Supplemental Digital Content is available in the text.
Keywords: anxiety, delirium, depression, intensive care unit, patient experience, virtual reality
Abstract
Objectives:
Patients’ stays in the ICU are often characterized by prolonged immobility, sedation, disrupted sleep, and extended periods of pain, which put ICU patients at greater risk for ICU-acquired weakness and delirium-related mortality. The aim of this study was to evaluate the feasibility and efficacy of using meditative virtual reality to improve the hospital experience of ICU patients.
Design:
Final report of prospective observational trial.
Setting:
Surgical and trauma ICUs of the University of Florida Health, an academic hospital.
Patients:
Fifty-nine nonintubated adult ICU patients without delirium at recruitment.
Interventions:
Patients were exposed to sessions of commercially available meditative virtual reality applications focused on calmness and relaxation, performed once daily for up to 7 days.
Measurements and Main Results:
Outcome measures included pain level, pain medication administration, anxiety, depression, sleep quality, heart rate, respiratory rate, blood pressure, delirium status, and patient ratings of the virtual reality system. Comparisons were made using paired t tests and mixed models. The virtual reality meditative intervention improved patients’ ICU experience with reduced levels of anxiety and depression; however, there was no evidence that virtual reality had significant effects on physiologic measures, pain, or sleep.
Conclusions:
The use of meditative virtual reality technology in the ICU was easily implemented and well-received by patients.
Patients’ stays in the ICU are often traumatic. Prolonged immobility and sedation are common during treatment and can lead to ICU-acquired weakness (ICUAW) (1). Disrupted sleep, long stays, and extended periods of pain put ICU patients at greater risk for delirium-related mortality (2). After ICU discharge, 50–70% of patients exhibit persistent cognitive dysfunction, physical weakness, and post-traumatic symptoms that can have indefinite impacts on the patient’s finances, independence, and daily life (3).
Many of these ICU-related complications are not the direct result of illness, injury, or treatment. Critical care professionals have raised attention to modifiable aspects of the ICU to improve patient recovery experience (4). Early regular exercise programs have shown promising results for preventing ICUAW (5). Clinical guidelines for delirium prevention emphasize strategies to orient patients, manage pain, control noise and light, and promote good sleep (6–8). Although modifiable risks have been identified, there are few feasible strategies for mitigating these risks within typical ICU constraints of time and resources.
We hypothesized that virtual reality (VR) can provide a platform for controlled, scalable, and effective environmental manipulation in the ICU. VR uses a head-mounted display to deliver immersive video and audio that enables interaction through tracking head, hand, and body movements (9). VR has been praised for mitigating some of the limitations of traditional therapies. VR experiences can help users feel safer, more in control, and more comfortable than in-person outpatient therapy through direct visualization without the stress of real stimuli (10). A meta-analysis of clinical outpatient exposure therapy in VR was demonstrated to be as effective as standard in situ treatment and perceived as more tolerable by patients (11). Preoperative VR relaxation has been shown to reduce anxiety and stress in child and adult patients (12, 13). Severe burn victims reported less pain when VR relaxation was used during wound debridement (14). Medical therapy with VR has been generally efficacious and accepted across a variety of treatment contexts. It remains important to expand VR applications toward improving the patient experience (15).
Recommendations for optimal ICU settings encourage early exercise, comfortable ambiance, pain management, and good sleep. In addition to the environmental adjustments provided by the healthcare team, VR may provide a system in which some of these recommendations can be enhanced. The purpose of this interdisciplinary study was to evaluate feasibility of VR relaxation therapies for ICU patients.
MATERIALS AND METHODS
Participants and Setting
This study was conducted on a single-center cohort of patients admitted to the surgical or trauma ICUs at University of Florida (UF) Health, a large academic quaternary care center in the Southeastern United States. Participants were greater than or equal to 18 years, negative for delirium at recruitment, not in contact isolation for infectious disease, likely to remain in the ICU for greater than or equal to 48 hours, not intubated, and without conditions that limit head or neck movement. All study procedures were performed in the patients’ ICU rooms. The study was approved by the UF Institutional Review Board (number 201703107).
Materials
The VR system consisted of a smartphone placed in a Google Daydream (vr.google.com/daydream) headset and a pair of Bluetooth headphones (Fig. 1A). The Google Daydream VR headset was selected because it was lightweight (< 1 pound), easy to operate and adjust, and simple to sanitize between uses. We used “Google Spotlight Stories’ Pearl” (atap.google.com/spotlight-stories) as an initial orientation to VR and “RelaxVR” (www.relaxvr.co; Fig. 1B) to provide patients with a calm immersive scene (e.g., rolling waves on a beach) with voice-guided meditation that promoted breath control and relaxation. Between uses, the hard surfaces of the VR equipment (i.e., headset and controller) were cleaned with medical disinfectant wipes and soft surfaces (i.e., face cushion and headphone ear pads) were affixed with disposable sanitary covers. The headset, smartphone, controller, VR applications, and earphones were collectively referred to as the Digital Rehabilitation Environment Augmenting Medical System (DREAMS).
Figure 1.

Virtual reality system. A, Digital Rehabilitation Environment Augmenting Medical System equipment: 1) Virtual reality sanitary mask; 2) Google Daydream headset; 3) Bluetooth headphones with sanitary covers; and 4) Android smartphone. B, RelaxVR menu screenshot.
Dependent Measures
The primary outcome measures were participants’ pain, sleep quality, affect, delirium, and responses to using DREAMS. Pain was measured with the Defense and Veterans Pain Rating Scale (DVPRS) (16), sleep quality with the Richards-Campbell Sleep Questionnaire (RCSQ) (17), affect with the Hospital Anxiety and Depression Scale (HADS) (18), delirium status with the Confusion Assessment Method for the ICU (CAM-ICU) (19), and patients’ qualitative responses to DREAMS with structured interviews (Supplement A, Supplemental Digital Content 1, http://links.lww.com/CCX/A174). Each patient’s heart rate (HR), respiration rate (RR), blood pressure (BP), and medication records were used to evaluate if the VR sessions had any effect on physiology and pain.
The CAM-ICU, DVPRS, HR, RR, BP, and medication records were recorded by healthcare staff during normal care. Records were retrieved from the UF Integrated Data Repository after sessions were concluded. The RCSQ, HADS, and DREAMS questionnaires were administered by study staff during sessions.
Session Procedures
Study staff administered the RCSQ and HADS on the first day of the study to establish baseline measures. Participants were then fitted with DREAMS and exposed to “Pearl” (5 min) to demonstrate the format of VR. Study staff then initiated guided meditation for breath control and progressive relaxation using the “RelaxVR” app. The meditations lasted between 5 and 20 minutes, depending on participant preference during each session. Once the session was completed, study staff removed the headset and interviewed participants with open-ended questions about their experience. At the end of the session, researchers asked participants to revisit the relaxation techniques provided by “RelaxVR” whenever they felt it could help.
Participants received up to seven sessions, each at least 24 hours apart. “Pearl” was only shown during the initial session, and subsequent sessions occurred in an otherwise identical manner.
Data Analysis
Results were summarized as frequencies and percentages for categorical variables, mean and sd for normally distributed variables, and median and interquartile ranges for non-normal continuous variables. Paired t tests with adjustments made for multiple comparisons were used to compare pre- and post-session numerical values. Mixed models were constructed to examine the changes in DVPRS, HR, RR, BP, opioid medication dosage, “pro re nata” (PRN) opioid medication dosage, PRN opioid medication frequency, RCSQ, and HADS across study days taking into account the correlation within the same subject’s measurements. As a sensitivity analysis for measures collected multiple times per day, we constructed models comparing pre- and post-DREAMS session values within 1, 2, 4, 6, 8, and 12 hours of the DREAMS session. Dosages of opioids were converted to oral morphine milligram equivalents (MMEs) prior to analysis. Medications received during an operation were excluded from the analysis. Statistical features were extracted from time series physiologic data including minimum, maximum, variance, and mean across study days for all time intervals. All significance tests were two-sided with α less than 0.05 considered statistically significant. Statistical analyses were performed with R v.3.6 (R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org).
RESULTS
Participants
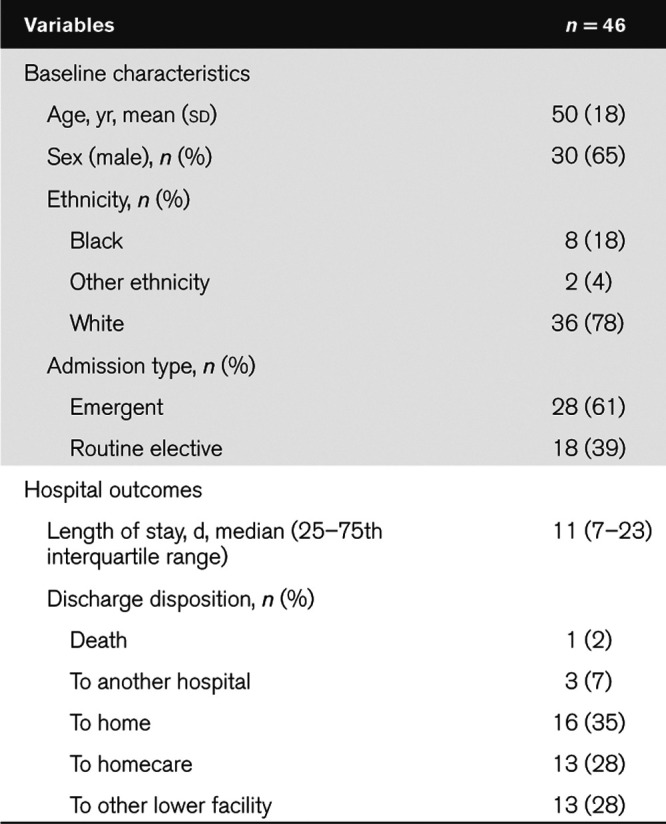
A total of 59 participants were recruited (Fig. 2). Thirteen participants did not complete the study due to emergent surgery or discharge from the ICU. The remaining 46 participants received either one (n = 17), two (n = 17) or three to seven (n = 12) DREAMS sessions. Participants were generally older (male = 50 yr, sd = 18) and male (65%) (Table 1). The median hospital stay was 11 days.
Figure 2.
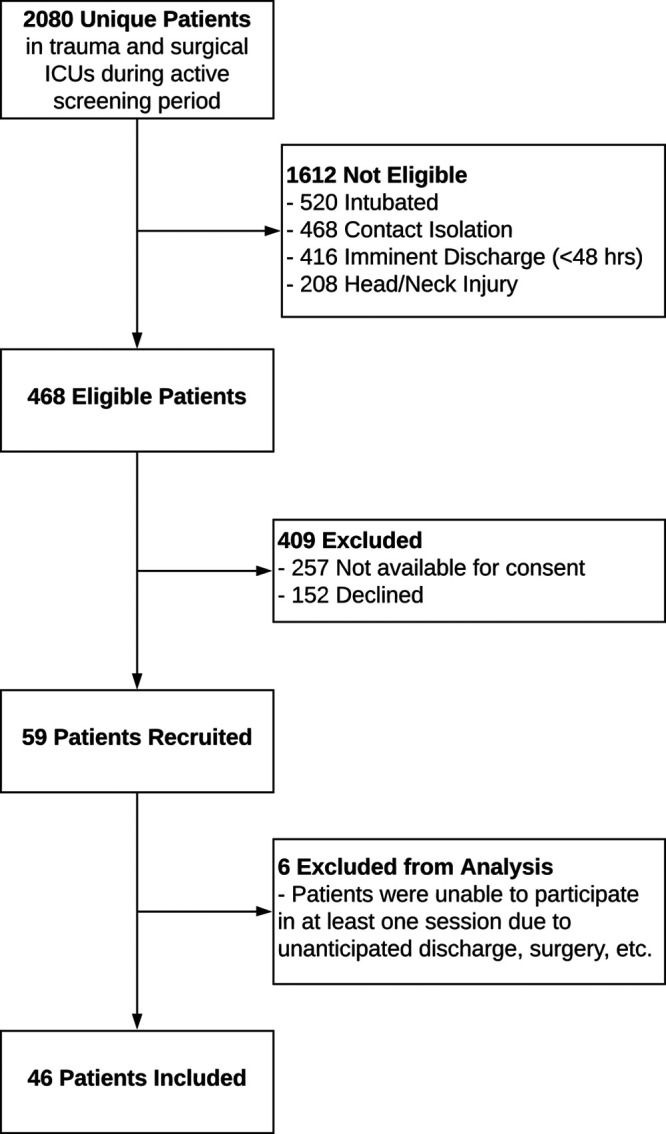
Screening consort diagram.
TABLE 1.
Cohort Age, Sex, Ethnicity, Admission Type, Hospital Length of Stay, and Discharge Disposition
Pain
The DVPRS is a self-reported visual analog scale that ranges from 0 (“no pain”) to 10 (so painful that “nothing else matters”). No statistically significant relationship was found between study day and DVPRS at any time point (p > 0.05; Fig. S1, Supplemental Digital Content 2, http://links.lww.com/CCX/A175; legend: mean DVPRS pain improvement before and after VR exposure). Despite no statistically significant improvement in DVPRS, 81% of patients agreed or strongly agreed with the statement, “I feel that I experienced less pain yesterday because of the DREAMS” (Fig. S2, Supplemental Digital Content 3, http://links.lww.com/CCX/A176; legend: patients’ perceptions of how DREAMS decreased their pain).
The dosage and frequency of opioid medications decreased over time at a rate of 12.9 (95% CI, 21.7–4.03) oral MMEs per study day. No statistically significant changes were found when comparing dosage or frequency before and after any intervention. Nonetheless, the observed decreases in PRN opioid dosages may be clinically significant with an average decrease from 54.8 MME after the first intervention to 11.5 MME after the third intervention (Table S1, Supplemental Digital Content 4, http://links.lww.com/CCX/A177).
Sleep
The RCSQ is a series of six questions about last night’s rest that patients scored from 0 to 100 (higher indicates better sleep). Participants’ RCSQ score improved by 4.56 (95% CI, 1.06–8.06) points each study day (Fig. S3, Supplemental Digital Content 5, http://links.lww.com/CCX/A178; legend: sleep improvement over time compared with baseline); however, there was no statistically significant difference observed when comparing successive nights sleep or baseline sleep quality to a given study day.
Affect
The HADS is a scale for patients to estimate their current anxiety and depression. A subscore of 0–7 is considered normal affect, 8–10 borderline, and 11–21 abnormal. We compared participants’ HADS’ subscores before their first DREAMS exposure to just before their second and third exposures. There was no statistically significant change in anxiety or depression before the second session; however, there were statistically significant decreases in anxiety (estimate = –2.17; 95% CI, –4.23 to –0.106) and depression (estimate = –1.25; 95% CI, –2.37 to –0.129) from before the first exposure to before the third exposure (Fig. 3). Ten of 13 patients with borderline depression improved to normal during the study. No patients transitioned to a worse depression classification during the study. Four of 10 patients with abnormal anxiety improved, with two of those patients reaching normal range during the study. Five of the 11 patients with borderline anxiety improved to normal during the study. Three patients experienced an increase in anxiety during the study.
Figure 3.
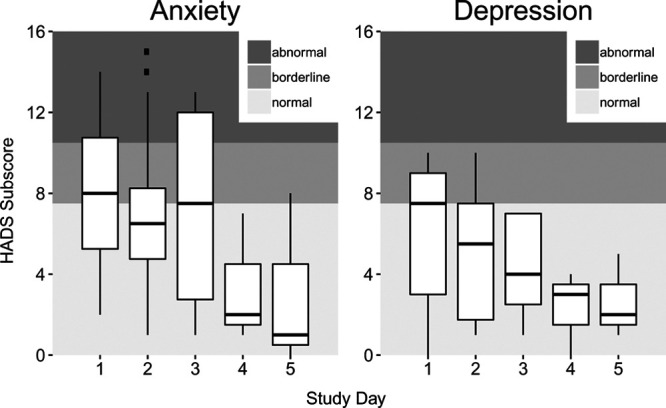
Hospital Anxiety and Depression Scale (HADS) subscores for Digital Rehabilitation Environment Augmenting Medical System participants.
Delirium
Of the 46 subjects that participated in a DREAMS session, 13 were delirious for at least 1 day during their admission. Seven participants were delirious prior to the study but recovered before enrollment and remained nondelirious until discharge. The other six patients became delirious after completing the DREAMS study and were diagnosed an average of 84 hours after their final DREAMS session. We found no reason to suspect DREAMS contributed to these participants’ delirium in patient interviews or informal check-ins with these participants’ nurses.
Vital Signs
Patients’ systolic BP, diastolic BP, mean arterial pressure, HR, or RR were compared at 1, 2, 4, 6, 8, and 12 hours before and after each DREAMS session. No statistically significant differences were observed in pre- versus post-session, minimum, maximum, mean, or variability at any time interval or session number.
Participants’ Reactions to DREAMS
Participants were asked to rate and discuss how much they agreed with statements about their use of DREAMS. They agreed that DREAMS was comfortable (Comfort), enjoyable (Enjoyment), helped them better manage their pain (Pain), and that they thought about DREAMS outside of sessions (Reflection). However, participants were mixed on whether DREAMS helped them sleep better (Sleep) (Fig. 4). Transcripts of audio recordings were analyzed qualitatively to identify themes in participants’ responses.
Figure 4.
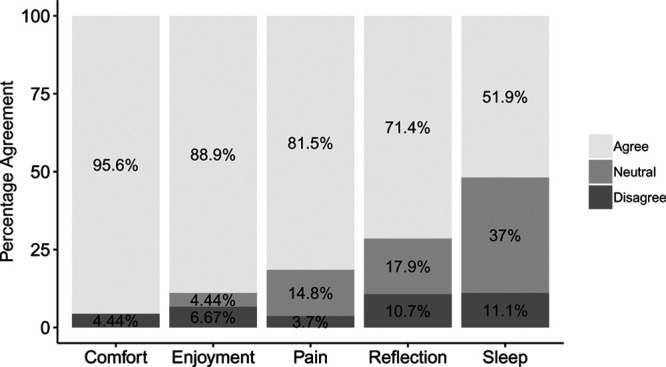
Participants’ reaction to the Digital Rehabilitation Environment Augmenting Medical System (DREAMS): Comfort = “I thought using the DREAMS was comfortable,” Enjoyment = “I liked the experience of using the DREAMS,” Pain = “I feel that I experienced less pain yesterday because of the DREAMS,” Reflection = “I found myself thinking about the DREAMS after the session was over,” Sleep = “I feel that I slept better last night because of the DREAMS.”
Novelty of Virtual Reality.
Although only one participant had prior experience with VR, the enjoyment of DREAMS was universal. Participants were often observed smiling, laughing, and giving positive remarks during use of DREAMS. Several participants recommended wider deployment of DREAMS. “[Other ICU patients] would be crazy if they didn’t [want to try DREAMS]…she was telling you how to breathe, and I could see how that could be beneficial for us in here, or for anybody.” Participants enjoyed DREAMS enough to inquire about purchasing. “I was going to ask you if I could buy one,” “Is this on iPhone? You should put it on an app!” Participants also volunteered feedback for improvements of DREAMS. Most comments involved better visuals and audio for improved immersion. “The waves were good, don’t get me wrong…But to get the authenticity of it all, you need [seagulls] just in the distance.” Participants were generally interested in the novelty of VR and expressed excitement about its use in the ICU.
Emotionally Evocative
The immersive virtual environments depicted in “Pearl” and “RelaxVR” tended to evoke nostalgic feelings. One participant noted, “[the rocks] remind me of the shores of Maine” while another stated that the beach scene made her think of family vacations she would like to plan after discharge. Some emotional reactions to “Pearl” were unexpected. “Pearl” is a short VR film about a family told from the inside of their family car. Several participants reported feeling nervous about the depiction. “Not safe driving…Sure tells you what not to do, when [the father] jumped into the back seat. He’s stupid.” Another participant noted sarcastically, “It’s funny, he’s driving while playing the guitar. Seems totally safe.” One participant experienced an especially negative emotional reaction to “Pearl” due to a previous experience of a loved one lost in a car accident. The immersive properties of VR can be a powerful tool for health promotion. It is important, however, that researchers, clinicians, and developers be aware of potential negative reactions that can be particularly strong in VR.
Relaxing.
Participants would often vocalize statements about relaxation and drift into sleep during VR sessions. For some participants, DREAMS provided a welcomed feeling of privacy and escape from their ICU room. Participants noted that “I liked that I could enjoy it [by myself]” and “[VR] is better than the TV because you’re there [the beach].” The DREAMS provided participants with an isolated visual and auditory environment to focus on their relaxation and breathing, which may have provided an escape or distraction from their uncomfortable but necessary recovery situation.
Technical Frustrations.
Sometimes participants were provided with suboptimal VR experiences due to errors with the equipment or software. Headsets that shifted during sessions would allow light from the ICU to bleed into the screen and disrupt the viewing experience. A blinking notification light became activated during one participant’s session, who later noted feeling dizzy as a result.
Temporary Effects
Participants seemed aware that the physiologic effects of DREAMS were negligible. The DREAMS was reported to have distracted from pain, but only during VR exposure. Several participants noted that they received medication that affected their sleep and made it difficult to say if DREAMS contributed. “Unfortunately, it was probably the melatonin…I’m sure [DREAMS helped me sleep] because I did not have the same problem.” Participants were unsure if DREAMS helped with pain, mostly due to the critical nature of their status. “I can’t tell [if DREAMS helped with my pain] because yesterday was pretty painful.” Another participant noted, “I mean, my leg pain was real bad, so I can’t really put it on [DREAMS].” Despite this, participants still reported enjoying DREAMS. When asked if DREAMS helped with his pain, one participant responded, “Not really, but it’s a good part of my day.”
DISCUSSION
We demonstrated feasibility for the use of VR relaxation in ICU patients. Despite finding no clinically or statistically significant effects on physiology, pain, or sleep, participants overwhelmingly enjoyed the VR experiences provided by DREAMS. Our results show that ICU patients are eager to participate and that VR may serve as a welcomed distraction from unavoidable discomforts associated with ICU care. Collectively, these results show VR to be a promising option to help improve the ICU patient experience.
The ICU is a busy, noisy setting in which patients of the greatest need are closely monitored by highly trained staff. Proposed additions to the ICU must be effective, simple, and affordable. DREAMS equipment was easy to set up, intuitive to operate, and enjoyed by participants. Each component of DREAMS is widely available, and most people are already familiar with smartphone interfaces. Furthermore, DREAMS can be quickly and easily sanitized with common medical disinfectant wipes, disposable sanitary covers, and ultraviolet germicidal equipment such as Cleanbox (cleanboxtech.com). Combined with participants’ enjoyment of the VR, this makes DREAMS and similar systems remarkably portable and ideal candidates for deployment in the ICU.
The primary goal of this study was to assess feasibility of VR for ICU patients’ experience. Participants were enthusiastic about use of VR in the ICU, but we did not find clinically or statistically significant effects in health outcomes such as pain, vital signs, or sleep. It should be noted that participants only received 5 to 20 minutes of VR exposure each day and that granularity of vital signs data was limited. It seems unlikely that 5 to 20 minutes of VR exposure would produce large effects in a critical care environment, but this remains an important topic to evaluate in future research. Previous research has shown that 40 minutes of VR exposure repeated across three months has been implemented with positive results (15). There remains good reason to hypothesize that VR can help patients better manage stress and discomfort in the ICU (20). Additionally, participants’ objective measures of pain (DVPRS) did not change, while their subjective account of pain (DREAMS questionnaire) indicated favorable effects. This discrepancy could be due to confirmation bias in our questionnaires, demand characteristics of our intervention, or subtle effects of VR not identified in this study and requires further investigation.
Our results may have been influenced by selection bias. We recruited ICU patients who were conscious, not intubated, not in isolation, and not already delirious. Although VR would not be helpful for the unconscious or severely delirious, ICU patients who are otherwise awake should be included in future research as they are at the greatest risk for developing ICUAW and delirium. Technical and procedural difficulties can contribute to participant frustration and should be minimized with training and preparation to provide the best experience possible. It will also be important to tailor VR equipment for the unique demands of the ICU. Most commercially available VR apps require the user to walk or stand and rotate to fully engage with the VR experience, which are potentially uncomfortable or unsafe prospects for many ICU patients. It will be important for researchers, clinicians, developers, and ICU survivors to collaborate in the design of VR equipment and software specific to the ICU patient experience—including those who may be immobile, intubated, or in contact isolation.
ICU patients are likely to experience unease and uncertainty in their recovery. These patients are under constant observation and receive the best medical care available. However, the vast majority of their time in the ICU is spent in prolonged discomfort and sedentary in an austere environment. VR technologies are relatively affordable, increasingly easy to use, and enjoyed by patients. Therapies in VR can be tailored to the needs of ICU patients to help manage pain, reduce stress, and provide a welcome distraction from the uncomfortable nature of their current condition.
CONCLUSIONS
A VR meditative intervention improved patients’ experiences in the ICU by reducing anxiety and depression; however, there was no evidence that VR had significant effects on vital signs, pain, or sleep. The use of VR in the ICU was easily implemented and well-received by patients. The DREAMS project demonstrates that interdisciplinary collaborations between clinical researchers, artists, engineers, and psychologists can implement emerging technologies to improve patients’ experiences in the ICU.
ACKNOWLEDGMENTS
We thank Tyler Loftus contributed to data analyses and interpretation and writing of the revision. Sherry Brown and George Omalay for their valuable assistance in planning and conducting this research. Julia Cupka, Laura Velez, and Haleh Hashemighouchani managed data collection and contributed to data analyses and article preparation. We would also like to thank research assistants (Ria Bhaskar, Ryan Cherico, Elizabeth Ingersent, and Emilie Pearson) for their assistance during data collection and analysis. The authors would like to thank “RelaxVR” for allowing the use of the screenshot depicted in Figure 1B.
Supplementary Material
Footnotes
Dr. Ong, Mr. Ruppert, and Drs. Bihorac and Suvajdzic contributed equally to this article.
Dr. Ong and Mr. Ruppert contributed equally as first authors to this article, while Drs. Bihorac and Suvajdzic contributed equally as senior authors.
Dr. Ong contributed to study development, data analysis, and writing. Mr. Ruppert contributed to study development, managed data collection, data analysis, and writing. Dr. Ozrazgat-Baslanti contributed to data analyses and interpretation and writing. Ms. Akbar contributed to data analyses and interpretation and writing. Dr. Rashidi coordinated recruitment and data collection. Dr. Suvajdzic conceptualized, planned, and developed study, oversaw virtual reality technologies and session procedures, and contributed to writing. Dr. Bihorac conceptualized, planned, and developed study and all experiments, supervised medical conduct and ICU protocol, analyzed and interpret data, and contributed to writing. All authors read and approved the final article.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
This work was supported in part by the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Sciences Award to the University of Florida UL1 TR000064 and by internal funding from Digital Worlds Institute.
Drs. Rashidi, Ozrazgat-Baslanti, and Bihorac were supported by R01 GM110240 from the National Institute of General Medical Sciences. Drs. Ozrazgat-Baslanti and Bihorac were supported by Sepsis and Critical Illness Research Center Award P50 GM-111152 from the National Institute of General Medical Sciences. Ruppert and Dr. Bihorac were supported by Davis Foundation – University of Florida. Dr. Rashidi was supported by the National Science Foundation Faculty Early Career Development Program 1750192. Dr. Ozrazgat-Baslanti has received grant that was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427 and received grant from Gatorade Trust (127900), University of Florida. The remaining authors have disclosed that they do not have any potential conflicts of interest.
All procedures were reviewed and approved by University of Florida Institutional Review Board-02. All participants provided informed consent prior to participation.
The datasets generated and/or analyzed during the current study are not publicly available to protect patient confidentiality but are available from the corresponding author on reasonable request.
The trial was registered on December 29, 2017, with ClinicalTrials.gov with the identifier: NCT03385993.
REFERENCES
- 1.Zorowitz R. ICU-acquired weakness: A rehabilitation perspective of diagnosis, treatment, and functional management. Chest. 2016; 150:966–971 [DOI] [PubMed] [Google Scholar]
- 2.Kalabalik J, Brunetti L, El-Srougy R. Intensive care unit delirium: A review of the literature. J Pharm Pract. 2014; 27:195–207 [DOI] [PubMed] [Google Scholar]
- 3.Svenningsen H, Langhorn L, Ågård AS, et al. Post-ICU symptoms, consequences, and follow-up: An integrative review. Nurs Crit Care. 2017; 22:212–220 [DOI] [PubMed] [Google Scholar]
- 4.Wilson ME, Beesley S, Grow A, et al. Humanizing the intensive care unit. Crit Care. 2019; 23:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: New innovations in nutrition and exercise physiology. Crit Care. 2015; 19Suppl 3S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mistraletti G, Pelosi P, Mantovani ES, et al. Delirium: Clinical approach and prevention. Best Pract Res Clin Anaesthesiol. 2012; 26:311–326 [DOI] [PubMed] [Google Scholar]
- 7.Brummel NE, Girard T. Preventing delirium in the intensive care unit. Crit Care Clin. 2013; 29:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 9.Gigante MA, Jones H, Earnshaw R. Virtual Reality Systems. 1993First Edition, London, United Kingdom: Academic Press [Google Scholar]
- 10.Stetz MAJMC, Ries RI, Folen R. Brahnam S, Jain L. Virtual reality supporting psychological health. Advanced Computational Intelligence Paradigms in Healthcare 6. 2011, Berlin, Germany: Springer; 13–29 [Google Scholar]
- 11.Morina N, Ijntema H, Meyerbröker K, et al. Can virtual reality exposure therapy gains be generalized to real-life? A meta-analysis of studies applying behavioral assessments. Behav Res Ther. 2015; 74:18–24 [DOI] [PubMed] [Google Scholar]
- 12.Ryu JH, Park SJ, Park JW, et al. Randomized clinical trial of immersive virtual reality tour of the operating theatre in children before anaesthesia. Br J Surg. 2017; 104:1628–1633 [DOI] [PubMed] [Google Scholar]
- 13.Ganry L, Hersant B, Sidahmed-Mezi M, et al. Using virtual reality to control preoperative anxiety in ambulatory surgery patients: A pilot study in maxillofacial and plastic surgery. J Stomatol Oral Maxillofac Surg. 2018; 119:257–261 [DOI] [PubMed] [Google Scholar]
- 14.Faber AW, Patterson DR, Bremer M. Repeated use of immersive virtual reality therapy to control pain during wound dressing changes in pediatric and adult burn patients. J Burn Care Res. 2013; 34:563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dascal J, Reid M, IsHak WW, et al. Virtual reality and medical inpatients: A systematic review of randomized, controlled trials. Innov Clin Neurosci. 2017; 14:14–21 [PMC free article] [PubMed] [Google Scholar]
- 16.Buckenmaier CC, 3rd, Galloway KT, Polomano RC, et al. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med. 2013; 14:110–123 [DOI] [PubMed] [Google Scholar]
- 17.Richards KC, O’Sullivan PS, Phillips R. Measurement of sleep in critically ill patients. J Nurs Meas. 2000; 8:131–144 [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983; 67:361–370 [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001; 29:1370–1379 [DOI] [PubMed] [Google Scholar]
- 20.Villani D, Preziosa A, Riva G. Coping with stress using virtual reality: A new perspective. Annual Review of CyberTherapy and Telemedicine. 2006; 4:25–32 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


