Supplemental Digital Content is available in the text.
Keywords: ABCDEF bundle, agitation, ARDS, coronavirus disease 2019, delirium, intensive care
Abstract
Objectives:
The severe acute respiratory syndrome coronavirus 2 pandemic has stretched ICU resources in an unprecedented fashion and outstripped personal protective equipment supplies. The combination of a novel disease, resource limitations, and risks to medical personnel health have created new barriers to implementing the ICU Liberation (“A” for Assessment, Prevention, and Manage pain; “B” for Both Spontaneous Awakening Trials and Spontaneous Breathing Trials; “C” for Choice of Analgesia and Sedation; “D” for Delirium Assess, Prevent, and Manage; “E” for Early Mobility and Exercise; and “F” for Family Engagement and Empowerment [ABCDEF]) Bundle, a proven ICU care approach that reduces delirium, shortens mechanical ventilation duration, prevents post-ICU syndrome, and reduces healthcare costs. This narrative review acknowledges barriers and offers strategies to optimize Bundle performance in coronavirus disease 2019 patients requiring mechanical ventilation.
Data Sources, Study Selection, and Data Extraction:
The most relevant literature, media reports, and author experiences were assessed for inclusion in this narrative review including PubMed, national newspapers, and critical care/pharmacology textbooks.
Data Synthesis:
Uncertainty regarding coronavirus disease 2019 clinical course, shifts in attitude, and changes in routine behavior have hindered Bundle use. A domino effect results from: 1) changes to critical care hierarchy, priorities, and ICU team composition; 2) significant personal protective equipment shortages cause; 3) reduced/restricted physical bedside presence favoring; 4) increased depth of sedation and use of neuromuscular blockade; 5) which exacerbate drug shortages; and 6) which require prolonged use of limited ventilator resources. Other identified barriers include manageable knowledge deficits among non-ICU clinicians unfamiliar with the Bundle or among PICU specialists deploying pediatric-based Bundle approaches who are unfamiliar with adult medicine. Both groups have been enlisted to augment the adult ICU work force to meet demand. Strategies were identified to facilitate Bundle performance to liberate patients from the ICU.
Conclusions:
We acknowledge current challenges that interfere with comprehensive management of critically ill patients during the coronavirus disease 2019 pandemic. Rapid response to new circumstances precisely requires established safety mechanisms and protocols like the ABCDEF Bundle to increase ICU and ventilator capacity and help survivors maximize recovery from coronavirus disease 2019 as early as possible.
During the current severe acute respiratory syndrome coronavirus 2 pandemic, up to 5% of patients with coronavirus disease 2019 (COVID-19) will develop acute respiratory failure. Among patients needing ICU-level care, nearly 90% require intubation and mechanical ventilation, often for a prolonged period (1–3). These surges inpatient volume, acuity, and ICU length of stay may exceed ICU resources and bed capacity (4, 5). The prolonged need for mechanical ventilation and the use of deep sedation, with or without use of continuous neuromuscular blockade (NMB), results in a population of COVID-19 patients at high risk for numerous ICU and post-ICU sequelae including the Post-Intensive Care Syndrome (6, 7). In the absence of effective COVID-19 specific therapies, ICU clinicians must rely even more heavily on broad-based strategies to mitigate the negative consequences of ICU care and subsequent postdischarge morbidity in this population (8).
The ICU Liberation Campaign using the ICU Liberation (“A” for Assessment, Prevention, and Manage pain; “B” for Both Spontaneous Awakening Trials and Spontaneous Breathing Trials; “C” for Choice of Analgesia and Sedation; “D” for Delirium Assess, Prevent, and Manage; "E" for Early Mobility and Exercise; and “F” for Family Engagement and Empowerment [ABCDEF]) Bundle (hereafter referred to as “the Bundle”) (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A204) was launched in 2014 by the Society of Critical Care Medicine (SCCM) to promote the widespread adoption of SCCM’s 2013 Pain, Agitation, and Delirium guidelines (9, 10). The Bundle’s clearly positive impact on patient outcomes has fostered its global adoption (11). The Bundle, its use described elsewhere in detail (12–16), continues to be refined by new practice guidelines (17) and published evidence (18–20). In succinct terms, the Bundle strives to optimize pain management, avoid deep sedation, reduce delirium, shorten the duration of mechanical ventilation, minimize ICU-acquired weakness, and foster ICU patient and family involvement in care processes.
Although multiple studies demonstrate the salutary impact of Bundle use on ICU patient care, outcomes, and healthcare costs in non-COVID-19 patients (21–24), important COVID-19-related issues and barriers may preclude the routine use of the Bundle in this population, particularly at centers experiencing a surge of critically ill adults with COVID-19. The need to adopt social distancing and increased workload demands have pushed interprofessional team (IPT) rounds and collaboration from the ideal that promotes Bundle performance (25, 26).
This narrative review, which is intended neither as a guideline nor practice statement, is informed by authors with experience in managing critically ill COVID-19 adults admitted to both academic and community centers and builds on other recent COVID-19 papers focused solely on delirium (27, 28). This article does not address factors like clinician fear or ethical issues (e.g., ventilator shortages) that may influence Bundle use during the pandemic (29). Instead, we highlight key barriers to Bundle adoption during the pandemic and offer ways to reenvision ICU care using evidence-based strategies to optimize Bundle application to critically ill adults with COVID-19 requiring mechanical ventilatory support.
SOLUTIONS TO OVERCOME POTENTIAL GLOBAL BARRIERS TO BUNDLE DELIVERY
Barriers to Bundle delivery in mechanically ventilated adults with COVID-19 can be stratified by those affecting application of the bundle as whole (i.e., global barriers) and those primarily affecting delivery of individual bundle elements. Barriers to Bundle use, and the solutions to overcome them, are influenced by the degree to which the Bundle was implemented prior to the pandemic and the number of COVID-19 ICU admissions at any one time relative to institutional ICU capacity and staffing. This section of the paper highlights global barriers to Bundle use and practical solutions to overcome them.
Alterations in Critical Care Hierarchy and Care Priorities
Prior to the pandemic, Bundle implementation was a major focus of ICU critical care quality improvement at many hospitals (11, 23). Clinicians should remind themselves of the strong evidence behind the Bundle and take the opportunity to ensure the entire IPT refreshes their knowledge surrounding it. In the current crisis, where ICU beds and ventilator resources may be limited, the Bundle would seem to be tailor made to reduce duration of mechanical ventilation and shorten the ICU stay. However, the Bundle requires substantial clinician resources and time that many institutions often struggled to provide even before the pandemic (23, 24). Although ICU delirium assessment/reduction and rehabilitation/mobility are no less important than pain and sedation in optimizing ICU liberation and survivorship, it is realistic to assume these two former Bundle elements may be especially difficult to apply fully, particularly at centers experiencing a surge in COVID-19 admissions. Restrictions on visitation, even to dying patients, has substantially reduced the role of family as part of the ICU care team (30).
The importance of the intensivist-led IPT, in both academic and community hospitals, and its importance in facilitating Bundle completion, is well-established (25, 26). However, daily IPT rounds and interactions have been forced to change. The need to adopt social distancing and increased workload demands have pushed IPT rounds and collaboration during the COVID-19 pandemic away from the ideal that promotes Bundle performance (26). In its place, medical team behaviors have tended to revert back to isolated conversations and patient care decisions that are less informed by the robust input from multiple professionals and their expertise; real-time IPT discussions, an essential component of Bundle success, are compromised. Maintaining the IPT may require novel approaches. Virtual participation by members from other areas of the hospital, or even from home, can augment an approach that facilitates best practice.
Enlistment of Noncritical Care Clinicians and Pediatric Intensive Care Specialists and Resources
At many hospitals, the need to expand ICU resources has placed the critically ill in noncritical care locations; in these settings, clinicians have variable levels of Bundle knowledge and experience. For example, an anesthesiologist, who has been asked to help with ventilator management in the ICU but previously practiced solely in the operating room, likely has little familiarity or experience with the Bundle. Similarly, the focus of training for non-ICU registered nurses (RNs) has been necessarily focused on immediate tasks; the time for review of strategies and processes of care such as the Bundle may be limited.
The apparent lower frequency of severe critical illness from COVID-19 among pediatric patients (31) has resulted in some institutions enlisting PICU professionals and resources to expand adult ICU capacity. The PICU team brings the same skill set to treat and support critically ill patients, just in younger patients with a different range of baseline neurocognitive development and physiology. Optimal Bundle implementation in the PICU patient requires the same strategies of repeated assessments and IPT communication and collaboration needed with adults (32–34) but uses different tactical tools to accomplish these goals (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/CCX/A205) (35–46).
Established ICU IPT members should embrace new team members and prioritize just-in-time training about the Bundle. Adult ICU specialists should partner with their PICU colleagues when adults are admitted to PICUs, both to provide support for adult-specific medicine and also to foster mutual learning about different approaches to Bundle element performance. The adult ICU team can provide just-in-time training, guidance, and coaching to non-ICU or PICU clinicians. The amount of training and coaching could be high for a non-ICU professional who has never heard of the Bundle or low for the PICU team member who has experience with the Bundle but requires guidance about the chronic medical comorbidities of adults.
Care Delivery When Personal Protective Equipment Is Limited
High quality critical care during a pandemic is predicated by adequate personal protective equipment (PPE); shortages can impact every stage of ICU care. Initial decisions about how supportive care for acute hypoxemia should be delivered (e.g., noninvasive vs invasive ventilatory support) continue to be clouded by concerns about viral aerosolization (47). If the frequency of bedside patient assessments and interventions are reduced, the increased use of hand restraints that invariably results may lead to greater use of deep sedation and NMB therapy outside of usual therapeutic goals. The loss of repeated bedside exams and assessments may force care to become more monitor-based, leaving clinicians to focus more on vital signs, cardiopulmonary status, and laboratory results than on neurologic and musculoskeletal function.
Restricted PPE availability has also resulted in an overlap of bedside responsibilities among available ICU IPT members (25); entry to the patient room by a single ICU clinician at a time is more common. As a result, the ICU team loses the added layers of safety based on profession-specific education and experience. Examples include non-respiratory therapists (RTs) making directed ventilator adjustments but without the RT’s implicit knowledge of how changes in one ventilator variable might impact another. Non-RNs may be asked to administer medications but without the RN’s ingrained process for cleaning central line hubs to mitigate risk of central line-associated bloodstream infections. The requirement to use PPE at all times also removes the all-important human face-to-face connection and touch of critical care. Patients can no longer see the nonverbal cues that convey communication, compassion, and empathy in ways that garbled words behind a N-95 mask can never hope to replace (48). Patient fear and anxiety, particularly if delirium is present, may increase and the ICU environment becomes that much more foreign.
Consider repurposing strategies that enhance efficiency of care for patient comfort, rethink the ideal number of in-room team members, and ask front line staff for outside-the-box ideas. Adherence to strong IPT communication along with robust just-in-time training and coaching can facilitate Bundle performance while also promoting judicious use of PPE (25, 26). As patients clinically improve, the bedside frequency of patient monitoring and care may be able to be reduced throughout the day, in a similar fashion to approaches increasingly being used at night to reduce sleep disruption (17). ICU teams can also reimagine in-room presence to two-person teams that enter the room together and work in tandem to provide “boluses” of patient care. This approach will reduce the time any-one clinician spends in the room, improve efficiency of care, and the in-the-moment clinician-clinician interaction will promote a sense of team rather than isolation. It may also reduce PPE use as the longer a single clinician stays in the room, the more likely they may need to temporarily leave the room to change PPE (e.g., a sweaty N-95 mask).
SOLUTIONS TO OVERCOME POTENTIAL BARRIERS TO SINGLE ELEMENT BUNDLE DELIVERY
A number of unique barriers, over and above those already discussed, exist surrounding the performance of individual Bundle components in critically ill adults with COVID-19. This section highlights these potential barriers and offers practical solutions to overcome them.
A—Assess, Prevent, and Manage Pain
Pain assessment of the mechanically ventilated COVID-19 patient may be compromised because patients may be more often deeply sedated and/or receiving NMB therapy and the nurse may be spending less time at the bedside. The risk factors and causes of pain may be different from those of less critically ill adults. Prolonged periods of high-dose opioid therapy are common. Strategies to overcome these barriers and optimize patient comfort are highlighted in Table 1 (10, 12, 13, 15–17, 49).
TABLE 1.
Barriers and Solutions to A: Assess, Prevent, and Manage Pain in Critically Ill Adults With Coronavirus Disease 2019 (10, 12, 13, 15–17, 49)

B—Both Spontaneous Awakening Trials and Spontaneous Breathing Trials
Sedation assessment is challenging during NMB therapy and may be reduced if the nurse is less frequently at the bedside. Although patients with acute respiratory distress syndrome can often safely be managed at a lighter sedation goal (50), COVID-19 patients may be maintained at a deep level of sedation for a prolonged period. Attempts at spontaneous awakening trials may be reduced due to concerns about patient-initiated device removal (e.g., self-extubation). Spontaneous breathing trials may be reduced due to deeper sedation and/or reduced lack of RT presence. Strategies to overcome barriers to wakefulness and mechanical ventilation liberation are presented in Table 2 (10, 12, 13, 15–17, 49, 50).
TABLE 2.
Barriers and Solutions to B: Both Spontaneous Awakening Trial and Spontaneous Breathing Trials in Critically Ill Adults With Coronavirus Disease 2019 (10, 12, 13, 15–17, 49, 50)

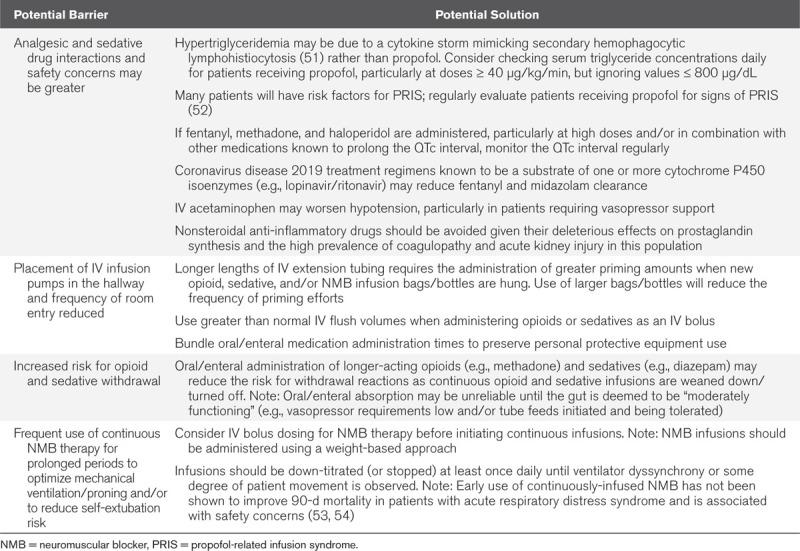
C—Choice of Analgesia, Sedation, and Neuromuscular Blockade
Use of analgesics, sedatives, and NMB, often for prolonged periods and at high doses, coupled with a high prevalence of multiple organ failure, will increase the risk for drug interactions, opioid/sedative withdrawal effects, and adverse drug events. Placement of IV infusion pumps in hallways and reduced room entry by nurses raise important medication administration-related concerns. Strategies to optimize analgesic, sedative, and NMB use and reduce safety concerns with their use are presented in Table 3 (12, 13, 15–17, 49, 51–54). Shortages of analgesic, sedative, and NMB medications are prevalent as the COVID-19 pandemic persists (55). Supplemental Table 3 (Supplemental Digital Content 3, http://links.lww.com/CCX/A206) provides suggestions for second-, third-, and fourth-line analgesics, sedatives, and NMB when first-line agents are not available (10, 17, 56–58). Supplemental Table 4 (Supplemental Digital Content 4, http://links.lww.com/CCX/A207) highlights the pharmacologic properties of these less commonly used ICU analgesics, sedatives, and NMB (57–59).
TABLE 3.
Barriers and Solutions to C: Choice of Analgesia, Sedation, and Neuromuscular Blockade in Critically Ill Adults With Coronavirus Disease 2019 (10, 12, 13, 15–17, 49, 51–54)
D—Delirium: Assess, Prevent, and Manage
The prevalence of delirium in critically ill adults with COVID-19 likely approaches 100% (27, 28, 60). Delirium screening may be compromised given the high proportion of patients managed at a very deep level of sedation and the reduced frequency of nurses at the bedside. In the potential absence of key IPT members and reduced entry into patient’ rooms, challenges to reducing potential modifiable delirium risk factors exist. Most COVID-19 patients will have disrupted sleep (17); multiple barriers prevent the routine application of nonpharmacologic strategies known to improve sleep and/or reduce delirium. Strategies to help better recognize and reduce delirium in this population are presented in Table 4 (10, 12, 13, 15–17, 25, 49).
TABLE 4.
Barriers and Solutions to D: Delirium: Assess, Prevent, and Manage in Critically Ill Adults With Coronavirus Disease 2019 (10, 12, 13, 15–17, 25, 49)

E—Early Mobility and Exercise
Critically ill adults with COVID-19 are at high risk for ICU-acquired weakness and compromised post-ICU physical function. Deep sedation, with or without NMB therapy, precludes out-of-bed rehabilitation (17, 50). Physical and occupational therapists may not be present in the ICU; the truncated time nurses are at the patient bedside may reduce in-bed rehabilitation efforts. As patients recover, contact precautions may preclude out of ICU room mobility efforts. Strategies to optimize rehabilitation and mobility in light of these barriers are presented in Table 5 (10, 12, 13, 15–17, 49).
TABLE 5.
Barriers and Solutions to E: Early Mobility and Exercise in Critically Ill Adults With Coronavirus Disease 2019 (10, 12, 13, 15–17, 49)

F—Family Engagement and Empowerment
In the face of contagion, evidence-based strategies known to support family engagement in ICU care have generally been abandoned (30). There is abundant sharing on social media by family members about their frustration, sadness, and grief over “not being there” for their loved ones. Families are rarely present in the hospital and almost never allowed at the bedside. For patients who are wakeful, daily telephone, facetime, or zoom communications with family is encouraged. Families should be encouraged to provide the ICU with family photos and provide clinicians with the E-stories and music the patient enjoys (30, 49).
CONCLUSIONS
The number of critically ill adults with COVID-19 requiring mechanically ventilatory support has dramatically affected the way critical care is delivered and has likely prompted a move at many centers away from evidence-based ICU practices such as ABCDEF Bundle delivery. Despite multiple factors affecting Bundle use in ICUs caring for COVID-19 adults, we have outlined multiple strategies to help operationalize the Bundle, regardless of PPE availability, the location of critical care, or patient severity of illness. Reemploying the use of evidence-based strategies developed over the past 20 years in critical care research, appropriately adapted for use in this new and trying time of the coronavirus pandemic, may be one of the best mechanisms by which to increase ventilator and ICU capacity and help critically ill adults with COVID-19 transition toward recovery and survivorship.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Devlin has received research funding from the National Institute of Aging, National Heart, Lung, and Blood Institute, and the Canadian Institute of Health Research; he is on the editorial board of Critical Care Medicine. Dr. Ely has received funding from the National Institute of Aging and National Heart, Lung, and Blood Institute. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zandella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS COV-2 admitted to ICUs of the Lombardy region of Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian MD, Devereaux AV, Dichter JR, et al. Introduction and executive summary: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. CHEST. 2014; 146Suppl8S–34S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpern NA, Tan K. 2020. United States ICU Resource Availability for COVID-19. Available at: https://www.sccm.org/Blog/March-2020/United-States-Resource-Availability-for-COVID-19. Accessed May 26, 2020. [Google Scholar]
- 6.Iwashyna TJ, Netzer G. The burdens of survivorship: An approach to thinking about long-term outcomes after critical illness. Semin Respir Crit Care Med. 2012; 33:327–338 [DOI] [PubMed] [Google Scholar]
- 7.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012; 40:502–509 [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Lamas D.ICU Doctors Already Know How to Get Covid-19 Patients Off Ventilators Faster. Washington Post. 2020. Available at: https://www.washingtonpost.com/outlook/2020/04/10/ventilators-icu-safety-bundle/. Accessed April 14, 2020.
- 9.Ely E. The ABCDEF bundle: Science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017; 45:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barr J, Fraser GL, Puntillo K, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 11.Morandi A, Piva S, Ely EW, et al. Worldwide survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) bundle. Crit Care Med. 2017; 45:e1111–e1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critical Illness, Brain Dysfunction and Survivorship (CIBS) Center at Vanderbilt University and the Nashville Veteran’s Administration Hospital ICU Delirium. 2020. Available at: https://www.icudelirium.org. Accessed April 14, 2020.
- 13.Society of Critical Care Medicine: ICU Liberation. 2020. Available at: https://www.sccm.org/ICULiberation. Accessed April 14, 2020.
- 14.Barnes-Daly MA, Pun BT, Harmon LA, et al. Improving health care for critically ill patients using an evidence-based collaborative approach to ABCDEF bundle dissemination and implementation. Worldviews Evid Based Nurs. 2018; 15:206–216 [DOI] [PubMed] [Google Scholar]
- 15.Balas MC, Pun BT, Pasero C, et al. Common challenges to effective ABCDEF bundle implementation: The ICU liberation campaign experience. Crit Care Nurse. 2019; 39:46–60 [DOI] [PubMed] [Google Scholar]
- 16.Stollings JL, Devlin JW, Pun BT, et al. Implementing the ABCDEF bundle: Top 8 questions asked during the ICU liberation ABCDEF bundle improvement collaborative. Crit Care Nurse. 2019; 39:36–45 [DOI] [PubMed] [Google Scholar]
- 17.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 18.Girard TD, Exline MC, Carson SS, et al. MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018; 379:2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shehabi Y, Howe BD, Bellomo R, et al. ANZICS Clinical Trials Group and the SPICE III Investigators. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019; 380:2506–2517 [DOI] [PubMed] [Google Scholar]
- 20.Olsen HT, Nedergaard HK, Strøm T, et al. Nonsedation or light sedation in critically ill, mechanically ventilated patients. N Engl J Med. 2020; 382:1103–1111 [DOI] [PubMed] [Google Scholar]
- 21.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014; 42:1024–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes-Daly MA, Phillips G, Ely E. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: Implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017; 45:171–178 [DOI] [PubMed] [Google Scholar]
- 23.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh SJ, Otusanya O, Gershengorn HB, et al. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med. 2019; 47:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan AL, Aldrich JM, Gross AK, et al. University of California, San Francisco Critical Care Innovations Group. Interprofessional care and teamwork in the ICU. Crit Care Med. 2018; 46:980–990 [DOI] [PubMed] [Google Scholar]
- 26.Stollings JL, Devlin JW, Lin JC, et al. Best practices for conducting interprofessional team rounds to facilitate performance of the ICU Liberation (ABCDEF) Bundle. Crit Care Med. 2020; 48:562–570 [DOI] [PubMed] [Google Scholar]
- 27.Kotfis K, Williams Roberson S, Wilson JE, et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020; 24:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaHue SC, James TC, Newman JC, et al. Collaborative delirium prevention in the age of COVID-19. J Am Geriatr Soc. 2020; 68:947–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ely E.Doctors Fear the Coronavirus. Is That Affecting Their Medical Decisions? Washington Post. 2020. Available at: https://www.washingtonpost.com/outlook/2020/04/14/doctors-fear-coronavirus-is-that-affecting-their-medical-decisions/. Accessed April 18, 2020.
- 30.Davidson JE, Aslakson RA, Long AC, et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017; 45:103–128 [DOI] [PubMed] [Google Scholar]
- 31.Ludvigsson J. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020; 109:1088–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieczorek B, Ascenzi J, Kim Y, et al. PICU Up!: Impact of a quality improvement intervention to promote early mobilization in critically ill children. Pediatr Crit Care Med. 2016; 35:405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simone S, Edwards S, Lardieri A, et al. Implementation of an ICU Bundle: An interprofessional quality improvement project to enhance delirium management and monitor delirium prevalence in a single PICU. Pediatr Crit Care Med. 2017; 18:531–540 [DOI] [PubMed] [Google Scholar]
- 34.Hanna ES, Zhao S, Shannon CN, et al. Changes in provider perceptions regarding early mobility in the PICU. Pediatr Crit Care Med. 2020; 21:e30–e38 [DOI] [PubMed] [Google Scholar]
- 35.Castarlenas E, Jensen MP, von Baeyer CL, et al. Psychometric properties of the numerical rating scale to assess self-reported pain intensity in children and adolescents: A systematic review. Clin J Pain. 2017; 33:376–383 [DOI] [PubMed] [Google Scholar]
- 36.Hicks CL, von Baeyer CL, Spafford PA, et al. The Faces Pain Scale-Revised: Toward a common metric in pediatric pain measurement. Pain. 2001; 93:173–183 [DOI] [PubMed] [Google Scholar]
- 37.Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997; 23:293–297 [PubMed] [Google Scholar]
- 38.Malviya S, Voepel-Lewis T, Burke C, et al. The revised FLACC observational pain tool: Improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. 2006; 16:258–265 [DOI] [PubMed] [Google Scholar]
- 39.Kerson AG, DeMaria R, Mauer E, et al. Validity of the Richmond Agitation-Sedation Scale (RASS) in critically ill children. J Intensive Care. 2016; 4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curley MA, Harris SK, Fraser KA, et al. State Behavioral Scale: A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006; 7:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vet NJ, de Wildt SN, Verlaat CW, et al. A randomized controlled trial of daily sedation interruption in critically ill children. Intensive Care Med. 2016; 42:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curley MA, Wypij D, Watson RS, et al. RESTORE Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators Network. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA. 2015; 313:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faustino EVS, Schneider JB, Sweberg T, et al. Sedation management in children supported on extracorporeal membrane oxygenation for acute respiratory failure. Crit Care Med. 2017; 45:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith HA, Boyd J, Fuchs DC, et al. Diagnosing delirium in critically ill children: Validity and reliability of the pediatric confusion assessment method for the intensive care unit. Crit Care Med. 2011; 39:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith HA, Gangopadhyay M, Goben CM, et al. The preschool confusion assessment method for the ICU: Valid and reliable delirium monitoring for critically ill infants and children. Crit Care Med. 2016; 44:592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traube C, Silver G, Kearney J, et al. Cornell assessment of pediatric delirium: A valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med. 2014; 42:656–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remy KE, Lin JC, Verhoef P. High-flow nasal cannula may be no safer than non-invasive positive pressure ventilation for COVID-19 patients. Crit Care. 2020; 24:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santana J.Behind All of the Protection, the Human Touch Fades Away. Anesthesiology News. 2020. Available at: https://www.anesthesiologynews.com/Correspondence/Article/04-20/The-COVID-19-Files-Behind-All-the-Protection-the-Human-Touch-Fades-Away/57877. Accessed April 20, 2020.
- 49.Posa P, Singh J, Stollings J. ICU Liberation. 2020Second EditionMount Prospect, IL: Society of Critical Care Medicine [Google Scholar]
- 50.Hager DN, Dinglas VD, Subhas S, et al. Reducing deep sedation and delirium in acute lung injury patients: A quality improvement project. Crit Care Med. 2013; 41:1435–1442 [DOI] [PubMed] [Google Scholar]
- 51.Martindale R, Patel JJ, Taylor B, et al. Nutrition Therapy in the Patient With COVID-19 Disease Requiring ICU Care. Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition. 2020. Available at: https://www.sccm.org/COVID19RapidResources/Resources/Nutrition-Therapy-in-the-Patient-with-COVID-19-Dis. Accessed April 20, 2020.
- 52.Roberts RJ, Barletta JF, Fong JJ, et al. Incidence of propofol-related infusion syndrome in critically ill adults: A prospective, multicenter study. Crit Care. 2009; 13:R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016; 44:2079–2103 [DOI] [PubMed] [Google Scholar]
- 55.Buckley B.Demand for Ventilator Drugs ‘Unprecedented.’ Pharmacy Practice News. 2020. Available at: https://www.pharmacypracticenews.com/Covid-19/Article/05-20/Demand-for-Ventilator-Drugs-%E2%80%98Unprecedented%E2%80%99/58203. Accessed May 4, 2020.
- 56.Gagnon DJ, Fontaine GV, Riker RR, et al. Repurposing valproate, enteral clonidine, and phenobarbital for comfort in adult ICU patients: A literature review with practical considerations. Pharmacotherapy. 2017; 37:1309–1321 [DOI] [PubMed] [Google Scholar]
- 57.The Medical Letter on Drugs and Therapeutics: Some Drugs for COVID-19. Available at: https://secure.medicalletter.org/w1919a. Accessed May 6, 2020 [Google Scholar]
- 58. Clinical Drug Information; American Society of Health System Pharmacists. Bethesda, MD. Accessed April 12, 2020. [Google Scholar]
- 59. Lexicomp Online; Lexicomp Corporation. Hudson, OH. Available at: https://www.wolterskluwercdi.com/lexicomp-online/. Accessed April 20, 2020. [Google Scholar]
- 60.Inouye S. The Epidemic Within the Pandemic: Delirium. New York Times. 2020. Available at: https://www.nytimes.com/2020/05/10/opinion/coronavirus-hospital-delirium.html. Accessed May 11, 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


