, which stimulate the expression of specific metabolic enzymes in the purine synthesis pathway or pathways that feed metabolites into purine synthesis130. For instance, mTORC1 was found to activate ATF4, in a manner distinct from its canonical activation as part of the integrated stress response, resulting in transcriptional induction of the enzymes catalyzing serine synthesis and its conversion through the mitochondrial tetrahydrofolate (mTHF) cycle to formate, which provides one carbon units essential for building the purine ring130 (Fig 4a,b).
It is interesting to note that in growing cells, including cancer cells, there is a robust increase in ribosome biogenesis. Over half of the mass of the ribosome is comprised of rRNA, which constitutes more than 80% of total cellular RNA. Thus, ribosome biogenesis underlying cell growth places a substantial increased demand on the cell for more nucleotides to support rRNA synthesis. It is well established that mTORC1 and MYC serve as key drivers of ribosome biogenesis in both normal and cancer cells131,132, thereby providing the logic for their parallel induction of nucleotide synthesis. Indeed, the newly synthesized pyrimidine and purine nucleotides produced in response to mTORC1 activation can be traced into rRNA128,130.
While inhibitors of enzymes involved in nucleotide synthesis were the first chemotherapeutics introduced in the 1940s133 and have become part of established cancer therapy regiments, the factors that dictate vulnerability to such inhibitors remain poorly understood. As immune cells, like cancer cells, upregulate de novo nucleotide synthesis to support cell proliferation upon activation, several immunosuppressants that target nucleotide synthesis pathways have been developed for clinical use. The growth of cancer cells in vitro and tumor models in vivo with uncontrolled mTORC1 or MYC activation has been found to be very sensitive to inhibitors of the enzyme inosine monophosphate dehydrogenase (IMPDH), such as mizoribine, which is a widely used immunosuppressant in Asia134,135. Interestingly, IMPDH is required for the synthesis of guanylates, which are disproportionately represented in pre-rRNA (37%). Therefore, the process of rRNA synthesis more rapidly depletes guanylates in cells with active ribosome biogenesis, thereby depriving cells of nucleotides for DNA synthesis, resulting in replication stress and apoptosis134. Thus, it is possible that such agents, despite their immunosuppressive effects, might be effective and selective anti-tumor agents in settings with strong increases in ribosome biogenesis, such as those with active mTORC1 or MYC. Interestingly, widely used chemotherapeutic agents that target nucleotide synthesis, including methotrexate and 6-mercatopurine, have been found to inhibit mTORC1 signaling through a mechanism involving depletion of purine, but not pyrimidine, nucleotides136,137. Whether some of the anti-cancer effects of these agents are due to inhibition of mTORC1 remains to be determined.
[H2] Protein synthesis
The growth program of PI3K-AKT signaling involves a robust increase in protein synthesis, with much of this driven by mTORC1 activation. mTORC1 activation leads to an increase in the protein synthesis capacity of cells through multiple mechanisms13 (Box 1). As a complement to the control of translation by mTORC1, MYC transcriptionally induces the expression of multiple components of the protein synthesis machinery, including ribosomal proteins and translation initiation factors132. MYC can directly stimulate Pol-I and Pol-III to transcribe rDNA and also promotes the expression of rRNA processing enzymes required to produce the mature forms that are assembled into ribosomes132. Interestingly cancers with high MYC are characterized by elevated rates of protein synthesis and ribosome biogenesis, and inhibition of protein synthesis has been shown to confer synthetic lethality in these cancers138,139. Future studies are needed to understand whether cancer cells with oncogenic PI3K signaling share this vulnerability.
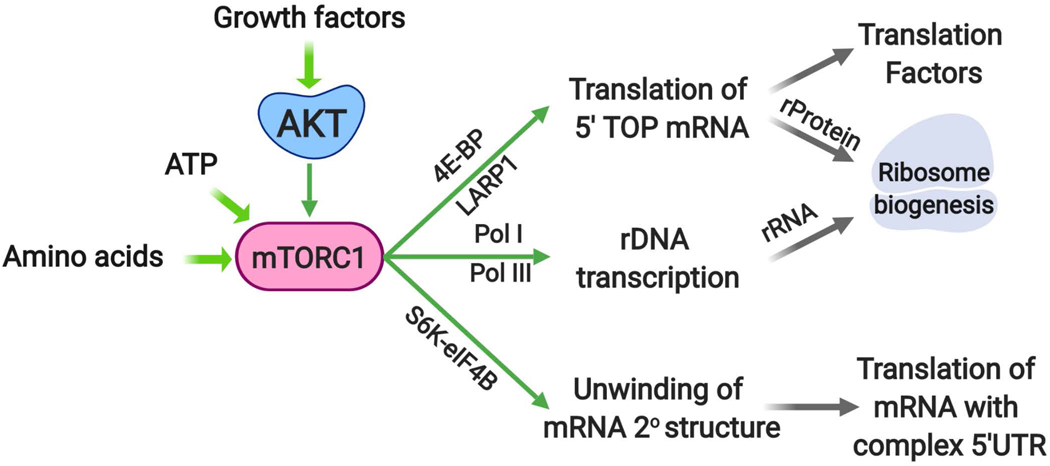
Box 1. Protein synthesis downstream of mTORC1.
Protein synthesis is a highly nutrient-and energy-costly process that is induced downstream of AKT signalling through mTORC113. Much of this regulation occurs through phosphorylation of its two best established substrates, S6K and eukaryotic translation initiation factor 4E (eIF4E) binding protein (4E-BP). The mTORC1-mediated phosphorylation of 4E-BP triggers its release from eIF4E at the 5’-cap of mRNAs, allowing assembly of the translation initiation complex189. This regulation has been found to be particularly important for the translation of mRNAs that have 5’-terminal oligopyrimidine (5’-TOP) or 5’-TOP-like sequences at the 5’-end of their 5’-UTRs, which encompass transcripts encoding the translation machinery, including ribosome proteins (rProteins) and translation factors190,191 (Box Figure). Oncogenic activation of mTORC1 is believed to enhance the rate of protein synthesis largely through the 4E-BP1-eIF4E axis, to support cancer cell growth138. Phosphorylation of another mTORC1 substrate, La-related protein 1 (LARP1), has also been implicated in the selective induction of translation of 5’-TOP mRNAs downstream of mTORC1192–194. In addition to mTORC1, both AKT and S6K have been reported to phosphorylate LARP1 and relieve its inhibitory effect on translation by dissociating it from 5’-UTRs192. A key target of S6K in the induction of translation is eIF4B, which upon phosphorylation forms an active heterodimer with the RNA helicase eIF4A, thereby enhancing the unwinding of 5’-UTRs with complex secondary structures77,195,196. Interestingly, translation of the MYC mRNA is particularly sensitive to the S6K-mediated phosphorylation of eIF4B77. Finally, mTORC1 signaling enhances rDNA transcription through both Pol-I and Pol-III, thus producing the rRNA needed for assembly with newly translated rProteins into ribosomes131.
[H1] PI3K-AKT signaling and redox homeostasis
One consequence of the metabolic changes underlying cancer cell proliferation is an increase in the production of reactive oxygen species (ROS). Accumulating evidence suggest that moderate levels of ROS support cancer cell growth, proliferation, and survival, whereas too much ROS is detrimental to the macromolecular constituents of the cell140. In addition to promoting anabolic metabolism and cell growth, the PI3K-AKT pathway also regulates multiple metabolic processes that modulate ROS levels.
[H2] Production of cellular reducing power
NADPH, a pyridine dinucleotide cofactor, is a key reservoir of electrons required for reductive biosynthesis and defence against oxidative stress (Fig. 5). Cancer cells upregulate NADPH-producing pathways to support an increased anabolic demand and to enhance their antioxidant capacity122,141,142. Recent studies have addressed the routes of consumption and production of NADPH using quantitative flux analysis [G]143–145. These analyses revealed that the majority of cytosolic NADPH is consumed by anabolic metabolism and particularly by fatty acid synthesis144 (Fig. 5). Indeed, de novo synthesis of a single molecule of one of the most abundant fatty acids in human plasma, palmitate, by the multifunctional enzyme fatty acid synthase (FASN) requires 14 molecules of NADPH. Ribonucleotide reductase (RNR) requires NADPH to catalyse the reduction of ribonucleotide 5’-diphosphates (NDPs) to deoxyribonucleotides diphosphates (dNDP), which are subsequently converted to the dNTPs required for DNA replication146. Additionally, NADPH is required for the activity of dihydrofolate reductase to produce the tetrahydrofolate for the reactions of one carbon metabolism. NADPH is also required for synthesis of the amino acid proline, which has emerged as an important process for cancer cell growth and survival147. There are several metabolic enzymes that can reduce NADP+ to replenish NADPH, the levels of which can be influenced by PI3K-AKT signaling (Fig. 5). The majority of the cytosolic NADPH pool is derived from reactions within the oxidative branch of PPP145, which can be stimulated downstream of AKT signaling through the mTORC1-SREBP axis68 (Fig. 5). SREBP also promotes the expression of malic enzyme 1 (ME1), which can contribute to the cytosolic NADPH pool148. Interestingly, both G6PD and ME1 expression are reciprocally repressed by the tumor suppressor p53, and their induction contributes to tumor growth in mouse models149,150. Finally, SREBP151, as well as the FOXO transcription factors152, have been found to control the expression of isocitrate dehydrogenase 1 (IDH1), another cytosolic enzyme that can produce NADPH. The relative importance of these three major sources of cytosolic NADPH to the oncogenic effects of PI3K-AKT signaling requires further research.
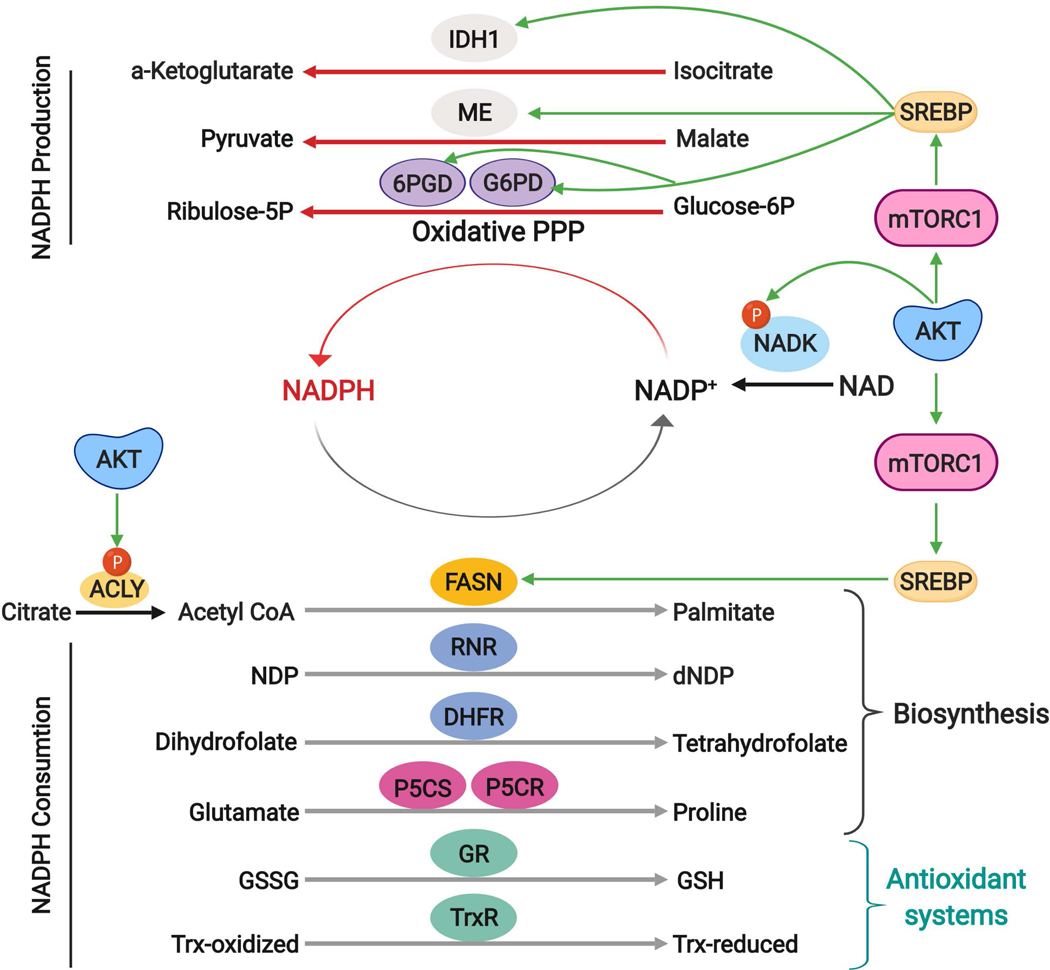
Fig. 5: AKT signaling and control of NADPH production and consumption.
NADPH serves as a major electron donor for reductive biosynthesis and defence against ROS, reactions that yield its oxidized form, NADP+. In the cytosol, NADPH can be regenerated from NADP+ through reactions involving two enzymes of the oxidative PPP (G6PD and PGD), isocitrate dehydrogenase 1 (IDH1) and malic enzyme (ME). Downstream of AKT and mTORC1, SREBP induces expression of these enzymes to enhance NADPH production. AKT-mediated phosphorylation of NADK serves to boost NADP+ abundance to further enhance the production of NADPH through these redox reactions. Many cellular reactions consume a large quantity of NADPH as reducing power. NADPH is required for key reactions in the biosynthesis of macromolecules, including fatty acid synthase (FASN) to produce palmitate, ribonucleotide reductase (RNR) to convert ribonucleotide diphosphates (NDPs) into deoxyribonucleotide diphosphates (dNDPs), dihydrofolate reductase (DHFR) for tetrahydrofolate synthesis, and the proline synthesis enzymes pyrroline-5-carboxylate (P5C) synthase (P5CS) and pyrroline-5-carboxylate reductase (P5CR). NADPH also serves as essential reducing power for antioxidant enzymes including glutathione reductase (GR), which reduces the oxidized glutathione disulfide (GSSG) to glutathione (GSH), and thioredoxin reductase (TrxR), which transfers electrons from NADPH to reduce thioredoxin for subsequent reduction of oxidized cysteines.
ROS, reactive oxygen species; PPP, pentose phosphate pathway; NADPH, nicotinamide adenine dinucleotide phosphate (reduced); G6PD, glucose 6-phosphate dehydrogenase; PGD, phosphogluconate dehydrogenase; SREBP: Sterol regulatory element binding proteins; NADK, NAD kinase.
The size of the cellular pool of NADP+ and NADPH available for interconversion through reduction and oxidation (redox) reactions is determined, in part, by the activity of NAD kinase (NADK). NADK catalyzes the phosphorylation of NAD+ to produce NADP+, which is the rate-limiting substrate for NADPH-producing enzymes. In response to growth factors, or in a growth factor-independent manner in cancer cells, AKT directly phosphorylates and acutely stimulates an increase in NADK activity, resulting in increased production of NADP+ and, subsequently, NADPH153 (Fig. 2 and Fig. 5). The AKT-mediated phosphorylation of NADK facilitates the anchorage-independent growth of cancer cells153. This finding might help explain the key role of PI3K signaling in the survival of cells detached from the extracellular matrix, which requires a robust anti-oxidant response154. The stimulated increase in NADK activity is also likely to serve as a key part of the broader anabolic program induced by the PI3K-AKT pathway, providing reducing cofactors in high demand for the biosynthetic processes underlying cell growth. Loss of NADK attenuates tumor growth in xenograft models155,156, and an activating mutation in NADK, identified in pancreatic ductal adenocarcinoma, was found to increase tumor growth in a xenograft model155. Future research is needed to define the role of NADK as a downstream target of AKT signaling in cancer development and progression and whether this enzyme is a viable metabolic target for cancer therapies.
[H2] Oxidative stress response
Activation of the PI3K-AKT pathway stimulates both ROS producing and scavenging mechanisms that vary between cellular settings. For instance, in neutrophils and macrophages, activation of PI3K-AKT signaling stimulates ROS production through regulation of the p47phox component of the NADPH oxidase NOX, thereby generating the respiratory burst required to eliminate extracellular and phagocytosed pathogens157,158. In addition, AKT directly phosphorylates and activates endothelial nitric oxide (NO) synthase (eNOS), another NADPH oxidase, which produces NO in endothelial cells to control vascular tone but can also generate ROS159,160. However, whether these or other specialized mechanisms downstream of PI3K-AKT signaling contribute to ROS production within cancer cells is unknown. Instead, most of the evidence to date, support an anti-oxidant role for PI3K-AKT signaling in cancer.
Elevated ROS, including superoxide (O2−) and hydrogen peroxide (H2O2), can oxidize and damage lipids, protein, and nucleic acids and have detrimental consequences on cell growth and survival140. Cells have multiple enzymes and systems to neutralize ROS, including superoxide dismutase (SOD), which converts O2- to H2O2, catalase, which reduces H2O2 into water, and the peroxiredoxin (Prx)/thioredoxin (Trx) and glutathione peroxidase (GPx)/glutathione (GSH) antioxidant systems, which utilize NADPH to reduce H2O2 into water and to repair macromolecules oxidized by exposure to ROS140. It is interesting to note that the FOXO transcription factors, which are negatively regulated by AKT, can also function to detoxify ROS through the induction of several ROS scavenging systems161–163. However, more research is necessary to understand the significance of the PI3K-AKT-FOXO circuit in redox control in the context of cancer.
Consistent with PI3K signaling playing an important role in the cellular response to ROS, an increase in ROS levels can activate the pathway through various mechanisms. H2O2 can influence cell signaling events by oxidizing cysteine residues on proteins, including the catalytic cysteine of protein and lipid phosphatases. These ROS-sensitive phosphatases include negative regulators of PI3K signaling, such as protein tyrosine phosphatase 1B, protein phosphatase 2A, and PTEN164–166, the inhibitory oxidation of which can activate AKT in response to a rise in H2O2165,167 (Fig. 6). Thus, together with downstream mechanisms to mitigate ROS levels, the PI3K-AKT pathway can serve as part of an adaptive oxidative stress response pathway.
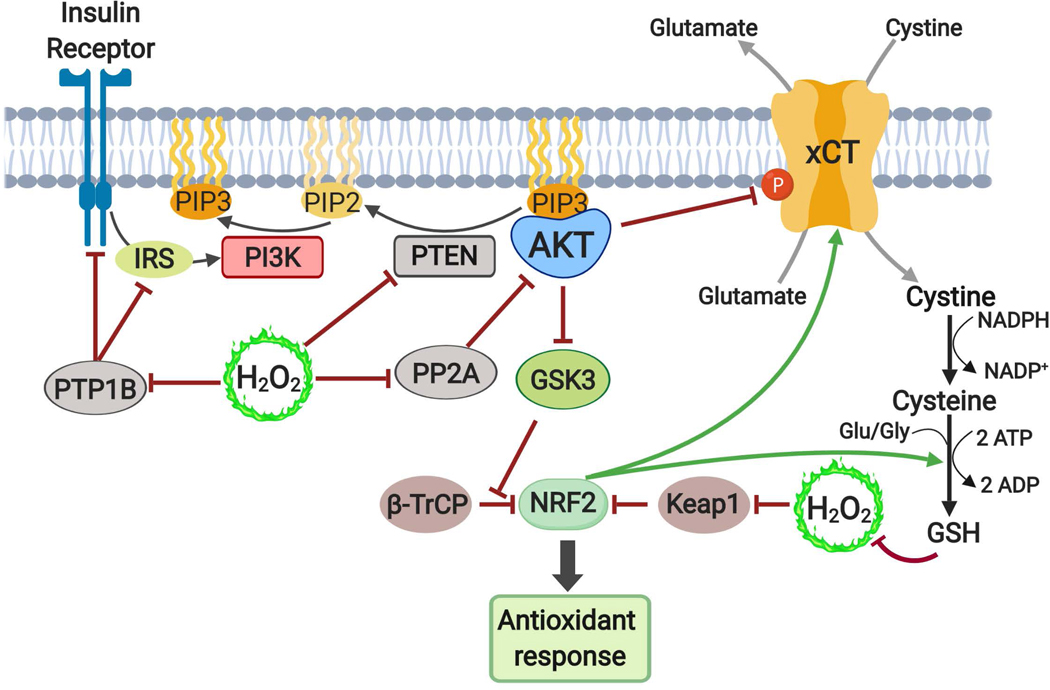
Figure 6: Interplay between ROS and the PI3K-AKT pathway.
ROS in the form of hydrogen peroxide can activate the PI3K-AKT pathway through inactivation of protein phosphatases, including PTP1B, which attenuates the activity of insulin receptor and insulin receptor substrate (IRS), and PP2A, which normally dephosphorylates T308 on AKT, thus leading to enhanced AKT phosphorylation and activation in response to ROS. ROS also inhibits the lipid phosphatase PTEN, resulting in accumulation of PIP3 and activation of AKT. AKT activation can facilitate the adaptation to ROS by activating the NRF2 transcription factor, which stimulates numerous enzymes to mount an antioxidant response. Downstream of AKT, GSK3 phosphorylates NRF2 and marks it for ubiquitination by the β-TRCP E3 ligase, leading to subsequent proteasomal degradation. Thus, through its inhibition of GSK3, AKT signaling can stabilize NRF2. NRF2 is stabilized and activated by ROS via cysteine oxidation of the E3 ligase Keap1, thereby disrupting its binding of NRF2. AKT also directly phosphorylates and inhibits the cystine-glutamate antiporter xCT, which transports cystine into cells that, upon NADPH-dependent reduction to cysteine, can be used to produce glutathione for ROS neutralization. xCT and the enzymes of glutathione synthesis are encoded by gene targets of NRF2 as part of its anti-oxidant response.
ROS, Reactive oxygen species; PTP1B, protein tyrosine phosphatase 1B; PP2A, protein phosphatase 2A; PTEN, phosphatase and tensin homolog; PDK1, phosphoinositide-dependent protein kinase 1; NRF2, nuclear factor erythroid 2-related factor 2; GSK3, Glycogen synthase kinase 3; NADPH, nicotinamide adenine dinucleotide phosphate (reduced), Keap1, kelch-Like ECH-associated protein 1; β-TRCP, β-transducin repeat containing protein.
As described above, AKT signaling increases cellular reducing power (NADPH) available for anti-oxidant responses (Fig. 5). However, the PI3K-AKT pathway also contributes to ROS detoxification through sustained activation of NRF2 (nuclear factor erythroid 2-related factor 2), a transcription factor that controls the expression of numerous genes involved in the antioxidant response, including the enzymes of glutathione synthesis and function, the thioredoxin system, NADPH regeneration, and ROS detoxification168. NRF2 is cellular sensor of both oxidative stress and growth factor signaling through mechanisms that control its protein stability. NRF2 is targeted for rapid proteasome-mediated degradation through two distinct E3 ubiquitin ligases, Keap1 and β-TrCP (Fig. 6). Oxidative stress directly inhibits the binding of Keap1 to NRF2, leading to increased abundance of NRF2169,170. Activation of the PI3K pathway also results in stabilization and activation of NRF2. AKT inhibits GSK3, which directly phosphorylates NRF2 and targets it for degradation by β-TrCP171 (Fig. 6). In addition, AKT has been proposed to phosphorylate and stabilize p21Cip1/WAF1, allowing it to compete with KEAP1 for NRF2 binding, thus leading to NRF2 stabilization and activation of anti-oxidant gene targets172,173.
Accumulating evidence indicates that NRF2 confers an advantage for aggressive cancer cell survival and proliferation by upregulating metabolic and antioxidant pathways174. Recently, the PI3K-AKT-NRF2 axis was reported to contribute to both anabolic metabolism and ROS detoxification through control of metabolic genes involved in NADPH regeneration175. Activation of AKT signaling through loss of PTEN induces NRF2 target genes to support proliferation and tumorigenesis175,176. Moreover, oncogenic activation of the PI3K-AKT pathway in breast cancer induces an NRF2-dependent transcriptional program, which enhances glutathione biosynthesis to support tumor growth and confer resistance to oxidative stress177. Interestingly, inhibition of glutathione biosynthesis was found to synergize with the chemotherapeutic agent cisplatin and induce tumor regression in PI3K-driven breast cancer models177, suggesting that the anti-oxidant response downstream of PI3K-AKT signaling represents a targetable metabolic vulnerability.
NRF2 also enhances glutathione synthesis through transcriptional induction of the cystine-glutamate antiporter xCT (or SLC7A11)178, which imports cystine molecules (oxidized dimers of cysteine) that are subsequently reduced to cysteine, an essential substrate for glutathione synthesis. Paradoxically, xCT function has been shown to be attenuated downstream of growth factor-stimulated and oncogenic PI3K signaling179,180. AKT was found to directly phosphorylate xCT and decrease its cystine transport activity179, thereby rendering cells with oncogenic PI3K signaling dependent on endogenous cysteine synthesis (Fig. 6). Since cystine reduction to cysteine requires NADPH181, acute inhibition of xCT by AKT could serve to preserve NADPH for use in lipid synthesis downstream of AKT-mediated activation of ACLY. Inhibition of the xCT antiporter could also serve to spare glutamate and glutamine nitrogen to be used for nitrogen-dependent synthesis of amino acids and nucleotides. Perturbations in the balance between anti-oxidant and biosynthetic activities of cancer cells imposed by oncogenic PI3K-AKT signaling is a particularly interesting and active area of investigation with potential to reveal new therapeutic approaches.
[H1] Clinical perspective
The PI3K-AKT pathway has emerged as one of the most frequently activated drivers of human cancer, making it a prime candidate for therapeutic intervention. Specific inhibitors targeting both PI3K and AKT have been developed as cancer therapies, with most trials demonstrating limited therapeutic benefit as single agents182. Due to the critical role of PI3K-AKT signaling in insulin-responsive glucose uptake into tissues, such as skeletal muscle, pan-PI3K inhibitors inevitably cause hyperglycemia, with the consequent hyperinsulinemia being shown to overcome pathway inhibition and reactivate PI3K signaling in tumors183. Dietary interventions, such as a ketogenic diet, have been found to alleviate this hyperglycemia and hyperinsulinemia and improve responses to these inhibitors in mouse cancer models183. Furthermore, resistance to PI3K inhibitors can arise due to redundant regulation of key downstream effectors such as mTORC1184. Importantly, patient stratification for oncogenic PIK3CA mutations, which are found in approximately 40% of ER+, HER2- breast cancers, together with use of a p110α-selective PI3K inhibitor (BYL719, trade named Piqray) yielded improved clinical responses when used in combination with an estrogen receptor antagonist185. Preclinical and clinical studies continue to focus on understanding the tumor response to PI3K inhibitors and to identify both combination therapies and unique vulnerabilities arising from uncontrolled PI3K signaling in distinct cancer settings. As discussed above for nucleotide synthesis, pharmacological targeting of specific metabolic enzymes and pathways induced downstream of PI3K-AKT signalling in cancer may offer effective alternative therapies to PI3K inhibitors for cancer treatment.
[H1] Conclusion
Research in the past two decades has defined how aberrant activation of the PI3K signaling pathway drives tumorigenesis, at least in part, through the control of metabolism. Future research aimed at refining our molecular map of the critical regulatory nodes connecting the oncogenic PI3K signaling network to metabolic networks in different cancers will help reveal metabolic dependencies and novel therapeutic strategies. One example of this is the finding that PI3K inhibitors can deplete intracellular nucleotide pools and cause DNA replication stress, which when combined with inhibitors of poly ADP-ribose polymerase (PARP), an enzyme involved in DNA repair, increases the anti-cancer efficacy of PI3K inhibitors in tumor models and a subset of cancer patients118,186–188. Metabolic enzymes are inherently druggable and offer a wealth of new targets to which specific pharmacological inhibitors can be developed, as new metabolic outcomes and vulnerabilities are identified. Finally, while we focused here on the cancer cell intrinsic regulation of metabolism, it is clear that the physiological features of the tissue of origin for a given tumor and the stromal cell milieu, the nutritional and metabolic status of the host, and distinct metabolic niches of sites of distant metastases will all differentially influence cancer cell metabolism and metabolic dependencies as they relate to cancers with oncogenic PI3K-AKT signaling.
Acknowledgements:
We apologize to our colleagues whose work we were unable to discuss due to space constraints. Research in the Manning lab related to the subject of this review was supported by grants to B.D.M. from the NIH (R35-CA197459 and P01-CA120964), DOD (W81XWH-18–1-0370 and W81XWH-18–1-0659), and a Rothberg Courage Award from the Tuberous Sclerosis Alliance.
Glossary:
- redox homeostasis
Maintaining proper levels of cellular NAD(P)+ and NAD(P)H for metabolic reduction and oxidation (redox) reactions
- metabolic flexibility
The ability of a cell to adapt its metabolism in response to changing environmental conditions, such as nutrient and energy availability
- anaplerotic metabolism (or anaplerosis)
Metabolic reactions that replenish TCA cycle intermediates used for biosynthetic processes
- glutaminolysis
The two-step removal of the amide and amine nitrogens from glutamine to produce the TCA cycle intermediate α-ketoglutarate, reactions that can serve as one form of anaplerosis
- anabolic processes
Metabolic processes and pathways that utilize nutrients and ATP to generate macromolecules such as proteins, lipids and nucleotides
- quantitative flux analysis
Measurement of the rate of consumption and production of metabolites in specific metabolic pathways, often achieved through the tracing of stable isotope-labelled nutrients and quantification via mass spectrometry
Footnotes
Competing Interests: B.D.M. is a shareholder and scientific advisory board member of Navitor Pharmaceuticals and LAM Therapeutics. G.H declares no competing interests.
References
- 1.Lawrence MS et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fruman DA et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorpe LM, Yuzugullu H & Zhao JJ PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 15, 7–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alessi DR et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15, 6541–6551 (1996). [PMC free article] [PubMed] [Google Scholar]
- 5.Alessi DR et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol 7, 261–269 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Guertin DA, Ali SM & Sabatini DM Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Dummler B & Hemmings BA Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans 35, 231–235 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Manning BD & Toker A AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell 31, 820–832.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandoth C et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zack TI et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet 45, 1134–1140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxton RA & Sabatini DM mTOR Signaling in Growth, Metabolism, and Disease. Cell 169, 361–371 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Valvezan AJ & Manning BD Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat. Metab. 1, 321–333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon S et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 156, 771–785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross DA, Alessi DR, Cohen P, Andjelkovich M & Hemmings BA Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Embi N, Rylatt DB & Cohen P Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem 107, 519–527 (1980). [PubMed] [Google Scholar]
- 17.Frame S, Cohen P & Biondi RM A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell 7, 1321–1327 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Sutherland C What Are the bona fide GSK3 Substrates? Int J Alzheimers Dis 2011, 505607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer EL & Brunet A FOXO transcription factors in ageing and cancer. Acta Physiol (Oxf) 192, 19–28 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Brunet A et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Hornsveld M, Dansen TB, Derksen PW & Burgering BMT Re-evaluating the role of FOXOs in cancer. Semin Cancer Biol 50, 90–100 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Warburg O, Wind F & Negelein E The metabolism of tumors in the body. J Gen Physiol 8, 519–530 (1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunt SY & Vander Heiden MG Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27, 441–464 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Rathmell JC et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23, 7315–7328 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elstrom RL et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64, 3892–3899 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Plas DR, Talapatra S, Edinger AL, Rathmell JC & Thompson CB Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem 276, 12041–12048 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Gottlob K et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15, 1406–1418 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edinger AL & Thompson CB Antigen-presenting cells control T cell proliferation by regulating amino acid availability. Proc Natl Acad Sci U S A 99, 1107–1109 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzzai M et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene 24, 4165–4173 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Augustin R The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life 62, 315–333 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Adekola K, Rosen ST & Shanmugam M Glucose transporters in cancer metabolism. Curr Opin Oncol 24, 650–654 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calera MR et al. Insulin increases the association of Akt-2 with Glut4-containing vesicles. J Biol Chem 273, 7201–7204 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Ng Y, Ramm G, Lopez JA & James DE Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab 7, 348–356 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Sano H et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278, 14599–14602 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Eguez L et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2, 263–272 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Wieman HL, Wofford JA & Rathmell JC Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18, 1437–1446 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siska PJ et al. Suppression of glut1 and glucose metabolism by decreased akt/mtorc1 signaling drives T cell impairment in B cell leukemia. J Immunol 197, 2532–2540 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldhart AN et al. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep 19, 2005–2013 (2017).demonstrate that both Akt
- 39.Wu N et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 49, 1167–1175 (2013).AMPK phosphorylate TXNIP on the same site (S308) to induce glucose uptake by inhibiting the endocytosis of GLUT1 or GLUT4 in response to growth signals or energy stress.
- 40.Parikh H et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4, e158 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong SY, Yu F-X, Luo Y & Hagen T Oncogenic activation of the PI3K/Akt pathway promotes cellular glucose uptake by downregulating the expression of thioredoxin-interacting protein. Cell Signal 28, 377–383 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Ancey P-B, Contat C & Meylan E Glucose transporters in cancer - from tumor cells to the tumor microenvironment. FEBS J (2018). doi: 10.1111/febs.14577 [DOI] [PubMed] [Google Scholar]
- 43.Roberts DJ, Tan-Sah VP, Smith JM & Miyamoto S Akt phosphorylates HK-II at Thr-473 and increases mitochondrial HK-II association to protect cardiomyocytes. J Biol Chem 288, 23798–23806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pastorino JG, Shulga N & Hoek JB Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277, 7610–7618 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Majewski N, Nogueira V, Robey RB & Hay N Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol Cell Biol 24, 730–740 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majewski N et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 16, 819–830 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Liu Y et al. Prognostic Significance of the Metabolic Marker Hexokinase-2 in Various Solid Tumors: A Meta-Analysis. PLoS ONE 11, e0166230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf A et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med 208, 313–326 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson M, Marayati R, Moffitt R & Yeh JJ Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget 8, 56081–56094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patra KC et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeWaal D et al. Author Correction: Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun 9, 2539 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep 8, 1461–1474 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nogueira V, Patra KC & Hay N Selective eradication of cancer displaying hyperactive Akt by exploiting the metabolic consequences of Akt activation. elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raez LE et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71, 523–530 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Deprez J, Vertommen D, Alessi DR, Hue L & Rider MH Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem 272, 17269–17275 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Hue L & Rider MH Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues. Biochem J 245, 313–324 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Houddane A et al. Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes. Cell Signal 34, 23–37 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Manning BD & Cantley LC AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miralpeix M, Azcon-Bieto J, Bartrons R & Argiles JM The impairment of respiration by glycolysis in the Lewis lung carcinoma. Cancer Lett 50, 173–178 (1990). [DOI] [PubMed] [Google Scholar]
- 60.Nissler K, Petermann H, Wenz I & Brox D Fructose 2,6-bisphosphate metabolism in Ehrlich ascites tumour cells. J Cancer Res Clin Oncol 121, 739–745 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu H et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 164, 433–446 (2016).This study demonstrates a new mode of regulation of glycolytic flux by PI3K through an AKT-independent, RAC-dependent mechanism involving cytoskeletal remodeling and release of actin-bound Aldolase A.
- 62.Jiang Z, Wang X, Li J, Yang H & Lin X Aldolase A as a prognostic factor and mediator of progression via inducing epithelial-mesenchymal transition in gastric cancer. J Cell Mol Med 22, 4377–4386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai L et al. High expression of ALDOA and DDX5 are associated with poor prognosis in human colorectal cancer. Cancer Manag Res 10, 1799–1806 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grandjean G et al. Definition of a Novel Feed-Forward Mechanism for Glycolysis-HIF1α Signaling in Hypoxic Tumors Highlights Aldolase A as a Therapeutic Target. Cancer Res 76, 4259–4269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du S et al. Fructose-bisphosphate aldolase a is a potential metastasis-associated marker of lung squamous cell carcinoma and promotes lung cell tumorigenesis and migration. PLoS ONE 9, e85804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semenza GL Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3, 721–732 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Chae YC et al. Mitochondrial akt regulation of hypoxic tumor reprogramming. Cancer Cell 30, 257–272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Düvel K et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39, 171–183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong H et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res 60, 1541–1545 (2000). [PubMed] [Google Scholar]
- 70.Hudson CC et al. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22, 7004–7014 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majumder PK et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10, 594–601 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Laughner E, Taghavi P, Chiles K, Mahon PC & Semenza GL HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21, 3995–4004 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas GV et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 12, 122–127 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Arsham AM, Plas DR, Thompson CB & Simon MC Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res 64, 3500–3507 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Stine ZE, Walton ZE, Altman BJ, Hsieh AL & Dang CV MYC, metabolism, and cancer. Cancer Discov 5, 1024–1039 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West MJ, Stoneley M & Willis AE Translational induction of the c-myc oncogene via activation of the FRAP/TOR signalling pathway. Oncogene 17, 769–780 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Csibi A et al. The mTORC1/S6K1 pathway regulates glutamine metabolism through the eIF4B-dependent control of c-Myc translation. Curr Biol 24, 2274–2280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Welcker M et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A 101, 9085–9090 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gregory MA, Qi Y & Hann SR Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem 278, 51606–51612 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Sears R et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14, 2501–2514 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang W et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281, 10105–10117 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Jensen KS et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. EMBO J 30, 4554–4570 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouchard C, Marquardt J, Brás A, Medema RH & Eilers M Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J 23, 2830–2840 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kress TR et al. The MK5/PRAK kinase and Myc form a negative feedback loop that is disrupted during colorectal tumorigenesis. Mol Cell 41, 445–457 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Wiegering A et al. Targeting translation initiation bypasses signaling crosstalk mechanisms that maintain high MYC levels in colorectal cancer. Cancer Discov 5, 768–781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brondfield S et al. Direct and indirect targeting of MYC to treat acute myeloid leukemia. Cancer Chemother Pharmacol 76, 35–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Safaroghli-Azar A, Bashash D, Kazemi A, Pourbagheri-Sigaroodi A & Momeny M Anticancer effect of pan-PI3K inhibitor on multiple myeloma cells: Shedding new light on the mechanisms involved in BKM120 resistance. Eur J Pharmacol 842, 89–98 (2019). [DOI] [PubMed] [Google Scholar]
- 88.DeBerardinis RJ & Chandel NS Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinberg F et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 107, 8788–8793 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wise DR et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105, 18782–18787 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Röhrig F & Schulze A The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer 16, 732–749 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Menendez JA & Lupu R Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7, 763–777 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Berwick DC, Hers I, Heesom KJ, Moule SK & Tavare JM The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem 277, 33895–33900 (2002). [DOI] [PubMed] [Google Scholar]
- 94.Potapova IA, El-Maghrabi MR, Doronin SV & Benjamin WB Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry 39, 1169–1179 (2000). [DOI] [PubMed] [Google Scholar]
- 95.Lee JV et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab 20, 306–319 (2014).This study demonstrates that active AKT promotes ACLY-dependent histone acetylation in cancer cells and in mouse models of breast and pancreatic cancer.
- 96.Carrer A et al. Acetyl-CoA Metabolism Supports Multistep Pancreatic Tumorigenesis. Cancer Discov 9, 416–435 (2019).This study reveals that KRAS promotes acetyl-CoA abundance to regulate histone acetylation and support the mevalonate pathway for cholesterol biosynthesis and loss of ACLY in the pancreas results in suppression of KRAS-driven pancreatic tumorigenesis.
- 97.Hatzivassiliou G et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C & Thompson CB ATP citrate lyase is an important component of cell growth and transformation. Oncogene 24, 6314–6322 (2005). [DOI] [PubMed] [Google Scholar]
- 99.Khwairakpam AD et al. ATP citrate lyase (ACLY): a promising target for cancer prevention and treatment. Curr Drug Targets 16, 156–163 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F & Kroemer G Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab 21, 805–821 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Ray KK et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 380, 1022–1032 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Granchi C ATP citrate lyase (ACLY) inhibitors: An anti-cancer strategy at the crossroads of glucose and lipid metabolism. Eur J Med Chem 157, 1276–1291 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Wei J et al. An allosteric mechanism for potent inhibition of human ATP-citrate lyase. Nature 568, 566–570 (2019). [DOI] [PubMed] [Google Scholar]
- 104.Horton JD, Goldstein JL & Brown MS SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109, 1125–1131 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Porstmann T et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24, 6465–6481 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Yecies JL et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 14, 21–32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owen JL et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A 109, 16184–16189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ricoult SJH, Yecies JL, Ben-Sahra I & Manning BD Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35, 1250–1260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim KH et al. Regulatory role of glycogen synthase kinase 3 for transcriptional activity of ADD1/SREBP1c. J Biol Chem 279, 51999–52006 (2004). [DOI] [PubMed] [Google Scholar]
- 110.Sundqvist A et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 1, 379–391 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Gouw AM et al. The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab 30, 556–572.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee G et al. Post-transcriptional Regulation of De Novo Lipogenesis by mTORC1-S6K1-SRPK2 Signaling. Cell 171, 1545–1558.e18 (2017).This study shows that S6K1 phosphorylates and activates SRPK2 to induce efficient splicing and translation of mRNAs encoding lipogenic enzymes.
- 113.Da Silva MR et al. Splicing regulators and their roles in cancer biology and therapy. Biomed Res. Int. 2015, 150514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lane AN & Fan TW-M Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 43, 2466–2485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tong X, Zhao F & Thompson CB The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev 19, 32–37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Villa E, Ali ES, Sahu U & Ben-Sahra I Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers (Basel) 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saha A et al. Akt phosphorylation and regulation of transketolase is a nodal point for amino acid control of purine synthesis. Mol Cell 55, 264–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Juvekar A et al. Phosphoinositide 3-kinase inhibitors induce DNA damage through nucleoside depletion. Proc Natl Acad Sci U S A 113, E4338–47 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ju HQ et al. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene 36, 6282–6292 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cascante M, Centelles JJ, Veech RL, Lee WN & Boros LG Role of thiamin (vitamin B-1) and transketolase in tumor cell proliferation. Nutr Cancer 36, 150–154 (2000). [DOI] [PubMed] [Google Scholar]
- 121.Kowalik MA, Columbano A & Perra A Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front Oncol 7, 87 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patra KC & Hay N The pentose phosphate pathway and cancer. Trends Biochem Sci 39, 347–354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmale Iii DG et al. Genetic Structure of Atmospheric Populations of Gibberella zeae. Phytopathology 96, 1021–1026 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Liu Y-C et al. Global regulation of nucleotide biosynthetic genes by c-Myc. PLoS ONE 3, e2722 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mannava S et al. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle 7, 2392–2400 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cunningham JT, Moreno MV, Lodi A, Ronen SM & Ruggero D Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell 157, 1088–1103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao P et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ben-Sahra I, Howell JJ, Asara JM & Manning BD Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013).demonstrate that mTORC1 induces de novo nucleotide synthesis.
- 129.Robitaille AM et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 339, 1320–1323 (2013).reveal that CAD, the enzyme that catalyzes the first three steps of the de novo pyrimidine synthesis pathway, is directly phosphorylated by S6K1 to stimulate pyrimidine synthesis.
- 130.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM & Manning BD mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).shows that mTORC1 enhances purine synthesis through transcriptional mechanisms, including the ATF4-mediated induction of the mitochondrial tetrahydrofolate cycle enzyme MTHFD2, which contributes one-carbon units to formation of the purine ring.
- 131.Iadevaia V, Liu R & Proud CG mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 36, 113–120 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Van Riggelen J, Yetil A & Felsher DW MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 10, 301–309 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Chabner BA & Roberts TG Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer 5, 65–72 (2005). [DOI] [PubMed] [Google Scholar]
- 134.Valvezan AJ et al. mTORC1 Couples Nucleotide Synthesis to Nucleotide Demand Resulting in a Targetable Metabolic Vulnerability. Cancer Cell 32, 624–638.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang F et al. Inosine monophosphate dehydrogenase dependence in a subset of small cell lung cancers. Cell Metab 28, 369–382.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoxhaj G et al. The mTORC1 Signaling Network Senses Changes in Cellular Purine Nucleotide Levels. Cell Rep 21, 1331–1346 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Emmanuel N et al. Purine Nucleotide Availability Regulates mTORC1 Activity through the Rheb GTPase. Cell Rep 19, 2665–2680 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Truitt ML & Ruggero D New frontiers in translational control of the cancer genome. Nat Rev Cancer 17, 332 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Pourdehnad M et al. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proc Natl Acad Sci U S A 110, 11988–11993 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schieber M & Chandel NS ROS function in redox signaling and oxidative stress. Curr Biol 24, R453–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nilsson R et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun 5, 3128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Locasale JW Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer 13, 572–583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lewis CA et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55, 253–263 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fan J et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen L et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 1, 404–415 (2019). [PMC free article] [PubMed] [Google Scholar]
- 146.Cory JG & Sato A Regulation of ribonucleotide reductase activity in mammalian cells. Mol Cell Biochem 53–54, 257–266 (1983). [DOI] [PubMed] [Google Scholar]
- 147.Tanner JJ, Fendt S-M & Becker DF The proline cycle as a potential cancer therapy target. Biochemistry 57, 3433–3444 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shimomura I, Shimano H, Korn BS, Bashmakov Y & Horton JD Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J Biol Chem 273, 35299–35306 (1998). [DOI] [PubMed] [Google Scholar]
- 149.Jiang P, Du W, Mancuso A, Wellen KE & Yang X Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 493, 689–693 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang P et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 13, 310–316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ricoult SJH, Dibble CC, Asara JM & Manning BD Sterol Regulatory Element Binding Protein Regulates the Expression and Metabolic Functions of Wild-Type and Oncogenic IDH1. Mol Cell Biol 36, 2384–2395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Charitou P et al. FOXOs support the metabolic requirements of normal and tumor cells by promoting IDH1 expression. EMBO Rep 16, 456–466 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoxhaj G et al. Direct stimulation of NADP+ synthesis through Akt-mediated phosphorylation of NAD kinase. Science 363, 1088–1092 (2019).This study demonstrates that Akt directly phosphorylates and activates NADK, which generates NADP+ from NAD+.
- 154.Schafer ZT et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113 (2009).This study demonstrates that treatment with antioxidants or activation of oncogenes such as ERBB2, PIK3CA, or AKT can rescue ROS-induced cell death caused by detachment of mammary cells from the extracellular matrix.
- 155.Tsang YH et al. Functional annotation of rare gene aberration drivers of pancreatic cancer. Nat Commun 7, 10500 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yau EH et al. Genome-Wide CRISPR Screen for Essential Cell Growth Mediators in Mutant KRAS Colorectal Cancers. Cancer Res 77, 6330–6339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomas DC The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol Lett 192, 88–96 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Chen Q et al. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol 170, 5302–5308 (2003). [DOI] [PubMed] [Google Scholar]
- 159.Lee MY et al. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci U S A 111, 12865–12870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang W et al. Superoxide production and reactive oxygen species signaling by endothelial nitric-oxide synthase. J Biol Chem 275, 16899–16903 (2000). [DOI] [PubMed] [Google Scholar]
- 161.Klotz L-O et al. Redox regulation of FoxO transcription factors. Redox Biol 6, 51–72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kops GJPL et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Honda Y & Honda S The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J 13, 1385–1393 (1999). [PubMed] [Google Scholar]
- 164.Salmeen A et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 (2003). [DOI] [PubMed] [Google Scholar]
- 165.Lee S-R et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277, 20336–20342 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Raman D & Pervaiz S Redox inhibition of protein phosphatase PP2A: Potential implications in oncogenesis and its progression. Redox Biol 101105 (2019). doi: 10.1016/j.redox.2019.101105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leslie NR et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 22, 5501–5510 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tonelli C, Chio IIC & Tuveson DA Transcriptional regulation by nrf2. Antioxid Redox Signal 29, 1727–1745 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cullinan SB, Gordan JD, Jin J, Harper JW & Diehl JA The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol 24, 8477–8486 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kobayashi A et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24, 7130–7139 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rada P et al. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31, 1121–1133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li Y, Dowbenko D & Lasky LA AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277, 11352–11361 (2002). [DOI] [PubMed] [Google Scholar]
- 173.Chen W et al. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell 34, 663–673 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee SB, Sellers BN & DeNicola GM The Regulation of NRF2 by Nutrient-Responsive Signaling and Its Role in Anabolic Cancer Metabolism. Antioxid Redox Signal 29, 1774–1791 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Mitsuishi Y et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22, 66–79 (2012).This study demonstrates that the PI3K-Akt pathway promotes nuclear accumulation of NRF2, which stimulates flux into the pentose phosphate pathway and nucleotide synthesis in proliferating cells.
- 176.Rojo AI et al. The PTEN/NRF2 axis promotes human carcinogenesis. Antioxid Redox Signal 21, 2498–2514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lien EC et al. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol 18, 572–578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sasaki H et al. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 277, 44765–44771 (2002). [DOI] [PubMed] [Google Scholar]
- 179.Lien EC, Ghisolfi L, Geck RC, Asara JM & Toker A Oncogenic PI3K promotes methionine dependency in breast cancer cells through the cystine-glutamate antiporter xCT. Sci Signal 10, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gu Y et al. mTORC2 Regulates Amino Acid Metabolism in Cancer by Phosphorylation of the Cystine-Glutamate Antiporter xCT. Mol Cell 67, 128–138.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pader I et al. Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc Natl Acad Sci U S A 111, 6964–6969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Janku F, Yap TA & Meric-Bernstam F Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 15, 273–291 (2018). [DOI] [PubMed] [Google Scholar]
- 183.Hopkins BD et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).This study demonstrates that systemic hyperinsulemia caused by PI3K inhibitors is sufficient to reactivate the PI3K-AKT pathway in tumor models and that the efficacy of PI3K inhibitors can be improved with anti-glycemic approaches that prevent hyperinsulemia.
- 184.Ilagan E & Manning BD Emerging role of mTOR in the response to cancer therapeutics. Trends Cancer 2, 241–251 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.André F et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 380, 1929–1940 (2019). [DOI] [PubMed] [Google Scholar]
- 186.Juvekar A et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov 2, 1048–1063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Konstantinopoulos PA et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol 20, 570–580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.González-Billalabeitia E et al. Vulnerabilities of PTEN-TP53-deficient prostate cancers to compound PARP-PI3K inhibition. Cancer Discov 4, 896–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ma XM & Blenis J Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10, 307–318 (2009). [DOI] [PubMed] [Google Scholar]
- 190.Thoreen CC et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hsieh AC et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485, 55–61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hong S et al. LARP1 functions as a molecular switch for mTORC1-mediated translation of an essential class of mRNAs. elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lahr RM et al. The La-related protein 1-specific domain repurposes HEAT-like repeats to directly bind a 5’TOP sequence. Nucleic Acids Res 43, 8077–8088 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fonseca BD et al. La-related Protein 1 (LARP1) Represses Terminal Oligopyrimidine (TOP) mRNA Translation Downstream of mTOR Complex 1 (mTORC1). J Biol Chem 290, 15996–16020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Raught B et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J 23, 1761–1769 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Holz MK, Ballif BA, Gygi SP & Blenis J mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123, 569–580 (2005). [DOI] [PubMed] [Google Scholar]