Supplemental Digital Content is available in the text.
Keywords: cardiac arrest, massive pulmonary embolism, pulmonary embolism, thrombolytic
Abstract
Objectives:
This survey sought to characterize the national prescribing patterns and barriers to the use of thrombolytic agents in the treatment of pulmonary embolism, with a specific focus on treatment during actual or imminent cardiac arrest.
Design:
A 19-question international, cross-sectional survey on thrombolytic use in pulmonary embolism was developed, validated, and administered. A multivariable logistic regression was conducted to determine factors predictive of utilization of thrombolytics in the setting of cardiac arrest secondary to pulmonary embolism.
Setting:
International survey study.
Subjects:
Physicians, pharmacists, nurses, and other healthcare professionals who were members of the Society of Critical Care Medicine.
Interventions:
None.
Measurements and Main Results:
Thrombolytic users were compared with nonusers. Respondents (n = 272) predominately were physicians (62.1%) or pharmacists (30.5%) practicing in an academic medical center (54.8%) or community teaching setting (24.6%). Thrombolytic users (n = 177; 66.8%) were compared with nonusers (n = 88; 33.2%) Thrombolytic users were more likely to work in pulmonary/critical care (80.2% thrombolytic use vs 59.8%; p < 0.01) and emergency medicine (6.8% vs 3.5%; p < 0.01). Users were more likely to have an institutional guideline or policy in place pertaining to the use of thrombolytics in cardiac arrest (27.8% vs 13.6%; p < 0.01) or have a pulmonary embolism response team (38.6% vs 19.3%; p < 0.01). Lack of evidence supporting use and the risk of adverse outcomes were barriers to thrombolytic use. Working in a pulmonary/critical care environment (odds ratio, 2.36; 95% CI, 1.24–4.52) and comfort level (odds ratio, 2.77; 95% CI, 1.7–4.53) were predictive of thrombolytic use in the multivariable analysis.
Conclusions:
Most survey respondents used thrombolytics in the setting of cardiac arrest secondary to known or suspected pulmonary embolism. This survey study adds important data to the literature surrounding thrombolytics for pulmonary embolism as it describes thrombolytic user characteristic, barriers to use, and common prescribing practices internationally.
Unexplained cardiac arrests have been attributed to pulmonary embolism (PE) in 5–13% of cases, with acute PE found to be a definitive cause in 5–6% of in-hospital cardiac arrests (1, 2). Massive PE leads to shock and cardiac arrest due to elevated right heart pressures that cause left ventricular septal shift and impaired cardiac output (3). As a result, electrical activity is preserved and routine acute cardiac life support (ACLS) interventions are largely ineffective (4). Thrombolytic agents bind to fibrin to convert plasminogen to plasmin, promoting rapid clot dissolution (5). However, use of thrombolytic agents is also associated with increased risk for bleeding and high cost with controversial mortality and morbidity benefits (1).
Multiple society guidelines agree on the rapid initiation of thrombolytic agents or mechanical therapies as the mainstays of treatment for massive or submassive PE but lack specific recommendations on agent choice, dosing, and infusion duration because of low quality of evidence (6–9). The decision to initiate thrombolytic therapy in this setting is based heavily on clinical judgment as patients often present with a limited history and nonspecific physical examination. Definitive diagnosis with imaging is seldom available. However, due to the high mortality associated with untreated massive PE, providing early resolution of pulmonary obstruction with thrombolytic therapy in a patient with low bleeding risk may result in favorable outcomes, especially when suspicion for a massive PE is high.
Although no robust prospective studies have shown a survival benefit with the use of thrombolytic therapy in cardiac arrest due to PE, numerous cases reports and cohort studies have observed increased rates of return of spontaneous circulation (ROSC), survival to discharge, and 30-day survival (4, 5, 10). Although these studies included a wide range of dosing practices with many utilizing the Food and Drug Administration approved dose for lysis of massive PE (alteplase 100 mg IV over 2 hr), recent most common treatment was generally given as alteplase 50 mg as a single bolus with or without a subsequent continuous infusion or bolus (11–13). Given the heterogeneity across studies regarding thrombolytic agent, dose, and threshold for initiation of therapy, prescribing practices are likely driven by clinician experience and practice site. This study sought to characterize the national prescribing patterns and barriers to the use of thrombolytic agents in the treatment of PE, with a specific focus on treatment during actual or imminent cardiac arrest.
MATERIALS AND METHODS
This was an international, cross-sectional survey administered to critical care or emergency medicine pharmacists, nurses, physicians, and other healthcare professionals who were members in the Society of Critical Care Medicine (SCCM).
A 19-item survey instrument was developed that contained the following domains: demographics, characterization of thrombolytic use for PE, and barriers to use of thrombolytics for PE. The survey instrument was developed according to the following steps: survey item generation, initial survey construction, pilot testing of survey items for face and content validity by 19 critical care and emergency medicine clinicians, incorporation of written and verbal suggestions from pilot testers based on consensus of the study investigators, and agreement from all study investigators on the final survey instrument (14, 15).
The survey instrument was distributed in May 2017 using an online survey software and insight platform (Opinion AS, Oslo, Norway). Data from each respondent were aggregated such that no personal information linking specific responses to a specific participant was retained. Participation was voluntary and could be stopped at any point during the survey. Respondents who completed at least 75% of the survey had their responses included. Survey questions pertaining to institutional practices were included one-time per center and evaluated for consistency.
Descriptive statistics, including frequencies, means, and sds, were used to describe study data. Categorical variables were compared using chi-square test or Fisher exact test, as appropriate. Participants were dichotomized into users versus nonusers of thrombolytics for PE in the last year. A multivariable logistic regression was conducted to determine factors predictive of utilization of thrombolytics in the setting of cardiac arrest secondary to PE. Candidate variables were selected for inclusion based on prior literature and evaluating variables in the univariate analysis with a p value of less than or equal to 0.2. Analyses were conducted using STATA Version 15 (StataCorp, College Station, TX). Institutional review board approval was obtained prior to survey dissemination.
RESULTS
Overall, 272 of the 7,900 invited participants completed the survey (response rate 3.4%). Of these, seven respondents were excluded from the analysis because of missing data, leaving 265 participants. All regions of the United States were well-represented; international respondents comprised of 13.9% participants (Table 1). A majority of the respondents practiced in pulmonary/critical care, followed by surgery/trauma, anesthesiology, and emergency medicine. Respondents were most commonly physicians (63.5%) or pharmacists (31.2%). Participants had a wide range of years in practice ranging from less than 3 years to greater than 14 years. Most respondents practiced in an academic center (55.8%), community teaching hospital (25.1%), or community nonteaching hospital (16.5%).
TABLE 1.
Baseline and Institutional Demographics
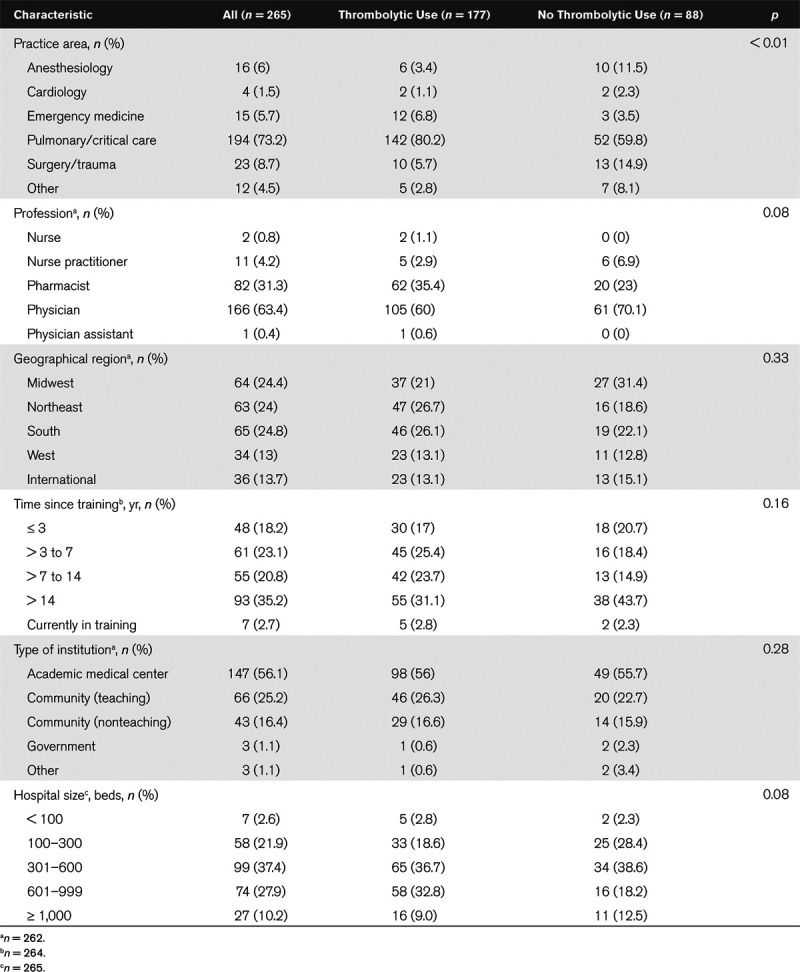
Most respondents (57%) reported using thrombolytics during cardiac arrest one to three times in the last year, followed by not using thrombolytics (33.2%) in this setting. A small proportion of respondents (9.8%) used thrombolytics more than three times in the last year. Groups were dichotomized according to thrombolytic use (n = 177; 66.8%) and no thrombolytic use (n = 88; 33.2%) for cardiac arrest in the last year. Thrombolytic users were more likely to work in pulmonary/critical care (80.2% thrombolytic use vs 59.8% no thrombolytic use; p < 0.01) and emergency medicine (6.8% vs 3.5%; p < 0.01) and less likely to work in a surgery/trauma or anesthesia. A higher proportion of utilizers were pharmacists (35.4% vs 23%; p = 0.08) and a lower proportion were physicians (60% vs 70.1%; p = 0.08). Groups were similar in terms of geographic region, type of hospital, and years of experience (Table 1).
Thrombolytic users were more likely to be very comfortable or somewhat comfortable in the setting of cardiac arrest secondary to known PE (98.3% vs 81.9%; p < 0.01) or cardiac arrest secondary to suspected PE (88% vs 60.2%; p < 0.01) (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A192). Users were more likely to have an institutional guideline or policy in place pertaining to the use of thrombolytics in the setting of cardiac arrest or have a PE response team (PERT) established or under development. Alteplase was on formulary commonly in both groups; however, tenecteplase was more likely to be a formulary agent in the thrombolytic use group (27.7% vs 11.4%; p < 0.01). Users were more likely to know where thrombolytics were stored in the hospital, and more likely to have access to thrombolytics in automated dispensing cabinets.
There was significant variation in dosing practices in both groups. The most common regimen specified for thrombolytic users was alteplase 50 mg IV bolus over 2 minutes with the ability to repeat in 10–30 minutes if no ROSC, followed by tenecteplase dosing based on weight category. For nonusers, the more common regimen specified was “other,” followed by alteplase 50 mg IV bolus over 2 minutes with the ability to repeat in 10–30 minutes if no ROSC. Anticoagulant use was similar between groups (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A192).
Thrombolytic users were more likely to report that they had no barriers to using thrombolytics (50.9% vs 39.8%; p = 0.09). Nonusers were more likely to cite a lack of evidence supporting use (14.1% vs 27.3%; p = 0.01) and the risk of adverse outcomes (11.9% vs 22.7%; p = 0.03) as barriers compared with users (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A192). Considerations for determining eligibility for thrombolytics were assessed. Significant right ventricular strain (59.5%), perceived risk of bleeding (72.8%), patient-specific factors (e.g., weight, comorbid conditions) (50.6%), and hemodynamic instability (92.5%) were most commonly cited as “always” considerations for initiation of thrombolytics. Most respondents (74.5%) reported that cost of therapy was a consideration less than half of the time. The most common factors considered to be very important in determining eligibility for thrombolytics in the setting of cardiac arrest were active bleeding (76.1%) and known positive venous thromboembolism (58.1%; Fig. 1).
Figure 1.
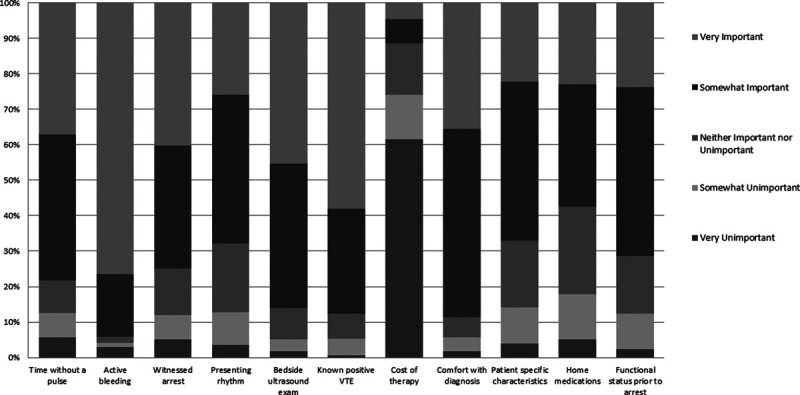
Important factors in determining thrombolytic eligibility. VTE = venous thromboembolism.
The following variables were included in the multivariable logistic regression: profession (physician, pharmacist, or other), practice area, PERT, years in practice, comfort level in treating cardiac arrest secondary to known PE, and the barriers assessed including lack of comfort, lack of evidence supporting use of thrombolytics for PE and adverse outcomes. Of these, working in a pulmonary/critical care environment (odds ratio [OR], 2.36; 95% CI, 1.24–4.52) and comfort level (OR, 2.77; 95% CI, 1.7–4.53) were predictive of thrombolytic use.
DISCUSSION
This study found that two-thirds of respondents used thrombolytics in the setting of cardiac arrest secondary to known or suspected PE. This is an important finding as, while clinical practice guidelines recommend the use of thrombolytics in the setting massive PE or selected submassive PE at risk of decompensation with low risk of bleeding (6–8, 16), they are largely silent as to whether thrombolytic agents are indicated once the patient has progressed from massive PE to cardiac arrest. The 2015 ACLS guidelines recommend that in patients with “confirmed” PE as the precipitating factor associated with cardiac arrest, thrombolysis, surgical embolectomy, and mechanical embolectomy are reasonable emergency treatment options (1). However, no guidance is available as to which patients should receive thrombolytics or timing, drug, or dose in this setting. This survey study adds important data to the current paucity of literature surrounding thrombolytics for PE. Although limited in design and scope, it describes thrombolytic user characteristics, barriers to use, and common prescribing practices internationally.
Respondents who used thrombolytics in the setting of PE were more likely to work in pulmonary/critical care or emergency medicine. Furthermore, working in a pulmonary/critical care was predictive of use of thrombolytics after adjustment in a multivariable analysis. Although this finding is to be expected as these providers may have frequent exposure to patients with PE, it also may pertain to the general demographic of SCCM, which is largely comprised of pulmonary/critical care clinicians. Respondents that used thrombolytics for cardiac arrest unsurprisingly reported being either very comfortable or somewhat comfortable with use in the setting of cardiac arrest secondary to both known and suspected PE compared with nonusers. A higher proportion of pharmacists were thrombolytic users, while a higher proportion of physicians were in the nonuser group. This could be due to a variety of factors, including a propensity to recommend pharmacologic agents given pharmacists’ expertise in pharmacology or their role in the policy development and education (17).
Thrombolytic users reported that institutional guidelines or policies were in place more frequently for thrombolytics in the setting of cardiac arrest. Furthermore, PERTs were more common at institutions where thrombolytics are used. PERTs mobilize the expertise a diverse groups of clinicians to evaluate and treat patients with massive and submassive PE (18). These teams design an individualized patient care plan in a time-dependent manner. Ideally, PERT teams consist of a multidisciplinary group, ranging considerable between institutions, that can bridge care from inpatient to outpatient (19). These teams typically comprise representatives from pulmonary/critical care, interventional cardiology, and emergency medicine, cardiac surgery, interventional radiology, noninterventional cardiology, hematology, and vascular medicine (20). They may also develop institutional guidelines and policies and disseminate education pertaining to PE (21). Users of thrombolytics were more likely to know where thrombolytics were stocked within the institution and report a higher frequency of availability within automatic dispensing cabinets in the emergency department or ICU. Furthermore, users were more likely to stock tenecteplase at their respective institutions, which may represent clinician and institutional preferences but could be confounded by a response bias. Respondents, particularly those who are users, may have a greater interest and experience with PE than nonrespondents and could suggest that utilizers have more advanced processes in place to facilitate the administration of thrombolytics.
Lack of evidence supporting thrombolytic agent use and adverse effects were common barriers cited by nonusers. Few high-quality studies have evaluated thrombolytic use in the setting of cardiac arrest secondary to known or suspected PE. Tenecteplase has been evaluated for treatment of PE in the prehospital setting in patients presenting with asystole or pulseless electrical activity. This study was the largest of its kind (n = 1,050) and found no difference in 30-day mortality (14.7% tenecteplase vs 17% placebo; p = 0.36) or ROSC (55.0% vs 54.6%; p = 0.96) (22). However, myocardial infarction precipitated most events, with only 8% of study participants experiencing PE (n = 37 confirmed PEs) as the etiology of cardiac arrest. Furthermore, intracranial hemorrhage occurred more frequently in the tenecteplase group (2.7% vs 0.4%; p < 0.01). A prospective cohort of out-of-hospital cardiac arrest for suspected cardiac etiology included 40 patients who received alteplase 50 mg IV over 2 minutes with an option for a second dose and heparin 5,000 units IV compared with 50 historical controls (12). The alteplase group had higher rates of ROSC (68% vs 44%; p = 0.03) and ICU admission (58% vs 30%; p < 0.01); however, no differences in mortality, bleeding, or other outcomes were observed. It is unclear how many of these patients experienced cardiac arrest due to PE. A subsequent small, retrospective study of 104 patients who received alteplase for cardiac arrest secondary to confirmed or “highly suspected” PE found the rate of ROSC to be 38.5% (23). Patients with ROSC received thrombolysis significantly earlier after cardiopulmonary resuscitation initiation than those without ROSC. Although other studies have also found higher rates of ROSC, admission to hospital and survival (5, 24–27), most available data are retrospective with a limited number of patients, not specific to PE, and with variable bleeding-related safety events reported. Given there is limited high-quality literature surrounding this disease state, it is unsurprising that lack of evidence was a commonly cited barrier to using thrombolytics.
There was significant heterogeneity for thrombolytic and anticoagulant regimens initiated in the setting of cardiac arrest secondary to PE. The most common regimens were alteplase 50 mg IV bolus over 2 minutes (may repeat in 10–30 min if no ROSC) and tenecteplase dose 30 to 50 mg IV bolus, depending on weight category. These regimens are supported by the largest clinical trials available in this setting, although limited data preclude definitive recommendations (10, 22, 23).
This study has several limitations that warrant discussion. First, although our response rate was less than ideal, it was representative of multiple professions and within the range of other published studies of critical care providers (28). Respondent self-selection among those who are more highly skilled and represent practices at institutions where clinicians provide bedside care to patients with cardiac arrest secondary to PE may have influenced our results, although the impact of this self-selection process is difficult to assess. Furthermore, users were grouped according to whether the respondent had actually used a thrombolytic in the setting of cardiac arrest secondary to known or suspected PE rather than asking case-based questions to assess opinions of use. We feel this approach is appropriate as PE is a common etiology of cardiovascular collapse and use was assessed over an entire year (2).
CONCLUSIONS
Most survey respondents used thrombolytics in the setting of cardiac arrest secondary to known or suspected PE. This survey study adds important data to the literature surrounding thrombolytics for PE as it describes thrombolytic user characteristic, barriers to use, and common prescribing practices internationally. In the absence of large, randomized controlled trials, this study helps to describe common practices pertaining to thrombolytics for PE in the setting of cardiac arrest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015; 132:S501–S518 [DOI] [PubMed] [Google Scholar]
- 2.Laher AE, Richards G. Cardiac arrest due to pulmonary embolism. Indian Heart J. 2018; 70:731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laher AE, Moolla M, Motara F, et al. Survival after cardiac arrest secondary to massive pulmonary embolism. Case Rep Emerg Med. 2018; 2018:8076808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan JK, Pantle H, Huiras P, et al. Evidence-based diagnosis and thrombolytic treatment of cardiac arrest or periarrest due to suspected pulmonary embolism. Am J Emerg Med. 2014; 32:789–796 [DOI] [PubMed] [Google Scholar]
- 5.Dirican A, Ozkaya S, Atas AE, et al. Thrombolytic treatment (alteplase; rt-Pa) in acute massive pulmonary embolism and cardiopulmonary arrest. Drug Des Devel Ther. 2014; 8:759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016; 149:315–352 [DOI] [PubMed] [Google Scholar]
- 7.British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax. 2003; 58:470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinides S. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35:3145–3146 [DOI] [PubMed] [Google Scholar]
- 9.Jaff MR, McMurtry MS, Archer SL, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation. 2011; 123:1788–1830 [DOI] [PubMed] [Google Scholar]
- 10.Böttiger BW, Martin E. Thrombolytic therapy during cardiopulmonary resuscitation and the role of coagulation activation after cardiac arrest. Curr Opin Crit Care. 2001; 7:176–183 [DOI] [PubMed] [Google Scholar]
- 11.Peppard SR, Parks AM, Zimmerman J. Characterization of alteplase therapy for presumed or confirmed pulmonary embolism during cardiac arrest. Am J Health Syst Pharm. 2018; 75:870–875 [DOI] [PubMed] [Google Scholar]
- 12.Böttiger BW, Bode C, Kern S, et al. Efficacy and safety of thrombolytic therapy after initially unsuccessful cardiopulmonary resuscitation: A prospective clinical trial. Lancet. 2001; 357:1583–1585 [DOI] [PubMed] [Google Scholar]
- 13.Kürkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: Presentation and outcome. Arch Intern Med. 2000; 160:1529–1535 [DOI] [PubMed] [Google Scholar]
- 14.Fowler F. Survey Research Methods. 2009Fourth Edition, Thousand Oaks, CA: SAGE Publications [Google Scholar]
- 15.Draugalis JR, Plaza C. Best practices for survey research reports revisited: Implications of target population, probability sampling, and response rate. Am J Pharm Educ. 2009; 73:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torbicki A, Perrier A, Konstantinides S, et al. ; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008; 29:2276–2315 [DOI] [PubMed] [Google Scholar]
- 17.William Dager SB, Gretchen B, Kamila D, et al. An opinion paper outlining recommendations for training, credentialing, and documenting and justifying critical care pharmacy services. Pharmacotherapy. 2011; 31:135e–175e [Google Scholar]
- 18.Dudzinski DM, Giri J, Rosenfield K. Interventional treatment of pulmonary embolism. Circ Cardiovasc Interv. 2017; 10:e004345. [DOI] [PubMed] [Google Scholar]
- 19.Rosovsky R, Borges J, Kabrhel C, et al. Pulmonary embolism response team: Inpatient structure, outpatient follow-up, and is it the current standard of care? Clin Chest Med. 2018; 39:621–630 [DOI] [PubMed] [Google Scholar]
- 20.Barnes GD, Kabrhel C, Courtney DM, et al. ; National PERT Consortium Research Committee. Diversity in the pulmonary embolism response team model: An organizational survey of the National PERT Consortium Members. Chest. 2016; 150:1414–1417 [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Lopez J, Channick R. The pulmonary embolism response team: What is the ideal model? Semin Respir Crit Care Med. 2017; 38:51–55 [DOI] [PubMed] [Google Scholar]
- 22.Böttiger BW, Arntz HR, Chamberlain DA, et al. ; TROICA Trial Investigators; European Resuscitation Council Study Group. Thrombolysis during resuscitation for out-of-hospital cardiac arrest. N Engl J Med. 2008; 359:2651–2662 [DOI] [PubMed] [Google Scholar]
- 23.Er F, Nia AM, Gassanov N, et al. Impact of rescue-thrombolysis during cardiopulmonary resuscitation in patients with pulmonary embolism. PLoS One. 2009; 4:e8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederer W, Lichtenberger C, Pechlaner C, et al. Recombinant tissue plasminogen activator during cardiopulmonary resuscitation in 108 patients with out-of-hospital cardiac arrest. Resuscitation. 2001; 50:71–76 [DOI] [PubMed] [Google Scholar]
- 25.Lederer W, Lichtenberger C, Pechlaner C, et al. Long-term survival and neurological outcome of patients who received recombinant tissue plasminogen activator during out-of-hospital cardiac arrest. Resuscitation. 2004; 61:123–129 [DOI] [PubMed] [Google Scholar]
- 26.Stadlbauer KH, Krismer AC, Arntz HR, et al. Effects of thrombolysis during out-of-hospital cardiopulmonary resuscitation. Am J Cardiol. 2006; 97:305–308 [DOI] [PubMed] [Google Scholar]
- 27.Renard A, Verret C, Jost D, et al. Impact of fibrinolysis on immediate prognosis of patients with out-of-hospital cardiac arrest. J Thromb Thrombolysis. 2011; 32:405–409 [DOI] [PubMed] [Google Scholar]
- 28.Hammond DA, McCreary EK, Rech MA, et al. Perceptions and practices for beta-lactam antibiotic dosing, administration, and monitoring in critically ill patients: Current views and use among critical care and infectious diseases pharmacists. J Am Coll Clin Pharm. 2019; 2:468–476 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


