Abstract
The severe acute respiratory syndrome-coronavirus-2 outbreak has rapidly reached pandemic proportions and has become a major threat to global health. Although the predominant clinical feature of coronavirus disease-2019 (COVID-19) is an acute respiratory syndrome of varying severity, ranging from mild symptomatic interstitial pneumonia to acute respiratory distress syndrome, the cardiovascular system can be involved in several ways. As many as 40% of patients hospitalized with COVID-19 have histories of cardiovascular disease, and current estimates report a proportion of myocardial injury in patients with COVID-19 of up to 12%. Multiple pathways have been suggested to explain this finding and the related clinical scenarios, encompassing local and systemic inflammatory responses and oxygen supply-demand imbalance. From a clinical point of view, cardiac involvement during COVID-19 may present a wide spectrum of severity, ranging from subclinical myocardial injury to well-defined clinical entities (myocarditis, myocardial infarction, pulmonary embolism, and heart failure), whose incidence and prognostic implications are currently largely unknown because of a significant lack of imaging data. Integrated heart and lung multimodality imaging plays a central role in different clinical settings and is essential in the diagnosis, risk stratification, and management of patients with COVID-19. The aims of this review are to summarize imaging-oriented pathophysiological mechanisms of lung and cardiac involvement in COVID-19 and to provide a guide for integrated imaging assessment in these patients.
Key Words: cardiac magnetic resonance, chest x-ray, computed tomography, coronavirus, COVID-19, echocardiography, lung ultrasound, multimodality imaging, SARS-CoV-2
Abbreviations and Acronyms: ACS, acute coronary syndrome(s); ARDS, acute respiratory distress syndrome; CMR, cardiac magnetic resonance; COVID-19, coronavirus disease-2019; CT, computed tomography; CXR, chest radiography; ED, emergency department; FoCUS, focused cardiac ultrasound; GGO, ground-glass opacity; ICA, invasive coronary angiography; ICU, intensive care unit; LUS, lung ultrasound; MI, myocardial infarction; MINOCA, myocardial infarction with nonobstructive coronary arteries; PE, pulmonary embolism; PPE, personal protection equipment; RT-PCR, reverse-transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography
Central Illustration
Highlights
-
•
Cardiac involvement is present in up to 12% of patients with COVID-19.
-
•
Multimodality imaging is essential in different clinical settings in COVID-19.
-
•
Multimodality imaging is useful in diagnosis, risk stratification, and management.
-
•
Strategies for preventing viral transmission during examinations must be adopted.
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) outbreak arisen in central China at the end of December 2019 has rapidly reached pandemic proportions and the associated coronavirus disease-2019 (COVID-19) has become a major threat to global health (1). As the pandemic grows, treating physicians are challenged with different and complex clinical scenarios. The most prominent feature of COVID-19 is an acute respiratory syndrome of varying severity, ranging from mild symptomatic interstitial pneumonia to acute respiratory distress syndrome (ARDS). However, several reports have directed attention toward possible cardiovascular involvement during SARS-CoV-2 infection: as many as 40% patients hospitalized with COVID-19 have history of cardiovascular disease, and current estimates report a proportion of myocardial injury in patients with COVID-19 of up to 12% (2, 3, 4). Identification of myocardial injury is associated with a dismal prognosis independently and in addition to coexisting cardiovascular diseases, so recognition of underlying mechanisms may offer a therapeutic opportunity (4). In this context, the use of multiple diagnostic imaging techniques may apply to both the heart and lungs to provide an integrated assessment of cardiac and pulmonary function and to refine diagnosis, risk stratification, and management among patients with COVID-19.
Pathogenesis and Clinical Manifestations of COVID-19
The pathogenesis of COVID-19 is characterized by 2 distinctive but synergistic mechanisms, the first related to viral replication and the second to the host immune response (5). The disease primarily involves the lungs and progresses through 3 stages of increasing severity, corresponding to distinct histopathologic, imaging, and clinical findings (6, 7, 8).
The first stage involves the incubation period, SARS-CoV-2 replication in the respiratory system, and potential spread to target organs. During this phase, alveolar and interstitial inflammation is mild and patchy and usually shows a bilateral, peripheral, and lower distribution, with patients presenting with mild respiratory and systemic symptoms.
The second stage is characterized by localized lung inflammation, which shows different grades of severity, ranging from severe interstitial inflammation and thickening to air-space consolidation. Patients develop symptoms of viral pneumonia and eventually hypoxia, leading to clinical deterioration and need for hospitalization.
In a subgroup of patients, transition to the third stage occurs. This phase is dominated by widespread lung inflammation and systemic inflammatory syndrome triggered by a dysregulated host immune response and cytokine storm, causing hyperinflammation, ARDS, shock, and multiorgan damage.
Clinical features of COVID-19 are variable. Although the majority of patients present with only mild respiratory and systemic symptoms, some progress to severe forms of viral pneumonia and eventually develop severe systemic inflammatory manifestations, with an increasingly higher case fatality rate (7). Cardiovascular adverse events may occur at different stages, complicating the course of the disease and leading to unfavorable outcomes (Central Illustration ).
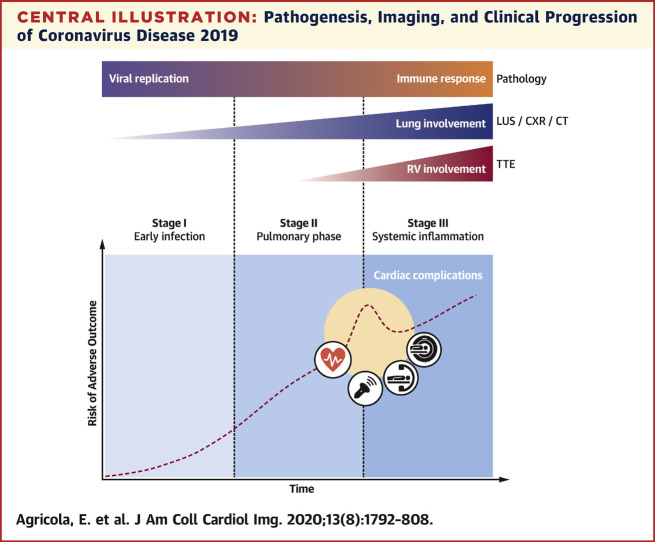
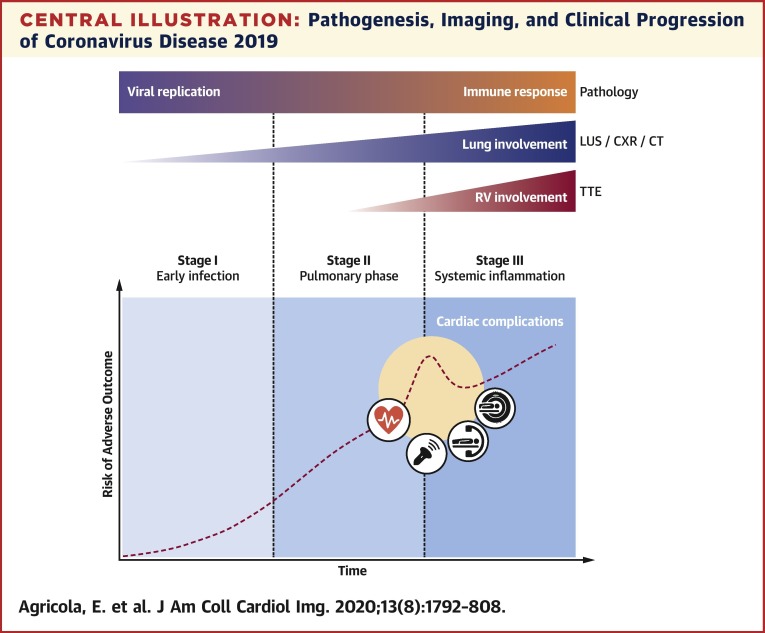
Central Illustration.
Pathogenesis, Imaging, and Clinical Progression of Coronavirus Disease 2019
Viral replication and host immune response synergistically determine coronavirus disease 2019 pathogenesis. As the disease progresses through its 3 stages, different chest imaging modalities (lung ultrasound, chest radiography, and computed tomography) demonstrate worsening lung involvement. In case of severe pneumonia, transthoracic echocardiography can identify increasing pulmonary hypertension and right ventricular impairment. Cardiovascular complications related to viral infection or to systemic inflammation can occur at different stages of the disease, increasing the risk for adverse outcome, and require specific multimodality imaging assessment. CT = computed tomography; CXR = chest radiography; LUS = lung ultrasound; RV = right ventricular; TTE = transthoracic echocardiography.
Cardiovascular Involvement in COVID-19
Definition of cardiac involvement in COVID-19 is challenging, as SARS-CoV-2 infection has multifaceted effects. From a clinical point of view, cardiac involvement during COVID-19 may present a wide spectrum of severity, ranging from subclinical myocardial injury to well-defined clinical entities. In a comprehensive understanding, the following clinical scenarios may be encountered: 1) primary cardiac involvement; 2) secondary cardiac involvement; and 3) worsening of previous cardiovascular diseases (Table 1 ).
Table 1.
Cardiovascular Involvement in Coronavirus Disease 2019
| Pathogenetic Mechanism | Clinical Manifestations | Imaging Modalities |
|---|---|---|
| Primary cardiac involvement | ||
| Direct viral damage (hypothesized) | Viral myocarditis | TTE CMR |
| Secondary cardiac involvement | ||
| Cytokine storm | Inflammatory myocarditis | TTE CMR |
| Oxygen supply-demand imbalance | Type 2 MI | TTE CT/ICA CMR |
| Inflammatory prothrombotic state Atherosclerotic plaque instability |
Type 1 MI | TTE CT/ICA |
| Inflammatory prothrombotic state | VTE and acute PE | TTE CT |
| Lung inflammation Hypoxic vasoconstriction High-PEEP mechanical ventilation Pulmonary thromboembolism |
RV increased afterload | TTE |
| Worsening pre-existing conditions | ||
| Infection-related metabolic demand Cytokine storm |
Heart failure exacerbation | TTE |
| Hypoxia Cytokine storm Drug side effects (QT interval prolongation: hydroxychloroquine and azithromycin alone or in combination with AADs) |
Arrhythmias | — |
AAD = antiarrhythmic drug; CMR = cardiac magnetic resonance; CT = computed tomography; ICA = invasive coronary angiography; MI = myocardial infarction; PE = pulmonary embolism; PEEP = positive end-expiratory pressure; RV = right ventricular; TTE = transthoracic echocardiography; VTE = venous thromboembolism.
Primary cardiac involvement
This may be a consequence of viral tropism for the endothelium and (presumably) for the myocardium. A link between the respiratory syndrome and the pleomorphic cardiovascular manifestations associated with COVID-19 could be identified in angiotensin converting enzyme 2, a membrane-bound enzyme that serves as cell-entry receptor for SARS-CoV-2 (9). This receptor is expressed in a variety of tissues, including lung alveolar epithelial cells and enterocytes of the small intestine, as well as arterial smooth muscle cells and endothelial cells (9). On the basis of previous data from the SARS-CoV epidemic, myocardial infection by coronavirus is a possibility: in an autopsy series, SARS-CoV ribonucleic acid was found in 35% of sampled hearts, along with macrophage infiltration and myocardial damage (10). The extent to which these finding may also apply to SARS-CoV-2 is unknown. To date, no cases of SARS-CoV-2 nucleic acid isolation from myocardial specimens have been described. However, several cases have reported on the occurrence of severe myocarditis during laboratory-proven COVID-19 (11, 12, 13, 14, 15). In all these cases, myocarditis caused severe left ventricular dysfunction but showed some degree of systolic function recovery following medical therapy, ranging from progressive improvement to complete myocardial function restoration. A single case of myopericarditis complicated by life-threatening cardiac tamponade has been reported, again without direct isolation of SARS-CoV-2 from the drained pericardial fluid (12). In the absence of proven SARS-CoV-2 infection of the myocardium, the clinical overlap of these case reports with other possible differential diagnoses calls for prudence in diagnosing SARS-CoV-2-related myocarditis.
Secondary cardiac involvement
This is the result of indirect myocardial damage during SARS-CoV-2 infection. Of note, it may represent the convergence of multiple different mechanisms. In a post-mortem examination from a patient with COVID-19 who developed ARDS, interstitial mononuclear inflammatory cells were noted in heart specimens without structural damage (16). A hyperinflammatory response in the advanced stage of the disease elicits a cytokine storm, mediated chiefly by interleukin-1 and interleukin-6 pathways, closely resembling hemophagocytic lymphohistiocytosis, a life-threatening hematologic disorder characterized by uncontrolled proliferation of activated lymphocytes and macrophages, with massive release of inflammatory cytokines (9). These cytokines have been implicated in myocardial injury and adverse remodeling in clinical and experimental models of acute coronary syndrome(s) (ACS) and may exhibit direct negative inotropic and metabolic effects on cardiomyocytes in sepsis-like settings (17). In addition, interleukin-1 plays a proven role in atherothrombosis, and the resulting hyperinflammatory milieu may provoke atherosclerotic plaque instability and a procoagulant state with increased risk for arterial and venous acute thrombotic events, including type 1 myocardial infarction (MI) and pulmonary embolism (PE). Indeed, there is increasing concern that patients with COVID-19 are more prone to develop thromboembolic venous events and disseminated intravascular coagulation (18,19). Secondary cardiac involvement may also be the consequence of hypoxia-induced myocardial damage, which could lead to type 2 MI. This condition could either unmask underlaying obstructive coronary artery disease (CAD) or present as MI with nonobstructive coronary arteries (MINOCA) in the presence of intense oxygen supply-demand imbalance (20). Moreover, altered pulmonary hemodynamic status may play a role in secondary cardiac involvement. In severe COVID-19 pneumonia, the use of higher positive end-expiratory pressure may be associated with increased right ventricular afterload and strain due to higher pulmonary arterial pressure and pulmonary vascular resistance. Pulmonary circulation hypoxic vasoconstriction and superimposed pulmonary thromboembolic events may further precipitate these effects.
Worsening of pre-existing cardiovascular diseases
This is frequently observed during COVID-19 and may explain the higher prevalence of patients with pre-existing cardiovascular comorbidities in nonsurvivor cohorts (3,4,21). Indeed, patients with heart failure are particularly vulnerable to hemodynamic decompensation during viral infections (22). Furthermore, in predisposed patients, arrhythmias may ensue as a result of multiple mechanisms, including hypoxia, systemic inflammation, and side effects of drugs used in the treatment of COVID-19 (i.e., hydroxychloroquine often combined with azithromycin) (2).
Multimodality Imaging in COVID-19
Chest radiography
Recent radiology research on COVID-19 has been molded by the Chinese experience, with the vast majority of reports focusing on the role of chest computed tomography (CT), almost neglecting the contribution of chest radiography (CXR). However, European hospitals have drawn diagnostic algorithms in which CXR is described as a first-line triage tool, mainly because of its availability and feasibility and long turnaround times for reverse-transcriptase polymerase chain reaction (RT-PCR) analysis. Furthermore, the American College of Radiology has pointed out that CT room decontamination after scanning patients with COVID-19 may disrupt radiological service availability and suggested that portable CXR might be considered the optimal tool to minimize the risk for cross-infection (23). As recently reported, CXR demonstrates typical radiographic features in the vast majority of patients with COVID-19, including ground-glass opacities (GGOs) and consolidation, while pleural effusion is not common (Table 2 , Figure 1 ). In a retrospective cohort of 64 patients, Wong et al. (24) found that the common computed tomographic findings of bilateral involvement, peripheral distribution, and lower zone dominance can also be assessed on CXR and that the severity of findings on CXR peaked at 10 to 12 days after symptom onset, consistent with previous reports with CT (24). Although 6 of 64 patients demonstrated abnormalities on CXR before eventually testing positive on RT-PCR, baseline CXR sensitivity was 69%, significantly lower than that reported for initial RT-PCR and baseline CT (25). Moreover, in contrast to what has been previously reported for chest CT, radiographic and virological recovery times were not significantly different, thus reducing the role of CXR in clinical monitoring (25). A retrospective analysis of 9 South Korean patients who underwent both chest CT and CXR further decreased the sensitivity of CXR in detecting COVID-19 pneumonia to 33.3% (26). However, the significance of this result is limited by the small sample size. Recently, Bandirali et al. (27) proposed a role for CXR in asymptomatic or minimally symptomatic patients in epidemic regions, who may have positive radiographic findings even after 14 days of quarantine. To date, there are no consistent findings accurately depicting the course of disease on serial CXR.
Table 2.
Integrated Multimodality Imaging Findings in COVID-2019 Pneumonia
| CXR | CT | LUS |
|---|---|---|
| — | Thickened pleura | Thickened pleural line |
| Blurred opacities∗ | Ground-glass opacities | Multiple B-lines (cometlike) |
| Patchy or diffuse opacities | Crazy paving pattern | Confluent B-lines (white lung) |
| Localized consolidation | Subpleural consolidation | Subpleural consolidation |
| Translobar consolidation | Translobar consolidation | Extensive consolidation with hypoechoic lung tissue (hepatization) and air bronchograms |
| Bilateral distribution of lung changes with predominance in lower and peripheral zones | ||
| Pleural effusion is rare | ||
CT = computed tomography; CXR = chest radiography; LUS = lung ultrasound.
The term “ground-glass opacities” is also used in CXR to refer to areas of blurred opacities.
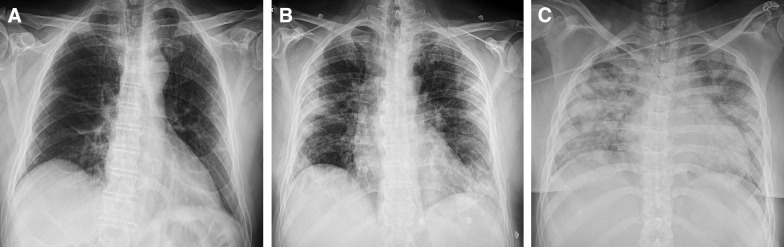
Figure 1.
Chest Radiographic Features of COVID-19 Pneumonia
(A) A 67-year-old-man presenting with sore throat: blurred peripheral ground-glass opacities (GGOs), mainly in the left medium to lower lung, with diffuse, blurred interstitial thickening. (B) A 59-year-old-man presenting with fever (39.5°C), cough, and diarrhea: diffuse, bilateral peripheral GGOs, consolidation areas mainly in the left lower lung and in the medium right lung. (C) A 43-year-old woman presenting with fever (40.5°C), cough, dyspnea, and severe hypoxia: bilateral consolidation areas occupying almost all lung parenchyma, with gross GGOs. No pleural effusion was noted in any case.
Chest CT
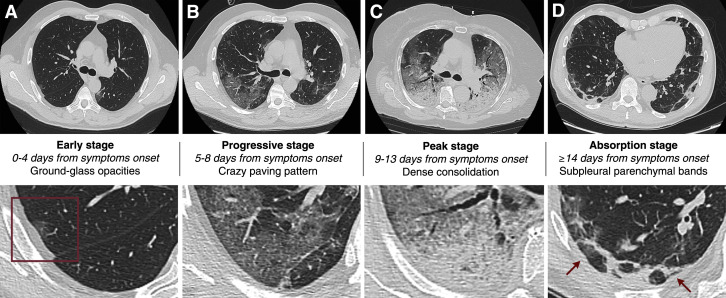
Chest CT is a highly accurate imaging modality for pneumonia identification and characterization. As recently reported, chest CT demonstrates typical imaging features in patients with COVID-19, including bilateral GGOs, crazy paving pattern (GGOs with superimposed interlobular or intralobular septal thickening), and/or consolidations, predominantly in subpleural locations in the lower lobes; typically, discrete pulmonary nodules, lung cavitation, pleural effusion, and lymphadenopathies are not present (28,29) (Table 2, Figure 2 ). Pan et al. (28) demonstrated that multiple computed tomographic scans could accurately depict the course of disease, summarized in 4 CT-based stages. Typical COVID-19 pneumonia often starts as small subpleural GGOs, mainly affecting the lower lobes (early stage, 0 to 4 days after symptom onset), which then rapidly develop into crazy paving pattern and consolidation areas, typically affecting both lungs (progressive stage, 5 to 8 days after symptom onset). Thereafter, dense consolidation becomes the most frequent finding (peak stage, 9 to 13 days after symptom onset). When infection resolves, the consolidation areas are gradually absorbed with residual GGOs and subpleural fibrotic parenchymal bands (absorption stage, >2 weeks after symptom onset) (Figure 2). Ai et al. (25) found that with RT-PCR as a reference, the sensitivity of chest CT for COVID-19 was 97%. Interestingly, these radiological findings are also observed in patients with clinical symptoms but negative RT-PCR results, and almost 50% and 33% of these patients were reconsidered as highly likely cases and as probable cases, respectively, in a comprehensive evaluation (25). Furthermore, 60% to 93% of patients had initial positive results on chest CT consistent with COVID-19, before the initial positive RT-PCR results (25). Finally, 42% of patients showed improvement on follow-up chest CT before RT-PCR results became negative (25). Nevertheless, it is worth emphasizing that patients with RT-PCR-confirmed COVID-19 might have normal findings on chest CT at admission, when disease is still subtle (30). Additionally, chest CT can be used for characterization of COVID-19 pneumonia severity. Yang et al. (31) proposed a CT-based severity score defined by summing individual scores from 20 lung regions; the individual scores in each lung, as well as the global severity score, were found to be higher in patients with severe COVID-19 compared with those with mild disease (sensitivity 83.3%, specificity 94%).
Figure 2.
Computed Tomographic Features and Staging of COVID-19 Pneumonia
The early stage (A) of typical COVID-2019 pneumonia is characterized by small subpleural ground-glass opacities (box), which then rapidly increase in number and develop into crazy paving pattern during the progressive stage (B). In the peak stage (C), dense consolidation becomes the most frequent finding. During the absorption stage (D), when the disease has a favorable course, consolidation areas are gradually absorbed, with residual subpleural fibrotic parenchymal bands (arrows).
Lung ultrasound
Lung ultrasound (LUS) is a widespread and validated technique for lung evaluation with features that make it very attractive for the assessment of patients affected by COVID-19 (32, 33, 34). LUS can be performed with any 2-dimensional scanner, including portable ones, using linear, convex, or phased-array probes. Specifically, a high-frequency linear probe is recommended to assess the pleural line, a phased-array low-frequency probe is suggested to evaluate deep consolidation, and a micro convex probe with a small footprint is useful for evaluating posterior fields in supine patients. The entire chest can be scanned with the probe oriented longitudinally or obliquely along the intercostal spaces. The scanning protocol consists of a 12-zone examination with 6 regions per hemithorax: the upper and lower parts of the anterior, lateral, and posterior chest wall, demarcated by the anterior and posterior axillary line (32,33).
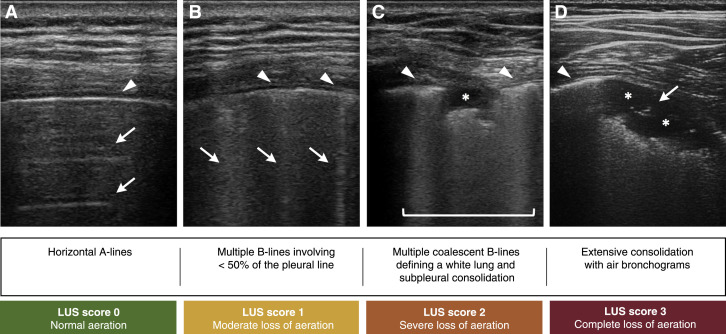
COVID-19 pneumonia is characterized by initial interstitial damage with a bilateral, peripheral, and posterior distribution followed by parenchymal involvement (34). LUS effectively detects the areas affected by subpleural interstitial syndrome with the appearance of B-lines, which increase in number as the pathology spreads, covering most of the pleural line. These findings correspond to GGOs and a reticular pattern on CT (Table 2). The characteristics of the B-lines help distinguish within interstitial syndrome between pneumonia or ARDS and cardiogenic pulmonary edema. Specifically, inflammatory patterns are characterized by the presence of bilateral, irregularly distributed B-lines with spared areas and coalescent B-lines mostly in posterior fields; furthermore, the pleural line appears typically thickened and irregular, with reduced or absent lung sliding (32). As the disease progresses, lung consolidations become frequent. The subpleural consolidation areas are identified as anechoic hemispheric areas close to the pleural line with a hyperechogenic base. Extensive consolidation appears as nontranslobar and translobar consolidation with hepatization of lung tissue and air bronchogram, which distinguish them from consolidations in resorptive atelectasis (Figure 3 ). However, LUS also presents limitations, as it is operator dependent, and abnormalities affecting the central regions surrounded by aerated lung are not detectable. With the aim of increasing reproducibility, it would be convenient to establish a scanning model and a severity score. The LUS score, validated with chest CT comparison, provides a numeric assessment of regional loss of aeration that can be used to assess the response to treatments (33) (Figure 3).
Figure 3.
Lung Ultrasound Features and Severity Grading of COVID-19 Pneumonia
Different patterns of lung involvement and corresponding lung ultrasound (LUS) severity score. (A) Normal lung: horizontal A-lines (arrows) arising from the pleural line (arrowhead) at regular intervals. (B) Moderate loss of aeration: multiple cometlike B-lines (arrows) arising from focally thickened pleural line (arrowheads). (C) Severe loss of aeration: multiple coalescent B-lines responsible for a white lung appearance (square sign) along with pleural line thickening (arrowheads); subpleural consolidation (asterisk) visible as a focal hypoechoic area. (D) Complete loss of aeration: pleural line thickening (arrowhead) and extensive lung consolidation visible as a large hypoechoic area (asterisks) with associated air bronchogram (arrow).
Transthoracic and transesophageal echocardiography
Although echocardiography should not routinely be performed in patients with COVID-19 and restricted to those in whom it is likely to result in a change in management, bedside echocardiography is a clinically useful tool in different clinical settings in emergency departments (EDs), intensive care units (ICUs), and non-ICU wards (35). Compact and highly mobile machines should be the ideal ultrasound systems to adopt, privileging dedicated probes and machines in infected areas. A miniaturized handheld ultrasound system that can be easily protected and cleaned may be an alternative option (35,36).
A pragmatic strategy based on the use of focused cardiac ultrasound (FoCUS) seems the most reasonable approach (37). FoCUS should be combined with LUS for the evaluation of patients with respiratory failure. The COVID-19 crisis highlights the need for imagers to be cross-trained (LUS and FoCUS) and nimbler: sonographers, cardiologists, and emergency physicians who are not familiar with LUS can learn quickly with initial support from expert colleagues and web resources (38). However, because FoCUS is not performed as the definitive diagnostic test, if no usable information is obtained, comprehensive echocardiography and/or other diagnostic testing must be considered (37). The aim of echocardiography is to reliably identify cardiac abnormalities and coexisting heart disease to facilitate triage and guide patient management. Echocardiography is also recommended for the evaluation of patients who develop symptoms consistent with a cardiac etiology. Information must quickly include biventricular function, gross valvular abnormalities, wall motion abnormalities, pericardial effusions, and surrogates of a patient’s volume status, including inferior vena cava collapsibility and ventricular size (37). Transthoracic echocardiography (TTE) is the standard technique, while transesophageal echocardiography (TEE) should be avoided because of the high risk for equipment and personnel contamination, unless there is a clearly defined indication that requires TE or inadequate imaging quality on TTE because of patient-specific factors (intubated patients, poor image quality, inability to position critically ill patients for optimal image acquisition) (35). The most common echocardiographic abnormalities encountered in our experience on patients with COVID-19 in the non-ICU setting are reported in Table 3 . Acute worsening of respiratory symptoms is a leading indication for performing echocardiography in these patients, frequently depicting a picture of acute cor pulmonale: right ventricular dilatation, paradoxical septal motion, and pulmonary hypertension. In this clinical setting PE seems relatively frequent (Figure 4 ). Echocardiography may expedite diagnosis of this condition.
Table 3.
Transthoracic Echocardiographic Findings in a Single-Center, Nonintensive Care Unit COVID-19 Cohort From San Raffaele Hospital, Milan, Italy (N = 209)
| Poor acoustic window | 11/209 (5.3) |
| LVEF (%) | 59 (55–63) |
| LVEF <50% | 12/198 (6.0) |
| RWMAs | 9/198 (4.5) |
| RV dilation∗ | 23/198 (11.6) |
| RV dysfunction† | 28/198 (14.1) |
| PH‡ | 24/198 (12.1) |
| sPAP (mm Hg) | 28 (23–33) |
| CVP 10–20 mm Hg | 5/198 (5.0) |
Values are n/N (%) or median (interquartile range).
CVP = central venous pressure; LVEF = left ventricular ejection fraction; PH = pulmonary hypertension; RV = right ventricular; RWMA = regional wall motion abnormality; sPAP = systolic pulmonary arterial pressure.
RV dilatation has been defined as RV mid diameter >35 mm.
RV dysfunction has been defined as either tricuspid annular plane systolic excursion <17 mm or Doppler tissue imaging S wave (S′ wave) <9.5 cm/s.
PH has been defined as sPAP >35 mm Hg.
Figure 4.
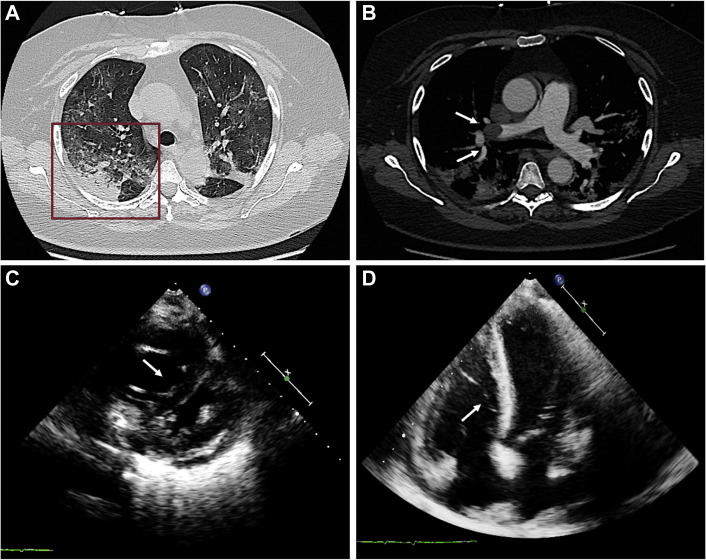
Multimodality Imaging of Pulmonary Embolism Complicating COVID-19 Pneumonia
A 61-year-old woman with reverse-transcriptase polymerase chain reaction swab results positive for severe acute respiratory syndrome coronavirus-2 presenting with sudden severe dyspnea associated with significant d-dimer increase. (A) Lung parenchyma windowing demonstrates bilateral, subpleural ground-glass opacities and consolidation areas (box), typical for coronavirus disease 2019 pneumonia. (B) Computed tomographic pulmonary angiography shows gross filling defect (arrows) in right pulmonary artery lobar branch for right upper lobe. (C, D) Transthoracic echocardiography shows right ventricular dilatation and septal shifting, indirect signs of severe pulmonary hypertension.
Cardiac CT and magnetic resonance
The clinical presentation of patients with suspected or confirmed COVID-19 may encompass a variable combination of dyspnea and chest pain, electrocardiographic alterations, and cardiac enzyme dispersion, challenging the differentiation between pulmonary and cardiovascular etiology of these findings and requiring advanced imaging to clarify the diagnosis. Several reports hint at an increased risk for venous and arterial thromboembolism, while our clinical experience and other reports suggest that some patients with COVID-19 presenting with ACS-like syndromes may not have obstructive CAD on invasive coronary angiography (ICA), falling into the wide spectrum of MINOCA (19,20). In this clinical scenario, CT may represent a valuable “one-stop shop” approach for the combined assessment of pneumonia, PE (Figure 4), and obstructive CAD (Figure 5 ) to guide further management, limiting the use of ICA to selected cases (Figure 6 ).
Figure 5.
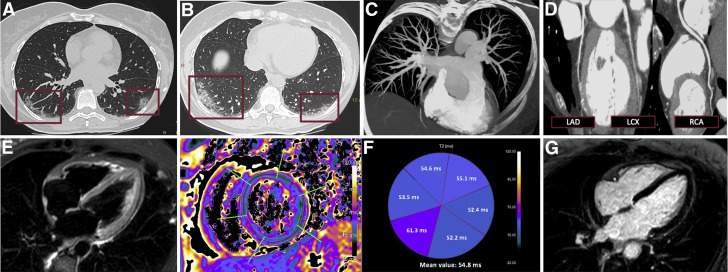
Multimodality Imaging of Myocardial Infarction With Nonobstructive Coronary Arteries Complicating COVID-19 Pneumonia
A 58-year-old woman with reverse-transcriptase polymerase chain reaction swab results positive for severe acute respiratory syndrome-coronavirus-2 presenting after 1 week of fever (38.5°C), cough, diarrhea with recent onset of typical chest pain, elevated cardiac markers (high-sensitivity troponin T 222 ng/l), ST-segment depression in the inferior and lateral leads on electrocardiography, and inferior septal hypokinesia on transthoracic echocardiography. Triple rule-out computed tomography shows peripheral lung opacities (A, B) characterized by crazy paving pattern involving both the inferior lobes, with posterior distribution, suggestive for coronavirus disease 2019 interstitial pneumonia (boxes), and demonstrates absence of pulmonary embolism (C) or coronary disease (D). Cardiac magnetic resonance shows slight diffuse myocardial hyperintensity on T2 short-tau inversion recovery image (E), consistent with a slight increase of T2 relaxation time on T2 mapping: mean value of 55 ms (normal range ≤50 ms) with a peak of 61 ms in the inferior septum (G); inversion recovery images do not show significant late gadolinium enhancement foci. LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; RCA = right coronary artery.
Figure 6.
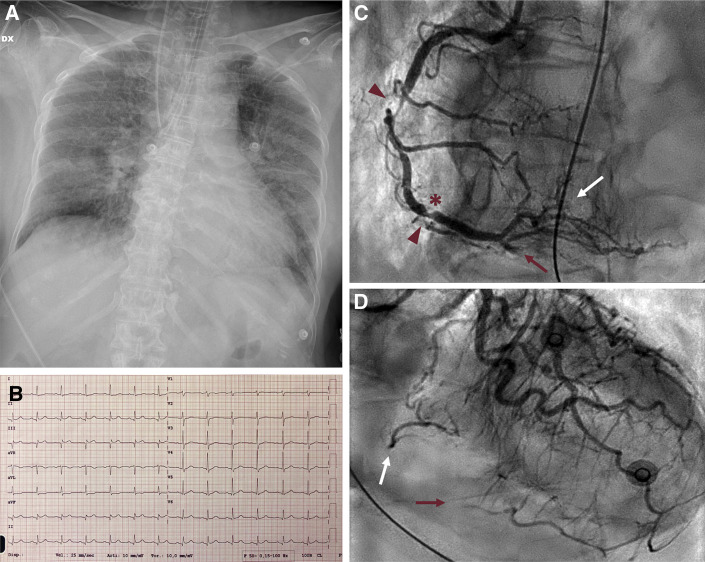
Type 1 Acute Myocardial Infarction Complicating COVID-19 Pneumonia
A 63-year-old woman with severe COVID-19 pneumonia requiring mechanical ventilation (A) presenting hypotension, with electrocardiogram (B) showing inferior ST-segment elevation acute myocardial infarction (high-sensitivity troponin T 579 ng/l, N-terminal pro–brain natriuretic peptide 8,441 pg/ml): invasive coronary angiography (C, D) demonstrates obstructive atherosclerotic disease of the right coronary artery (arrowheads) with haziness, hinting at thrombosis of ruptured plaque (asterisk) with distal embolization to both posterior descending artery (red arrow) and posterolateral branch (white arrow).
Computed tomographic coronary angiography is a well-established tool to effectively and safely rule out CAD in the setting of acute chest pain, thanks to its excellent negative predictive value (95% to 100%) (39). Of note, computed tomographic angiography can combine coronary artery, pulmonary artery, and thoracic aorta assessment using dedicated “triple-rule-out” protocols. In selected patients with variable degrees of respiratory symptoms, showing cardiac enzyme and d-dimer elevations, a dedicated triple-rule-out approach, with lung parenchyma instead of the thoracic aorta as the third focus of the examination, may solve different clinical questions in one sitting (40). Although most of the currently available computed tomographic scanners allow imaging of the coronary arteries with high resolution and limited motion artifacts, clinical judgment is advised, as dedicated scanners can improve image quality. Additionally, computed tomographic angiography can rule out left atrial appendage thrombus, allowing direct-current cardioversion in patients with atrial fibrillation, thereby limiting operator exposure deriving from TEE. Moreover, cardiac CT can provide advanced diagnostic assessment through myocardial characterization (41). Indeed, CT can be completed with a delayed iodine-enhanced scan to identify areas of myocardial necrosis or fibrosis. This further evaluation may be especially useful in patients with MINOCA, making it possible to differentiate MI from stress cardiomyopathy, which is typically characterized by an absence of myocardial late enhancement, and to diagnose acute myocarditis, detecting myocardial scar with typical nonischemic pattern. In this case, one can speak of “quadruple rule-out,” with a single examination looking for lung involvement, coronary and pulmonary artery patency, and myocardial scar (42). However, cardiac CT remains limited in the detection of myocardial edema, which represents the hallmark of acute myocardial inflammation (41).
Cardiac magnetic resonance (CMR) is the imaging modality of choice for the diagnosis of acute myocarditis, revealing with high sensitivity focal or diffuse myocardial edema through short-tau inversion recovery sequences and mapping techniques (T2 and native T1), potentially associated with necrotic foci visible with late gadolinium enhancement, diffuse expansion of extracellular volume fraction, and hyperemia (43,44) (Figure 5). The recent introduction of parametric mapping enables CMR to reveal diffuse myocardial edema that can be missed by conventional sequences, increasing its accuracy in the diagnosis of inflammatory cardiomyopathies. Currently, a few case reports have shown CMR findings consistent with acute myocarditis in patients with laboratory-proven SARS-CoV-2 infection (13, 14, 15). Myocardial edema was the key for CMR diagnosis in all of these cases, underscoring the importance of including mapping techniques in CMR protocols adopted in patients with COVID-19 with suspected myocarditis (43). Therefore, in selected patients with COVID-19 not requiring ICU support, when clinical presentation and biomarker alterations suggest acute-onset myocardial inflammation, if the diagnosis is likely to influence management, CMR may be considered to confirm acute myocarditis, after the exclusion of alternative relevant clinical conditions, including ACS and heart failure, by means of other rapidly available imaging modalities (i.e., cardiac CT or TTE).
Nuclear cardiology imaging
Nuclear cardiology encompasses several noninvasive imaging modalities and techniques that can be used for myocardial perfusion and viability assessment, as well as for the diagnosis of infective endocarditis, cardiac sarcoidosis, and amyloidosis. However, most of these conditions can be proficiently and safely evaluated using other imaging modalities after clinical resolution of COVID-19. Therefore, in patients with COVID-19, the use of nuclear cardiology tests should be restricted to very specific indications when they may yield diagnosis or directly influence clinical management and no alternative imaging modalities can be performed (i.e., suspected infective endocarditis of prosthetic valves or intracardiac devices), in order to reduce health care personnel exposure related to long protocols and imaging acquisition times (45).
Invasive cardiac imaging
When evaluating the role of invasive cardiac imaging modalities in patients with COVID-19, several aspects deserve consideration. In the complex rearrangement of the health care service, all efforts should be directed to ensure the standard of care and timely access to the catheterization laboratory for patients with acute cardiovascular conditions, irrespective of SARS-CoV-2 infection. Therefore, the use of ICA in patients with COVID-19 should be restricted to those presenting with clinical or hemodynamic instability, including acute MI, myocarditis, cardiogenic shock, and cardiac arrest (Figure 6). In these cases, an invasive strategy is pivotal to ensure diagnosis and interventional treatment (46). In addition, ICA eventually combined with coronary intravascular imaging or left ventriculography plays an important role in identification and differential diagnosis of MINOCA (9). On the basis of our direct experience, MINOCA accounts for >25% of ACS in patients with COVID-19. Nevertheless, patient status, severity of respiratory compromise, comorbidities, and the risk for futility should be carefully evaluated when considering indications for invasive strategies in patients with COVID-19.
The Imaging-Based Risk Assessment and Monitoring
Some clinical and laboratory risk factors for in-hospital death have already been identified in patients with COVID-19 (7,8). The quantification of lung and cardiac involvement by multimodality imaging could effectively delineate the severity of the disease and eventually the prognosis, providing a base for further clinical decision making.
Quantification of lung damage using a chest CT severity score has been proposed to identify patients who need hospital admission (31). This score sums individual scores from 20 lung regions: scores of 0, 1, and 2 were assigned if parenchymal opacification involved 0%, <50%, and ≥50%, respectively, of each region (severity score range 0 to 40). The individual scores for each lung as well as the total score were significantly higher in patients with clinically severe COVID-19 compared with mild cases. A severity score <19.5 was highly effective in ruling out severe COVID-19 pneumonia, with a negative predictive value of 96.3% (31).
In the same way LUS could be effective in evaluating COVID-19 pneumonia severity and monitoring its modifications over time. For this purpose the numeric assessment of regional loss of aeration measured by global LUS score could represent a useful tool (33). The global LUS score can be calculated as the sum of regional aeration scores attributed to each lung region during a standard 12-zone examination: 0 = A-lines or <3 B-lines are visualized, 1 = ≥3 B-lines involving ≤50% of the pleura, 2 = B-lines becoming coalescent or involving >50% of the pleura, and 3 = tissue-like pattern (33) (Figure 3). The global LUS score showed a good correlation with lung density as assessed on CT and has been applied in the ICU setting to quantify and monitor lung aeration in weaning from mechanical ventilation and in patients with ARDS on extracorporeal membrane oxygenation (33). So far, the implementation of the global LUS score to monitor disease evolution and to guide decision making in patients with COVID-19 has not been systematically investigated.
Similarly, despite growing evidence pointing at the negative prognostic impact of cardiovascular involvement in COVID-19, no specific risk scores have been developed and validated. Interestingly, although great emphasis has been placed on the link between myocardial injury and mortality, the actual incidence of specific cardiovascular clinical conditions (myocarditis, MI, PE, and heart failure) and the respective prognostic implications in different stages of COVID-19 are largely unknown because of a significant lack of imaging data (4). A systematic approach with the use of multimodality imaging to precisely characterize COVID-19-related cardiovascular manifestations should be warranted to provide clinicians with comprehensive risk stratification tools.
Application of Imaging Modalities in Different Clinical Settings
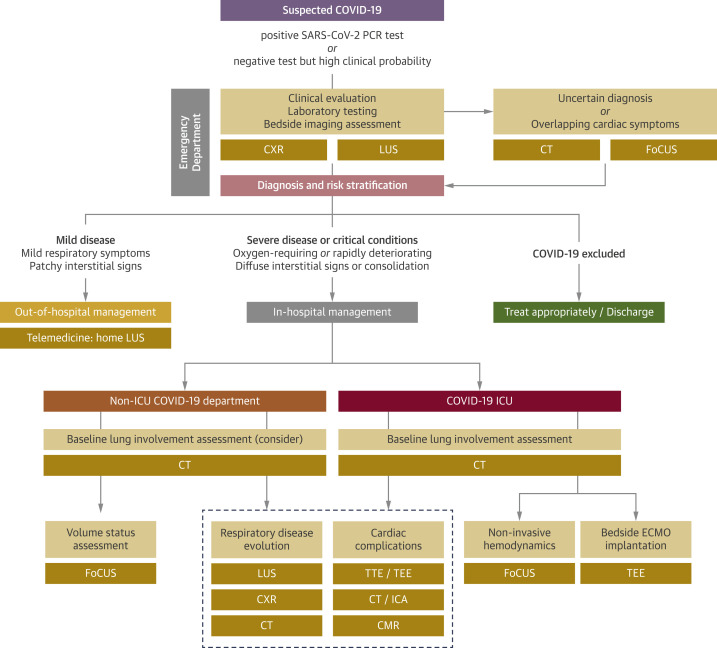
Imaging modalities are useful in the management of patients with COVID-19 in different clinical settings, from triage in the ED to ICU and non-ICU wards (Figure 7 ).
Figure 7.
Integrated Multimodality Imaging Pathways in Clinical Practice
Specific multimodality imaging pathways can be implemented in different clinical settings for diagnosis, risk stratification, management, disease progression monitoring, and detection of eventual cardiovascular complications. COVID-19 = coronavirus disease-2019; CMR = cardiac magnetic resonance; CT = computed tomography; CXR = chest radiography; ECMO = extracorporeal membrane oxygenation; FoCUS = focused cardiac ultrasound; ICA = invasive coronary angiography; ICU = intensive care unit; LUS = lung ultrasound; PCR = polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome-coronavirus-2; TEE = transesophageal echocardiography; TTE = transthoracic echocardiography.
ED and triage
A rapid and efficient diagnosis of COVID-19 is of paramount importance to accurately manage the large number of patients presenting to the ED with suspected SARS-CoV-2 infection. Considering the high probability of COVID-19 among patients currently accessing ED with fever and respiratory symptoms, the main goal is to stratify patients with positive SARS-CoV-2 RT-PCR results (or with clinically highly suspected infection despite negative results) to discharge those with mild symptoms and admit to non-ICU or ICU departments those with severe or life-threatening infection. A simultaneous clinical evaluation and LUS performed by the same visiting physician (reducing the number of operators exposed), combined with laboratory testing and CXR, allows fast diagnosis, risk stratification, and decision making regarding patient destination. In this context, LUS has the potential to rapidly discriminate initial forms of COVID-19 from advanced presentations (34). FoCUS is an adjunct to recognize specific ultrasound signs in patients with or suspected cardiac symptoms (37). This quick stratification could be subsequently confirmed by CXR, trying to limit the number of computed tomographic scans performed in the ED setting, reserving CT for cases with uncertain diagnosis or to rule out other causes of illness, such as PE. Of note, several patients have a severe form at ED presentation, rapidly becoming noninvasive ventilation dependent and, therefore, cannot easily undergo CT; in these patients, LUS is of paramount importance for rapid diagnosis and stratification. Despite its potential diagnostic utility, no unequivocal advantage has been demonstrated for an LUS-guided strategy over standard CXR and (if appropriate) computed tomographic evaluation in patients with suspected or confirmed COVID-19. Furthermore, LUS requires closer contact with the patient, potentially exposing clinicians to higher risk for aerosolized particle inhalation, mandates the use of more protective personal protection equipment (PPE), and should be performed by trained personnel. In this context, LUS application is a promising technique, although its role should not be overemphasized in the absence of solid evidence; on the contrary, CXR and clinical evaluation remain pivotal for initial patient assessment.
Beyond ED evaluation, an important approach to take care of patients and prevent transmission is the application of telemedicine (47). Telemedicine and e-visits could be combined with home triage for patients reporting worsening symptoms or self-monitored parameters, the latter being ideally performed by dedicated teams providing both clinical evaluation and LUS at the patient’s home, thus more accurately differentiating patients who could continue remote monitoring and medical therapy at home from those who need hospitalization.
Non-ICU COVID-19 department
Treatment of patients admitted to non-ICU COVID-19 departments is currently based on supportive care (i.e., oxygen therapy, noninvasive ventilation if necessary) and a combination of empirically prescribed drugs (i.e., hydroxychloroquine, antibiotic medications, antiviral medications, glucocorticoid agents, and anticytokine therapies). Along with clinical and laboratory evaluation, imaging is fundamental to assess COVID-19 evolution and response to therapy, both in daily clinical activity and in the context of controlled pharmacological and interventional trials. Baseline CT is frequently used to confirm diagnosis and to obtain detailed information on disease extent and severity, thus becoming also a reference for subsequent imaging follow-up (28). Of note, considering its known advantages (portability, bedside evaluation, safety), LUS seems particularly useful for serial assessments during hospital stay and may be useful to determine the timing of CT (34). Alongside with lung imaging, FoCUS could be useful to assess volume status and concomitant cardiac involvement, reserving cardiac CT, ICA, and CMR only for select cases, including suspected concomitant MI, PE, and myocarditis (37).
COVID-19 ICU
The ICU represents the most challenging setting in the management of patients with COVID-19. Ideally, baseline CT is needed in all critically ill patients requiring ICU admission to precisely describe morphological lung involvement. As in the previously described clinical settings, serial LUS and CXR are fundamental to monitor disease evolution in ICU patients, while CT could be used when clinical changes are observed, substantial modifications in morphological lung damage are suspected, or ventilator-related complications need to be excluded (32). Echocardiography could be useful to rule out concomitant cardiogenic causes of respiratory manifestations (37). Furthermore, FoCUS allows noninvasive hemodynamic monitoring in the ICU setting: assessment of biventricular function, estimated stroke volume, filling pressures, pulmonary pressures, and central venous pressure (37). Similarly, TTE helps in identifying patients at high risk for ventilator weaning failure and guides tailored therapeutic strategy. Finally, when mechanical respiratory and circulation support with extracorporeal membrane oxygenation is needed, both TTE and TEE are important to guide device selection (venovenous vs. venoarterial) on the basis of concomitant cardiogenic cause, assist during device placement (cannulation), and monitor cardiac function and device-related complications during support (48).
COVID-19-free patients
Patients with low clinical suspicion for COVID-19 and those with negative RT-PCR results deserve special consideration. As medical systems are overwhelmed, an accurate balance between infection prevention and adequate health care assistance delivery should be pursued. Besides clinical disease probability assessment, while serology tests are under development, current strategies to reduce in-hospital SARS-CoV-2 spread from asymptomatic patients rely on RT-PCR nasopharyngeal swab tests, which have important limitations (49). Therefore, adherence to international guideline recommendations and restriction of imaging tests to those with an important impact on patients’ clinical management are advocated (35,36). Triaging protocols should differentiate between patients requiring nondeferrable but schedulable imaging examinations, who can be appropriately managed after RT-PCR results are available, and those with urgent or emergent acute cardiovascular conditions, who should be considered SARS-CoV-2 positive until proved otherwise. Optimization of the health care network and patient pathways is required to avoid contamination between infected patients and SARS-CoV-2-negative patients, while maintaining adequate health assistance. Both patients and health care workers should be provided with standard PPE and keep social distance when possible. On the basis of our experience, RT-PCR testing should be performed according to local resources in select patients requiring hospitalization or undergoing aerosol-generating high-risk procedures, after body temperature measurement and a clinical triaging questionnaire evaluating history of fever, dyspnea, or cough and SARS-CoV-2 exposure in recent weeks (50).
Management Strategy for Cleaning, Protection, and Disinfection
The current COVID-19 pandemic has sharply increased the examination work load of imaging departments. The in-hospital infection rate was about 41% in a Chinese experience: 29% among hospital staff members and 12.3% among inpatients (2). In Italy, up to 9% of overall cases were reported among health care workers, with an estimated in-hospital infection rate of 10.8% (51). SARS-CoV-2 transmission occurs through the direct inhalation of droplets but also by touching the eyes, nose, or mouth after hand contact with contaminated surfaces. Imagers, nurses, and technicians are at especially high risk because of close patient contact while performing imaging studies. To prevent and mitigate transmission, preventive measures must be implemented, encompassing facilities, imaging equipment, PPE, and machine disinfection procedures (35).
Specific in-hospital routes between the imaging department and COVID-19 wards should be defined. The special environment for COVID-19-dedicated imaging should include a contaminated equipment area, a separated report room, and a staff cleaning room. The use of mobile equipment and dedicated scanners, ultrasound probes, and machines for infected patients should be encouraged (35).
Staff members must undergo rigorous nosocomial infection training and be equipped with high-quality PPE (Table 4 ), balancing the risk for transmission with the potential for scarcity of PPE, considering in some cases their reuse, with adequate precautions. The use of a checklist and a step-by-step process to ensure proper wearing (donning) and removing (doffing) is recommended. Imaging personnel not directly involved should avoid any contact, and the distance between technicians and patients must be, preferably, >1 to 2 m. All patients should wear surgical masks during imaging. Left-lateral patient positioning with the scanner on the right side of the bench may ensure the greatest distance between the patient’s face and the echocardiographer during TTE. Personnel involved in TEE should wear full PPE, as this procedure is aerosol generating. Although a cuffed endotracheal tube and closed-circuit ventilation could reduce the risk for aerosol generation in intubated patients, noninvasive ventilation carries a higher risk for droplet spreading. The level of protection during TEE should be full in both the ICU and the non-ICU context (35).
Table 4.
Personal Protection Equipment Needed at Different Protection Levels During Diagnostic Examinations in Patients With COVID-19
| TTE/CT/CMR |
TEE/ICA |
|||
|---|---|---|---|---|
| Respiratory Symptoms | No Respiratory Symptoms | Respiratory Symptoms | No Respiratory Symptoms | |
| Protective cap | X | X | X | |
| Surgical mask | X | X∗ | ||
| N-95/FFP2 and N-99/FFP3 respirator | X | X | X | |
| Goggles/face shield | X | X | X | |
| Nonsterile gloves | X | X∗ | X | |
| Sterile latex gloves | X | X | ||
| Disposable plastic gown | X | X∗ | X | |
| Isolation fluid-resistant gown | X | X | ||
| Shoe covers/protective boots | X | X | X | |
TEE = transesophageal echocardiography; TTE = transthoracic echocardiography; other abbreviations as in Tables 1 and 2.
Surgical mask, nonsterile gloves, and disposable plastic gown may be used in addition to N-95/FFP2 and N-99/FFP3 respirator, sterile gloves, and isolation fluid-resistant gown, respectively, to reduce personal protective equipment contamination.
Because SARS-CoV-2 is sensitive to most standard viricidal disinfectant solutions, imaging machines should be thoroughly cleaned. It is recommended to use soft cloth dipped in 2,000 mg/l chlorine-containing disinfectant or 75% ethanol for scanners disinfection (35). Generally, for echocardiographic probes, it is advised to immerse them for ≤1 h without using hot steam, cold gas, or abrasive agents, such as ethylene-oxide or glutaraldehyde-based methods. Automated disinfection solutions should be available. Air, object surfaces, and floor disinfection in the COVID-19-dedicated imaging department should be carried out according to daily operation specifications. In reading rooms, social distancing should be remembered and all nonessential items removed (35).
As of this writing, none of the health care workers in the cardiac imaging department of our hospital have been infected with SARS-CoV-2, underscoring the relevance of adequate PPE use and adherence to a rigorous safety protocol (52). Because PPE availability could be a significant issue, especially in hard-hit areas, the use of clinical judgment should be emphasized to avoid additional staff exposure deriving from performing imaging tests unlikely to yield clinically important information among COVID-19-positive or suspected positive patients. Thus, the need for procedures requiring stringent PPE (i.e., TEE or nuclear imaging) and the possibility to perform alternative imaging modalities (i.e., cardiac CT) or no procedure at all should be thoroughly assessed in order to optimize PPE use.
Conclusions
The SARS-CoV-2 outbreak has rapidly reached pandemic proportions and has become a major threat to global health. Although the predominant clinical feature of COVID-19 is an acute respiratory syndrome of varying severity, the cardiovascular system can be involved in several ways. Heart and lung multimodality imaging plays a central role in different clinical settings and is essential in diagnosis, risk stratification, and management of patients with COVID-19. To prevent and mitigate transmission, key preventive measures must be adopted, encompassing equipment, facilities, health care personnel, and disinfection procedures.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: To recognize COVID-19 pathogenesis, pulmonary and cardiovascular clinical manifestations, and the corresponding imaging findings in order to improve patient care.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: To apply integrated heart and lung multimodality imaging in different clinical settings to improve diagnosis, risk stratification, and management of COVID-19 patients. To select the proper personal protection equipment for health care workers protection during imaging examinations in COVID-19 era.
TRANSLATIONAL OUTLOOK: A systematic multimodality imaging approach to COVID-19-related cardiovascular manifestations could provide clinicians with comprehensive tools for risk stratification and decision making.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Imagingauthor instructions page.
References
- 1.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;80:656–665. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Mar 27 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. Mar 27 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Hear Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clerkin K.J., Fried J.A., Raikhelkar J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 10.Oudit G.Y., Kassiri Z., Jiang C. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. Mar 16 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua A., O’Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. Mar 27 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;19:30912. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sala S., Peretto G., Gramegna M. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grebe A., Hoss F., Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 19.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agewall S., Beltrame J.F., Reynolds H.R. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–153. doi: 10.1093/eurheartj/ehw149. [DOI] [PubMed] [Google Scholar]
- 21.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020 Mar 25 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 22.Kytömaa S., Hegde S., Claggett B. Association of influenza-like illness activity with hospitalizations for heart failure: the Atherosclerosis Risk in Communities Study. JAMA Cardiol. 2019;4:363–369. doi: 10.1001/jamacardio.2019.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Radiology ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection Available at:
- 24.Wong H.Y.F., Lam H.Y.S., Fong A.H.-T. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019 doi: 10.1148/radiol.2020201160. Mar 27 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2019 doi: 10.1148/radiol.2020200642. Feb 26 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon S.H., Lee K.H., Kim J.Y. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandirali M., Sconfienza L.M., Serra R. Chest x-ray findings in asymptomatic and minimally symptomatic quarantined patients in Codogno. Italy. Radiology. 2020;295:E7. doi: 10.1148/radiol.2020201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Dong C., Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020 doi: 10.1148/radiol.2020200843. Mar 19 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W., Yan F. Patients with RT-PCR confirmed COVID-19 and normal chest CT. Radiology. 2020;295:E3. doi: 10.1148/radiol.2020200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R., Li X., Liu H. Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpicelli G., Elbarbary M., Blaivas M. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 33.Chiumello D., Mongodi S., Algieri I. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit Care Med. 2018;46:1761–1768. doi: 10.1097/CCM.0000000000003340. [DOI] [PubMed] [Google Scholar]
- 34.Soldati G., Smargiassi A., Inchingolo R. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020;39:1459–1462. doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skulstad H., Cosyns B., Popescu B.A. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak. J Am Soc Echocardiogr. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer K.T., Flachskampf F.A. Focused cardiac ultrasonography. J Am Coll Cardiol Img. 2019;12:1243–1253. doi: 10.1016/j.jcmg.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Picard M.H., Weiner R.B. Echocardiography in the time of COVID-19. J Am Soc Echocardiogr. 2020;33:674–675. doi: 10.1016/j.echo.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann U., Truong Q.A., Schoenfeld D.A. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burris A.C., Boura J.A., Raff G.L., Chinnaiyan K.M. Triple rule out versus coronary CT angiography in patients with acute chest pain results from the ACIC consortium. J Am Coll Cardiol Img. 2015;8:817–825. doi: 10.1016/j.jcmg.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Esposito A., Palmisano A., Barbera M. Cardiac computed tomography in troponin-positive chest pain: sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. J Am Coll Cardiol Img. 2019;12:745–748. doi: 10.1016/j.jcmg.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Pontone G., Baggiano A., Conte E. “Quadruple rule out” with cardiac computed tomography in COVID-19 patient with equivocal acute coronary syndrome presentation. J Am Coll Cardiol Img. 2020 Apr 21 doi: 10.1016/j.jcmg.2020.04.012. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira V.M., Schulz-Menger J., Holmvang G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 44.Palmisano A., Benedetti G., Faletti R. Early T1 myocardial MRI mapping: value in detecting myocardial hyperemia in acute myocarditis. Radiology. 2020;295:316–325. doi: 10.1148/radiol.2020191623. [DOI] [PubMed] [Google Scholar]
- 45.Skali H., Murthy V.L., Al-Mallah M.H. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID-19) pandemic: an information statement from ASNC and SNMMI 2020. J Nucl Med. 2020;61:784–791. [PMC free article] [PubMed] [Google Scholar]
- 46.Mahmud E., Dauerman H.L., Welt F.G. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.039. Apr 21 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 48.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drake D.H., De Bonis M., Covella M. Echocardiography in pandemic: front-line perspective, expanding role of ultrasound, and ethics of resource allocation. J Am Soc Echocardiogr. 2020;33:683–689. doi: 10.1016/j.echo.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Istituto Superiore di Sanità COVID-19 integrated surveillance: key national data. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data Available at:
- 52.Zhan M., Qin Y., Xue X., Zhu S. Death from COVID-19 of 23 health care workers in China. N Engl J Med. 2020;382:2267–2268. doi: 10.1056/NEJMc2005696. [DOI] [PMC free article] [PubMed] [Google Scholar]