Abstract
Background
No therapy is approved for COVID-19 pneumonia. The aim of this study was to assess the role of tocilizumab in reducing the risk of invasive mechanical ventilation and death in patients with severe COVID-19 pneumonia who received standard of care treatment.
Methods
This retrospective, observational cohort study included adults (≥18 years) with severe COVID-19 pneumonia who were admitted to tertiary care centres in Bologna and Reggio Emilia, Italy, between Feb 21 and March 24, 2020, and a tertiary care centre in Modena, Italy, between Feb 21 and April 30, 2020. All patients were treated with the standard of care (ie, supplemental oxygen, hydroxychloroquine, azithromycin, antiretrovirals, and low molecular weight heparin), and a non-randomly selected subset of patients also received tocilizumab. Tocilizumab was given either intravenously at 8 mg/kg bodyweight (up to a maximum of 800 mg) in two infusions, 12 h apart, or subcutaneously at 162 mg administered in two simultaneous doses, one in each thigh (ie, 324 mg in total), when the intravenous formulation was unavailable. The primary endpoint was a composite of invasive mechanical ventilation or death. Treatment groups were compared using Kaplan-Meier curves and Cox regression analysis after adjusting for sex, age, recruiting centre, duration of symptoms, and baseline Sequential Organ Failure Assessment (SOFA) score.
Findings
Of 1351 patients admitted, 544 (40%) had severe COVID-19 pneumonia and were included in the study. 57 (16%) of 365 patients in the standard care group needed mechanical ventilation, compared with 33 (18%) of 179 patients treated with tocilizumab (p=0·41; 16 [18%] of 88 patients treated intravenously and 17 [19%] of 91 patients treated subcutaneously). 73 (20%) patients in the standard care group died, compared with 13 (7%; p<0·0001) patients treated with tocilizumab (six [7%] treated intravenously and seven [8%] treated subcutaneously). After adjustment for sex, age, recruiting centre, duration of symptoms, and SOFA score, tocilizumab treatment was associated with a reduced risk of invasive mechanical ventilation or death (adjusted hazard ratio 0·61, 95% CI 0·40–0·92; p=0·020). 24 (13%) of 179 patients treated with tocilizumab were diagnosed with new infections, versus 14 (4%) of 365 patients treated with standard of care alone (p<0·0001).
Interpretation
Treatment with tocilizumab, whether administered intravenously or subcutaneously, might reduce the risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia.
Funding
None.
Introduction
Since December, 2019, COVID-19 spread rapidly in Wuhan and throughout the Hubei province of China, and more recently in Europe and worldwide. Although a comparison of crude fatality rates across countries is made difficult by different testing policies, data from February and March, 2020, suggest that the fatality rate in Italy has been higher than that in China.1, 2
The clinical presentation of COVID-19 is highly heterogeneous, ranging from asymptomatic to severe pneumonia with respiratory failure that could lead to invasive mechanical ventilation or death.3, 4, 5 The disease is characterised by an initial phase of viral replication that can be followed by a second phase driven by the host inflammatory response.6 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection might cause a hyperimmune response that is associated with acute respiratory distress syndrome, as suggested by typical radiological findings.7 The most critical patients can develop a so-called cytokine storm, characterised by increased production of many cytokines that produce long-term damage and lung tissue fibrosis.8
No therapy has been approved for COVID-19 pneumonia, but current clinical approaches consider the combination of antiviral drugs and immunomodulatory drugs. Although lopinavir/ritonavir showed no benefit beyond the standard of care in an initial study,9 clinical trials on antivirals are ongoing. Drawing on a wider immunological perspective derived from rheumatology,10 immunomodulatory drugs have been considered, such as selective cytokine inhibitors, which leads to the inhibition of either the ligand or the receptor of a cytokine.11
Research in context.
Evidence before this study
No therapy is approved for COVID-19 pneumonia, but current clinical approaches consider the combination of antiviral and immunoactive drugs, including tocilizumab, a recombinant humanised monoclonal antibody against the interleukin-6 receptor. Literature research was done through PubMed, Embase, Cochrane Review, ISI Web of Science, and SCOPUS up to May 31, 2020. This research revealed an increasing interest in tocilizumab use in COVID-19 pneumonia, but no randomised clinical trial has been published so far. In a single centre study from Wuhan, China, that included 15 patients with COVID-19 pneumonia at risk for cytokine storm, treatment with tocilizumab appeared to have clinical benefit, although the doses were variable, ranging from 80 mg to 600 mg. A small, retrospective, case-control study from France found that death, intensive care unit admission, or both was higher in patients without tocilizumab than in the tocilizumab group (72% vs 25%; p=0·002). A randomised ongoing clinical study (CORIMUNO) anticipates a beneficial effect of tocilizumab compared with standard of care.
Added value of this study
In our multicentre, retrospective study of 544 patients with severe COVID-19 pneumonia, the use of tocilizumab administered either intravenously or subcutaneously was associated with reduced risk of mechanical ventilation and death (adjusted hazard ratio 0·61, 95% CI 0·40–0·92; p=0·020). We also found a strong association between the use of tocilizumab and reduced risk of death (adjusted hazard ratio 0·38, 0·17–0·83; p=0·015).
Implications of all the available evidence
Tocilizumab, administered intravenously or subcutaneously, might be capable of reducing the risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia.
Tocilizumab is a recombinant humanised monoclonal antibody of the IgG1 class, which is directed against both the soluble and membrane-bound forms of the interleukin-6 (IL-6) receptor.12, 13 Tocilizumab is recommended for the treatment of severe rheumatoid arthritis, systemic juvenile idiopathic arthritis, giant cell arteritis, and life-threatening cytokine release syndrome induced by chimeric antigen receptor T cell therapy.14, 15, 16 In a single-centre study from Wuhan, China,17 which included 15 patients with COVID-19 pneumonia at risk for cytokine storm, treatment with tocilizumab appeared to have a clinical benefit, although doses ranged from 80 mg to 600 mg. That study from China and other anecdotal observations18, 19 resulted in the opportunity for off-label use of tocilizumab to treat patients with COVID-19 severe pneumonia in Italy. By May, an increasing number of studies had reported use of tocilizumab in treating COVID-19.20, 21
The aim of this multicentre cohort study was to assess the role of tocilizumab in reducing the risk of invasive mechanical ventilation or death in a cohort of patients with severe COVID-19 pneumonia who received standard of care treatment.
Methods
Study design and participants
The Tocilizumab in Patients with Severe COVID-19 Pneumonia (TESEO) Study is a retrospective, observational cohort study done in three tertiary care centres in the Emilia-Romagna region, Italy, on patients with severe COVID-19 pneumonia. All centres contributed data on tocilizumab and standard of care treatment (appendix p 1). In the Modena cohort, we collected data on baseline signs, symptoms, comorbidities, blood count, and biochemical markers.
The study population was adults (≥18 years) with COVID-19, confirmed by PCR on nasopharyngeal swab, who were admitted to the centres in Bologna and Reggio Emilia between Feb 21 and March 24, 2020, and to the centre in Modena between Feb 21 and April 30, 2020 (after reviewers requested a follow-up extension, which was only possible at the Modena centre). Eligible patients had severe pneumonia, defined as at least one of the following: presence of a respiratory rate of 30 or more breaths per minute, peripheral blood oxygen saturation (SaO2) of less than 93% in room air, a ratio of arterial oxygen partial pressure (PaO2) to fractional inspired oxygen (FiO2) of less than 300 mm Hg in room air, and lung infiltrates of more than 50% within 24–48 h, according to Chinese management guidelines for COVID-19 (version 6.0).3, 22
Exclusion criteria for the use of tocilizumab were coexistent infection other than COVID-19; a PaO2/FiO2 ratio greater than 300 mm Hg; chronic or current glucocorticoid use; history of severe allergic reactions to monoclonal antibodies; less than 500 per μL neutrophils or less than 50 × 109 platelets; active diverticulitis, inflammatory bowel disease, or another symptomatic gastrointestinal tract condition that might predispose patients to bowel perforation; severe haematological, renal, or liver function impairment.
The study was approved by the Regional Ethical Committee of Emilia Romagna. All patients who received tocilizumab provided verbal, not written, informed consent because of isolation precautions.
Procedures
All patients received standard of care treatment at the time of hospital admission according to the regional COVID-19 guidelines of Emilia Romagna23 and updated data on treatment of COVID-19.24 Standard of care treatment included oxygen supply to target SaO2 reaching at least 90%, hydroxychloroquine (400 mg twice on day 1, followed by 200 mg twice per day on days 2–5, eventually adjusted for creatinine clearance estimated by a chronic kidney disease algorithm), azithromycin (500 mg once per day for 5 days) at the physician's discretion when suspecting a bacterial respiratory superinfection, lopinavir–ritonavir (400/100 mg twice per day) or darunavir–cobicistat (800/150 mg once per day) for 14 days, and low molecular weight heparin for prophylaxis of deep vein thrombosis according to bodyweight and renal function.
In addition to receiving the standard of care treatment, a non-randomly selected subset of patients also received tocilizumab treatment. Patients were considered eligible for tocilizumab treatment if they showed SaO2 of less than 93% and a PaO2/FiO2 ratio of less than 300 mm Hg in room air or a more than 30% decrease in their PaO2/FiO2 ratio in the previous 24 h during hospitalisation. Tocilizumab was administered by the intravenous or subcutaneous route depending on the availability of specific formulation at time of treatment. It should be mentioned that during the observation period, the high national requirements created an intermittent shortage of both formulations of the drug. Therefore, a random subset of patients who were eligible for tocilizumab never received the drug because of unavailability. Intravenous tocilizumab was administered at 8 mg/kg bodyweight (up to a maximum of 800 mg) administered twice, 12 h apart. The second dose was given because pharmacokinetic data suggested that adequate plasma levels of the drug could be obtained only after two doses, based on the results of pharmacokinetic models for severe or life-threatening chimeric antigen receptor T cell-induced cytokine storm in adult and paediatric patients.25
The subcutaneous formulation was used when there was a shortage of the intravenous formulation, at a dose of 162 mg administered in two simultaneous doses, one in each thigh (ie, 324 mg in total). This approach was used to mimic, as much as possible, the pharmacokinetic activity of the intravenous formulation to achieve similar levels of drug exposure. Because the site and depth of subcutaneous injection can influence absorption and distribution,26 the rate of absorption might vary markedly between dosing sites.26 The peak plasma concentration might take a few days to be reached after a single subcutaneous dose because of slow absorption through the lymphatic system into the systemic circulation.27 Because the monoclonal antibody might undergo proteolytic degradation by the cells of the reticuloendothelial system,28 higher doses than usual subcutaneous dosing for other indications were provided through separate injections.27 This decision was supported by a comparative pharmacokinetic and pharmacodynamic study of subcutaneous versus intravenous tocilizumab, which showed that after a single 162 mg dose in healthy subjects, subcutaneous bioavailability was 48·8%, whereas the pharmacodynamic activity of subcutaneous and intravenous tocilizumab against a soluble IL-6 receptor was similar during 1 week.28
The patients' full medical history, chronic comorbidities (including the Charlson Comorbidity Index29), demographic and epidemiological data, and baseline PaO2/FiO2 ratio were obtained at hospital admission. Other treatments were recorded, including glucocorticoids for acute respiratory distress syndrome. The risk of multiorgan failure and mortality was assessed with a standardised Subsequent Organ Failure Assessment (SOFA) score.30 Clinical data, including symptoms, complete blood count, coagulation, inflammatory, and biochemical markers were routinely registered in the electronic patient charts for the Modena cohort only.
Outcomes
The primary outcome of the study was a composite of death or invasive mechanical ventilation. The indications for mechanical ventilation were neurological failure (ie, altered consciousness with a Glasgow Coma Scale score of <10), cardiovascular failure (ie, vasopressor requirement or major electrocardiogram changes, including arrythmia or changes in repolarisation phase), and respiratory failure, defined by the presence of at least two of the following criteria: respiratory rate of 30 or more breaths per minute, respiratory distress with activation of accessory respiratory muscles, the need for FiO2 at 80% or more to maintain a SaO2 level of 90%, or a PaO2/FiO2 ratio of less than 100 mm Hg.31, 32
Statistical analysis
We compared the baseline characteristics of the participants treated with standard of care with tocilizumab and those treated with standard of care alone, including signs and symptoms, existing comorbidities, and blood count markers. Continuous variables were expressed as median (IQR) and compared by the Mann-Whitney U test for two groups and Kruskal Wallis test for three groups. Categorical variables were expressed as numbers (%) and compared by the χ2 test or Fisher's exact test across the groups. In a secondary analysis, the group treated with standard of care and tocilizumab was further split into those who received subcutaneous or intravenous formulations of tocilizumab.
We did a standard survival analysis, following up participants from the date of entry into clinics until initiation of invasive mechanical ventilation or death. We compared the time to invasive mechanical ventilation or death by treatment group using unweighted Kaplan-Meier curves and univariable and multivariable Cox regression analysis with baseline fixed covariates. The effect of treatment was shown using an unadjusted and adjusted hazard ratio (HR) with 95% CI. Key confounders were identified as sex, age, recruiting centre, duration of symptoms, and baseline SOFA score, which were the most probable causes of both treatment assignment and outcome risk.
To control for potential additional sources of time-fixed and time-varying confounding, we did various additional analyses. First, we adjusted the analysis for the baseline level of inflammation and coagulation in a subset of participants with available C-reactive protein and D-dimer values. Second, we replaced the SOFA score with alternative measures of the extent of concomitant morbidities at baseline, such as a binary indicator (≥1 comorbidity among diabetes, hypertension, cardiovascular disease, chronic renal insufficiency, or cancer) and the Charlson Comorbidity Index. Third, with the aim of emulating a randomised trial with similar characteristics and appropriately controlling for the time-varying confounder of glucocorticoid use, we fitted a marginal structural Cox regression model with stabilised inverse probability weights, which were constructed using sex, age, SOFA score, recruiting centre, duration of symptoms, time-varying use of glucocorticoids, and inverse probability of censoring.33 A secondary analysis with an endpoint of death alone used both the cause-specific hazard approach (assuming non-informative censoring) and a competing risk approach, in which deaths that occurred after the initiation of invasive mechanical ventilation were included as events.
Finally, to test the hypothesis that the difference between treatment groups might vary according to the baseline PaO2/FiO2 ratio, we formally included an interaction term in the Cox regression model. The results were shown after categorising the population into two strata using a clinical threshold of the PaO2/FiO2 ratio being 150 mm Hg. We also did a similar stratification analysis using age (18–64 years vs ≥65 years) to further investigate the possible confounding or effect modification caused by age.
In the subset of participants from the Modena cohort, we compared the mean trajectories of IL-6 (on a log10 scale) and of aspartate aminotransferase (raw scale) over time between tocilizumab and standard of care, using a linear mixed model with random intercept and slope.
We considered a two-sided p value test of less than 0·05 to be statistically significant. Statistical analyses were performed using the SAS software, version 9.4 (Cary, NC, USA).
Results
Of 1351 patients admitted to the recruiting centres, 544 (40%) patients with severe pneumonia were included in our analysis (figure 1 ). Overall, 359 (66%) of the patients were male, with a median age of 67 years (IQR 56–77; table 1 ). All patients showed clinical deterioration, with a median SOFA score of 2 (IQR 1–4), mainly driven by respiratory failure and requirement of oxygen support. The SOFA scores and PaO2/FiO2 ratios at baseline differed substantially across centres, with patients in Modena being the most compromised (appendix p 1).
Figure 1.
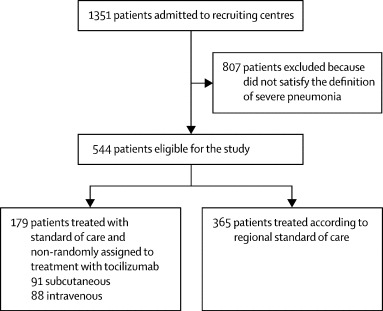
Overview of participants included in the TESEO cohort
Table 1.
Characteristics of patients from all centres combined
|
Tocilizumab plus standard care group (n=179) |
Standard care group (n=365) | p value | All patients (n=544) | |||||
|---|---|---|---|---|---|---|---|---|
| Subcutaneous (n=91) | Intravenous (n=88) | Overall (n=179) | ||||||
| Baseline characteristics | ||||||||
| Age (years) | 67 (55–73) | 63 (54–72) | 64 (54–72) | 69 (57–78) | 0·0064 | 67 (56–77) | ||
| Sex | .. | .. | .. | .. | 0·088 | .. | ||
| Female | 28 (31%) | 24 (27%) | 52 (29%) | 133 (36%) | .. | 185 (34%) | ||
| Male | 63 (69%) | 64 (73%) | 127 (71%) | 232 (64%) | .. | 359 (66%) | ||
| Baseline PaO2/FiO2 (mm Hg) | 199 (123–262) | 145 (102–229) | 169 (106–246) | 277 (191–345) | <0·0001 | 239 (139–306) | ||
| Baseline SOFA score | 2 (1–3) | 3 (2–4) | 3 (2–4) | 2 (0–3) | 0·0004 | 2 (1–4) | ||
| Duration of symptoms (days from symptom onset) | 8 (5–10) | 4 (3–8) | 7 (4–10) | 5 (2–9) | 0·0017 | 6 (3–9) | ||
| Outcomes | ||||||||
| Follow-up (days) | 12 (6–17) | 13 (7–18) | 12 (6–17) | 8 (4–14) | <0·0001 | 9 (4–15) | ||
| Events | ||||||||
| Mechanical ventilation | 17 (19%) | 16 (18%) | 33 (18%) | 57 (16%) | 0·41 | 90 (17%) | ||
| Deaths after mechanical ventilation* | 2 (12%) | 3 (19%) | 5 (15%) | 14 (25%) | 0·51 | 19 (21%) | ||
| Death | 7 (8%) | 6 (7%) | 13 (7%) | 73 (20%) | 0·0007 | 86 (16%) | ||
Data are median (IQR) or n (%) unless otherwise indicated. The p values refer to differences between overall tocilizumab and standard of care and were calculated using the χ2 test or Kruskal-Wallis test as appropriate. PaO2/FiO2=ratio of arterial oxygen partial pressure to fractional inspired oxygen. SOFA=Subsequent Organ Failure Assessment.
Percentages show the proportion of those who were mechanically ventilated.
365 (67%) patients received standard of care treatment alone and 179 (33%) received treatment with tocilizumab in addition to standard of care (88 [16%] received intravenous and 91 [17%] subcutaneous tocilizumab; table 1). The standard of care group included older patients with less severe disease, and the group treated with intravenous tocilizumab included the most compromised patients, as shown by their PaO2/FiO2 ratios and SOFA scores (table 1). After baseline, 53 (30%) of 179 patients treated with tocilizumab started glucocorticoids versus 61 (17%) of 365 patients in the standard of care group.
Data for comorbidities, signs, and symptoms were available for the patients from the Modena cohort only, which accounted for 354 (65%) of all patients (table 2 ). Among these patients, those treated with tocilizumab had a higher burden of hypertension and diabetes, and more had symptoms such as headache and cough (table 2). Biochemical markers were available for 304 (86%) of the patients in Modena, and showed that patients treated with tocilizumab had higher lactate dehydrogenase and worse inflammatory profiles at baseline, with higher C-reactive protein and IL-6 concentrations (table 3 ).
Table 2.
Baseline signs, symptoms, and comorbidities for patients at the Modena centre only
|
Tocilizumab plus standard care group (n=132) |
Standard care group (n=222) | p value | All patients (n=354) | |||
|---|---|---|---|---|---|---|
| Subcutaneous (n=84) | Intravenous (n=48) | |||||
| Age (years) | 67 (56–73) | 61 (52–74) | 67 (55–78) | 0·34 | 66 (55–76) | |
| Sex | .. | .. | .. | 1·0 | .. | |
| Female | 26 (31%) | 15 (31%) | 68 (31%) | .. | 109 (31%) | |
| Male | 58 (69%) | 33 (69%) | 154 (69%) | .. | 245 (69%) | |
| Any comorbidity | 39 (46%) | 24 (50%) | 36 (16%) | <0·001 | 99 (28%) | |
| Comorbidities | ||||||
| Diabetes | 11 (13%) | 6 (13%) | 7 (3%) | 0·0008 | 24 (7%) | |
| Hypertension | 37 (44%) | 22 (46%) | 30 (14%) | <0·0001 | 89 (25%) | |
| Cardiovascular disease | 9 (11%) | 6 (13%) | 12 (5%) | 0·12 | 27 (8%) | |
| Chronic renal insufficiency | 2 (2%) | 5 (10%) | 7 (3%) | 0·045 | 14 (4%) | |
| Cancer | 2 (2%) | 0 | 8 (4%) | 0·38 | 10 (3%) | |
| Disease duration | ||||||
| Days from symptoms onset to hospitalisation | 8 (6–11) | 5 (3–9) | 5 (2–9) | 0·0016 | 7 (3–10) | |
| Days from hospitalisation to intubation | 3 (0–5) | 2 (1–4) | 2 (0–3) | 0·49 | 2 (0–4) | |
| Sign and symptoms | ||||||
| Fever (°C) | 37 (36–38) | 37 (36–38) | 37 (36–37) | 0·54 | 37 (36–37) | |
| Cough | 42 (50%) | 20 (42%) | 55 (25%) | <0·0001 | 117 (33%) | |
| Myalgia | 5 (6%) | 5 (10%) | 7 (3%) | 0·088 | 17 (5%) | |
| Sputum | 5 (6%) | 0 | 4 (2%) | 0·059 | 9 (3%) | |
| Headache | 5 (6%) | 7 (15%) | 10 (5%) | 0·032 | 22 (6%) | |
| Haemoptysis | 0 | 1 (2%) | 2 (1%) | 0·45 | 3 (1%) | |
| Systolic pressure (mm Hg) | 130 (118–138) | 120 (110–135) | 124 (110–140) | 0·36 | 125 (110–138) | |
Data are median (IQR) or n (%) unless otherwise indicated. p values were calculated using the χ2 test or Kruskal-Wallis test as appropriate.
Table 3.
Baseline blood count and biochemical markers for patients at the Modena centre only
|
Tocilizumab plus standard care group (n=125) |
Standard care group (n=179) | p value | All patients (n=304) | ||
|---|---|---|---|---|---|
| Subcutaneous (n=78) | Intravenous (n=47) | ||||
| Haemoglobin (g/dL) | 12·8 (11·5–13·7) | 13·0 (11·6–13·7) | 12·7 (11·2–14·2) | 1·0 | 12·7 (11·4–14·0) |
| White cells (mm3) | 7195 (5470–10380) | 6840 (5140–9380) | 6200 (4570–9360) | 0·22 | 6700 (4890–9560) |
| Lymphocytes (%) | 22·1 (9·7–36·8) | 18·1 (12·5–25·8) | 23·1 (9·9–39·7) | 0·60 | 20·6 (9·9–36·6) |
| Total lymphocytes (mm3) | 1580 (1390–2142) | 2459 (1852–3348) | 1390 (1000–2815) | 0·30 | 1852 (1120–2726) |
| Platelets (109/L) | 257·5 (183·0–374·0) | 211·0 (156·0–294·0) | 209·0 (155·0–298·0) | 0·0027 | 221·5 (163·0–317·0) |
| Alanine aminotransferase (U/L) | 37·0 (27·0–71·0) | 35·0 (21·5–62·5) | 31·0 (19·0–48·0) | 0·0070 | 33·0 (22·0–56·0) |
| Bilirubin (mg/dL) | 0·6 (0·4–0·7) | 0·6 (0·4–0·8) | 0·6 (0·4–0·8) | 0·74 | 0·6 (0·4–0·8) |
| Calcium (mg/dL) | 8·6 (8·4–9·1) | 8·5 (8·1–8·9) | 8·6 (8·3–9·1) | 0·26 | 8·6 (8·3–9·1) |
| Creatine kinase (U/L) | 63·0 (33·0–159·0) | 130·0 (41·5–312·0) | 71·0 (39·0–202·0) | 0·051 | 76·0 (38·0–197·5) |
| Chloride (mmol/L) | 101·0 (98·0–105·0) | 100·0 (99·0–103·0) | 101·0 (97·0–103·0) | 0·91 | 101·0 (98·0–103·0) |
| Creatinine (mg/dL) | 0·8 (0·6–0·9) | 0·9 (0·7–1·2) | 0·9 (0·7–1·1) | 0·0050 | 0·8 (0·7–1·1) |
| D-dimer (mg/mL) | 1210 (820·0–2290) | 1000 (780·0–2730) | 1240 (610·0–2480) | 0·64 | 1200 (690·0–2480) |
| Lactate dehydrogenase (U/L) | 600·0 (505·0–761·0) | 676·0 (536·0–765·0) | 507·5 (419·5–705·5) | 0·0002 | 564·0 (454·0–745·0) |
| Potassium (mmol/L) | 3·9 (3·5–4·3) | 3·8 (3·6–4·0) | 3·9 (3·5–4·3) | 0·34 | 3·9 (3·5–4·2) |
| Sodium (mmol/L) | 137·5 (136·0–139·0) | 137·0 (135·0–138·0) | 138·0 (135·0–141·0) | 0·019 | 138·0 (135·0–140·0) |
| Ferritin (mg/mL) | 1168 (543–1214) | .. | 423 (355–993) | 0·14 | 447 (355–1141) |
| C-reactive protein (mg/dL) | 3·4 (0·6–7·8) | 6·1 (1·8–15·3) | 5·4 (1·8–14·6) | 0·022 | 5·3 (1·4–13·6) |
| Interleukin-6 (pg/mL) | 190·2 (86·6–401·0) | 238·3 (140·2–731·9) | 144·1 (41·1–385·8) | 0·045 | 178·6 (67·6–402·0) |
Data are median (IQR). p values were calculated using the Kruskal-Wallis test.
Overall, invasive mechanical ventilation was started in 90 (17%) of 544 patients, including 57 (16%) of 365 patients in the standard care group versus 33 (18%) of 179 patients treated with tocilizumab (p=0·41; table 1). 86 (16%) of 544 patients died, including 73 (20%) patients in the standard care group versus 13 (7%; p=0·0007) patients treated with tocilizumab (table 1). The percentage of patients who were ventilated differed by centre (p=0·028), but risk of mortality did not (p=0·49; appendix p 1). 19 further deaths occurred after the date of initiation of mechanical ventilation (two in the subcutaneous tocilizumab group, three in the intravenous tocilizumab group, and 14 in the standard of care group), giving a total of 105 deaths, which we analysed using a competing risk approach.
At 14 days from hospital admission, the cumulative probabilities estimated with Kaplan-Meier analyses for all groups were 36·1% (95% CI 31·2–40·9) for the primary composite endpoint of invasive mechanical ventilation or death, 18·8% (15·1–22·5) for mechanical ventilation, and 21·1% (16·3–25·8) for death (appendix p 7; figure 2 ).
Figure 2.
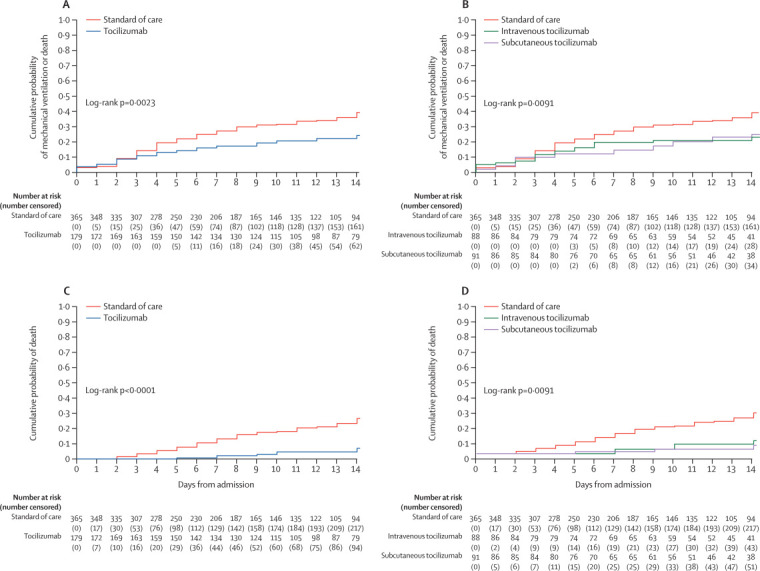
Kaplan-Meier estimates of the cumulative probability of mechanical ventilation or death (A, B) and death (C, D) by treatment group
Unweighted Kaplan-Meier estimates showed the beneficial effect of treatment with tocilizumab compared with standard of care only (figure 2). At day 14 after hospital admission, the proportion of patients with the composite outcome was 22·6% (95% CI 16·2–29·0) for the tocilizumab group versus 36·5% (30·7–42·2) for the standard of care only group (log rank p=0·0023; figure 2A; appendix p 7). The difference was larger and the association stronger for the mortality endpoint, both with the cause-specific hazard approach (log rank p<0·0001; figure 2C) and the competing risk approach (p<0·0001). After splitting the tocilizumab group by administration route, both groups showed a benefit compared with standard of care, with no marked difference between the intravenous and subcutaneous groups (log rank p=0·0091 [figure 2B]; log rank p<0·0001 [figure 2D]).
Patients who received tocilizumab showed a significant reduction in risk of invasive mechanical ventilation or death when compared with those receiving standard of care only, as estimated from the unadjusted Cox regression model (HR 0·60, 95% CI 0·43–0·84; p=0·0030; table 4 ). After controlling for the key identified confounders of sex, age, SOFA score, recruiting centre, and duration of symptoms, the treatment effect was even larger (adjusted HR [aHR] 0·61, 0·40–0·92; p=0·020; table 4). These results were supported by various analyses that aimed to control for further sources of confounding, namely after adjusting for baseline C-reactive protein values (aHR 0·57, 0·38–0·84; p=0·0048) and baseline d-dimer levels (aHR 0·66, 0·42–1·05; p=0·082), after replacing the SOFA score with the Charlson Comorbidity Index (aHR 0·64, 0·46–0·91; p=0·012), and after controlling for time-varying confounding of using glucocorticoids and informative censoring (aHR=0·53, 0·31–0·89; p=0·016; table 4).
Table 4.
Unadjusted and adjusted relative hazards of the composite of the initiation of invasive mechanical ventilation or death
|
Unadjusted analysis |
Adjusted analysis* |
Adjusted analysis† |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Overall (two-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Tocilizumab (any) | 0·60 (0·43–0·84) | 0·0030 | 0·64 (0·45–0·91) | 0·012 | 0·61 (0·40–0·92) | 0·020 |
| Tocilizumab (any)‡ | 0·54 (0·37–0·78) | 0·0009 | .. | .. | 0·53 (0·31–0·89)§ | 0·016 |
| Baseline PaO2/FiO2≤150 mm Hg (two-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Tocilizumab (any)¶ | 0·30 (0·17–0·52) | .. | 0·20 (0·11–0·36) | .. | 0·19 (0·08–0·44) | .. |
| Interaction p value ¶ | .. | .. | .. | .. | .. | 0·011 |
| Baseline PaO2/FiO2>150 mmHg (two-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Tocilizumab (any)¶ | 0·31 (0·16–0·59) | .. | 0·39 (0·20–0·77) | .. | 0·46 (0·21–0·99) | .. |
| Overall comparison (three-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Subcutaneous tocilizumab | 0·63 (0·41–0·97) | 0·036 | 0·69 (0·44–1·08) | 0·102 | 0·65 (0·39–1·11) | 0·11 |
| Intravenous tocilizumab | 0·57 (0·36–0·90) | 0·016 | 0·60 (0·38–0·95) | 0·030 | 0·55 (0·31–0·98) | 0·042 |
| Intravenous tocilizumab | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Subcutaneous tocilizumab | 1·10 (0·61–1·95) | 0·76 | 1·14 (0·63–2·05) | 0·67 | 1·18 (0·59–2·36) | 0·64 |
| Standard of care | 1·75 (1·11–2·75) | 0·016 | 1·66 (1·05–2·62) | 0·030 | 1·80 (1·02–3·19) | 0·042 |
Data are n (95% CI) unless otherwise indicated. Data obtained using a Cox regression model. 152 patients with missing PaO2/FiO2 were not included in the stratified analysis. PaO2/FiO2=ratio of arterial oxygen partial pressure to fractional inspired oxygen.
Adjusted for age, sex, and recruiting centre.
Adjusted for age, sex, recruiting centre, duration of symptoms, and Subsequent Organ Failure Assessment (SOFA) score.
Using a weighted Cox instead of standard Cox model.
Adjusted for age, sex, recruiting centre, duration of symptoms, SOFA score, use of steroids after baseline, and censoring using inverse probability weighting.
Some p values intentionally left out as p values in the subsets are not interpretable.
The largest difference was found when comparing intravenously administered tocilizumab with standard of care only. After adjusting for the same set of identified confounders (ie, sex, age, and SOFA score, recruiting centre, and duration of symptoms), we estimated a reduction in the risk of invasive ventilation or death with an aHR of 0·55 (95% CI 0·31–0·98; p=0·042; table 4). For the composite endpoint, we found no evidence for a difference between subcutaneous and intravenous tocilizumab (aHR 1·18, 0·59–2·36; p=0·64; table 4). Finally, the main results for the composite endpoint were similar after restricting the analysis to people enrolled in Modena only (aHR 0·65, 0·43–0·99; p=0·044).
The formal test for interaction and the stratified analyses showed evidence that the different risk between tocilizumab and standard of care varied by baseline PaO2/FiO2 value (p=0·011). In particular, the effect of tocilizumab was greater in people with baseline PaO2/FiO2 value of less than 150 mm Hg (aHR 0·19, 95% CI 0·08–0·44; table 4). No difference in the results was found after controlling for age using stratification (aged 18–65 years vs ≥65 years; data not shown).
With regard to mortality, a significant reduction in risk of death was found for tocilizumab treatment compared with standard of care treatment alone after controlling for sex, age, SOFA score, recruiting centre, and duration of symptoms (aHR 0·38, 95% CI 0·17–0·83; p=0·015; table 5 ). We found no statistical evidence for a differential benefit of tocilizumab by baseline PaO2/FiO2 value (interaction p=0·12; table 5), but the reduction in risk of death was stronger in people with a baseline PaO2/FiO2 ratio of less than 150 mm Hg.
Table 5.
Unadjusted and adjusted relative hazards of death (all-cause mortality)
|
Unadjusted analysis |
Adjusted analysis* |
Adjusted analysis† |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Overall comparison (two-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Tocilizumab (any) | 0·28 (0·15–0·50) | <0·0001 | 0·36 (0·20–0·66) | 0·0009 | 0·38 (0·17–0·83) | 0·015 |
| Baseline PaO2/FiO2≤150 mmHg (two-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Tocilizumab (any)‡ | 0·11 (0·04–0·27) | .. | 0·09 (0·03–0·24) | .. | 0·03 (0·00–0·24) | .. |
| Interaction p value‡ | .. | .. | .. | .. | .. | 0·12 |
| Baseline PaO2/FiO2>150 mmHg (two-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Tocilizumab (any)‡ | 0·22 (0·08–0·63) | .. | 0·39 (0·12–1·20) | .. | 0·44 (0·11–1·73) | .. |
| Overall comparison (three-way contrast) | ||||||
| Standard of care | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Subcutaneous tocilizumab subcutaneous | 0·30 (0·14–0·66) | 0·0025 | 0·37 (0·17–0·83) | 0·016 | 0·44 (0·17–1·14) | 0·091 |
| Intravenous tocilizumab | 0·25 (0·11–0·58) | 0·0012 | 0·35 (0·15–0·80) | 0·013 | 0·29 (0·09–0·99) | 0·048 |
Data are n (95% CI) unless otherwise indicated. Data obtained using a Cox regression model. 152 patients with missing PaO2/FiO2 were not included in the stratified analysis. PaO2/FiO2=ratio of arterial oxygen partial pressure to fractional inspired oxygen.
Adjusted for age, sex, and recruiting centre.
Adjusted for age, sex, recruiting centre, duration of symptoms, and Subsequent Organ Failure Assessment (SOFA) score.
Some p values intentionally left out as p values in the subsets are not interpretable.
We also repeated this analysis after controlling for the Charlson Comorbidity Index instead of SOFA, and the results were similar (aHR 0·36, 95% CI 0·19–0·65; p=0·0008). Finally, after including the additional 19 deaths that occurred after the date of initiation of invasive ventilation, the results from the competing risk analysis were similar to those of the main analysis (aHR 0·27, 0·16–0·47; p<0·0001).
The mixed linear models showed that IL-6 plasma levels were slightly higher at study entry in the tocilizumab group than in the standard of care group (2·46 log10 mg/mL vs 2·25 log10 mg/L; p=0·091; appendix p 3). Over time, IL-6 was stable in the tocilizumab group and decreased in the standard of care group with a difference in slope of −0·02 log10 mg/L (95% CI −0·03 to −0·00; p=0·0042; appendix pp 3–4).
Adverse events were carefully monitored during the study period. In the tocilizumab group, one (<1%) patient had an episode of injection site reaction, with spontaneous resolution in a few hours. One (<1%) episode of severe neutropenia required granulocyte-colony stimulating factor administration. Finally, there was no evidence for a difference in the rate of increase of aspartate aminotransferase between treatment groups (appendix pp 5–6). There was only one (<1%) patient in the standard of care group whose aspartate aminotransferase concentrations increased from 19 U/L before treatment level to 139 U/L at 6 days after treatment, and none in the tocilizumab group.
We paid careful attention to new episodes of infections in the tocilizumab and standard of care groups, which included bloodstream infections (three vs four), bacterial pneumonia (eight vs six), candidemia (two vs two), urinary tract infection (one vs one), Pneumocistis jirovecii pneumonia (one vs one), invasive aspergillosis, (four vs none), hepatitis B virus reactivation (one vs none), and herpes simplex virus 1 reactivation (four vs none). Of note, one (<1%) severe adverse event occurred in the tocilizumab group 12 days after subcutaneous injection, consisting of severe liver failure due to herpes simplex virus 1 reactivation, leading to death. This patient received high-dose glucocorticoids after the administration of tocilizumab. Overall, 24 (13%) of 179 patients treated with tocilizumab were diagnosed with new infections, versus 14 (4%) of 365 patients treated with standard of care alone (p<0·0001).
Discussion
In the real-life setting of the TESEO cohort, we found a significant reduction in risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia who were treated with either intravenous or subcutaneous tocilizumab and standard of care, compared with those treated with standard of care only. The association with the use of tocilizumab was stronger when overall mortality risk was analysed alone.
Our results are consistent with those of a smaller, retrospective, case-controlled French study by Klopfenstein and colleagues,20 in which death or intensive care unit (ICU) admissions were higher in patients who did not receive tocilizumab than those who did (72% vs 25%; p=0·002). The CORIMUNO randomised clinical trial, from which some data are already available, also anticipates a beneficial effect of tocilizumab when compared with standard of care.34
The natural history of severe COVID-19 pneumonia is thought to be driven by a so-called cytokine storm.14 Nevertheless, current recommendations do not include any immunologically active drug in routine clinical practice, and use of glucocorticoids is controversial.35, 36 Tocilizumab, administered intravenously or subcutaneously, can be considered together with anakinra as one of the immunomodulatory drugs that have been tested in clinical care for the treatment of severe COVID-19 pneumonia.19, 20, 21, 37 In the present study, IL-6 levels remained stable after tocilizumab administration but decreased in people receiving standard of care only. This finding was expected because tocilizumab competitively blocks IL-6 receptors and leaves free IL-6 in plasma. Longer follow-up and larger sample sizes are needed to better understand the prognostic role of IL-6 concentrations and other biomarkers in patients with COVID-19 pneumonia who are treated with tocilizumab.
The real-life setting, including three different hospitals, accounted for the heterogenicity in clinical characteristics and disease severity across intervention groups. As expected, the comparator group showed a higher baseline PaO2/FiO2 value than did the intervention group. Thus, in the unadjusted analysis the magnitude of the beneficial effect associated with the use of tocilizumab could have been underestimated. We attempted to control for this confounding bias by adjusting for SOFA score, which includes baseline PaO2/FiO2, and the difference was indeed larger after adjustment. In addition, the effect of tocilizumab was at least two times higher in people with a baseline PaO2/FiO2 ratio of less than 150 mm Hg, implying that the benefit of tocilizumab could be greater in patients with a greater risk of death or mechanical ventilation. Further studies are needed to evaluate the optimal timing of tocilizumab initiation on the basis of PaO2/FiO2 values and severity of disease stage.
Our results were similar after further adjusting for post-baseline use of glucocorticoids. This analysis opens the discussion for the combination of immunomodulatory drugs (ie, monoclonal antibodies) with anti-inflammatory drugs (ie, glucocorticoids and non-steroid anti-inflammatory drugs). Importantly, very similar results were obtained regardless of the route of tocilizumab administration. Nevertheless, we cannot rule out the presence of other time-varying confounders that were affected by the chosen treatment strategy and were not accounted for in the analysis. Of note, antiviral drugs (protease inhibitors such as lopinavir–ritonavir and darunavir–cobicistat) were used in both groups and were never started after baseline in the tocilizumab group.
A major concern is adverse events. We observed a significantly higher prevalence of infection in the tocilizumab group than in the standard of care only group. The study design and short follow-up period do not allow us to make conclusions regarding the early and long-term side-effects of receiving tocilizumab followed by glucocorticoids; data from ongoing randomised clinical trials are required. Nevertheless, the case of severe herpes simplex virus 1 hepatitis in the tocilizumab group suggests the importance of screening for herpes virus reactivation, especially if glucocorticoids are added.
We chose a composite outcome including both invasive mechanical ventilation and all-cause mortality. The crude fatality rate in our cohort was 16% (86 deaths before mechanical ventilation and 105 [19%] deaths in total among 544 patients diagnosed with severe pneumonia). A large multicentre cohort study from China showed a fatality rate of 28% among hospitalised patients, despite patients being younger by a median of 15 years compared with our study.3 Moreover, in a study conducted in Wuhan, China, 84 (42%) of 201 patients developed acute respiratory distress syndrome, and 44 (52%) of them died.1 In the European setting, a recent large study38 with 1591 patients admitted to ICUs in the Lombardy region of Italy showed that 1150 (88%) received mechanical ventilation, 137 (11%) received non-invasive ventilation, and the fatality rate was 26%.38 However, this analysis did not exclude patients who were still hospitalised and did not evaluate patients outside of the ICU setting and is therefore not fully comparable with our findings.
Our composite endpoint allowed us to describe not only the most critical clinical events, but also the most burdensome issue for health-care systems that need to rapidly increase the availability of their ICU resources. It is important to note that many countries are facing a shortage of mechanical ventilators. This shortage could lead to difficult clinical choices about which patients to prioritise for treatment. Consequentially, a treatment that reduces ICU admission is highly relevant not only to ameliorate the prognosis of the hospitalised patients, but also to give more patients the opportunity to receive intensive care when needed. However, the largest effect of the tocilizumab treatment in the present study was for mortality, and rates of mechanical ventilation alone made little contribution to the difference observed.
Our study has some limitations. First, it is not a randomised comparison, and therefore unmeasured confounding cannot be ruled out. In addition, the results rely on the usual assumptions about the model being correctly specified (ie, that the assumed underlying causal structure is correct and we have adjusted for all sources of measured confounding). The participants who received standard of care only were older and therefore at higher baseline risk of invasive ventilation and death. However, these patients were also more likely to be women, and female sex has been shown to be associated with better outcomes.39 The patients who received tocilizumab in addition to standard of care treatment were mainly selected based on the availability of the drug (which was intermittent over the recruitment phase because of shortages), and they were more compromised patients with lower PaO2/FiO2 ratios and higher SOFA scores compared with those treated with standard of care alone. We adjusted the analysis for SOFA, which controls for respiratory function (baseline PaO2/FiO2 ratio) and separately for the Charlson Comorbidity Index (which controls for the extent of comorbidities present at admission). In the tocilizumab group, there were two patients with cancer and two patients with renal insufficiency, and in the standard of care group, there were eight patients with cancer and seven with chronic renal insufficiency. Therefore, we cannot rule out residual confounding that cannot be controlled by regression interpolation.
Another limitation is that although the key confounder measurements (for sex, age, SOFA score, duration of symptoms, and Charlson Comorbidity Index) were available for all participants, this was not true for some of the biomarkers of inflammation and coagulation, which were available for only the participants in Modena. However, the results were similar when we repeated the analysis using the Modena centre dataset alone. Importantly, when we used a marginal structural model instead of the standard estimate of the hazard ratio that is conditioned on covariates, which additionally controlled for glucocorticoid use after baseline, the difference in risk between treatment strategies was even larger. This is a key result, given the wide use of these methods, the time-varying structure of the data, and our attempt to emulate the results of randomised comparisons.33
The study was also open label, so that staff involved knew which patients were receiving tocilizumab. This knowledge might have led to variability in the decision of when to move a patient to invasive ventilation (ie, a quicker decision for those receiving standard of care only). Moreover, it should be acknowledged that the indication for mechanical ventilation, even if suggested by guidelines, still relies on clinical judgment, which might vary according to experience and the availability of resources. The ICU staff involved in the study shared similar protocols and resources. Finally, because of the short follow-up period, we were not able to assess long-term safety and adverse effects. Further studies are needed to define appropriate dosing and minimise the side-effects.
Our study has also strengths. First, it was a large study that included patients from a real-life hospital setting. Second, key confounding factors were collected daily in a standardised way for a minimum of 14 days and were linked to the electronic charts of blood counts and clinical data.
Many questions remain open. The generalisability of the results must be considered in relation to different epidemiological settings, particularly regarding the tocilizumab dose and use at the appropriate time point of the disease course. Other drugs that act directly in the inflammatory response pathway triggered in COVID-19 are being tested. Tocilizumab use in severe COVID-19 pneumonia is still in its infancy, and the best treatment strategies have yet to be developed. For instance, our experience also described subcutaneous tocilizumab use, which warrants future studies in out-patient settings.
In conclusion, both intravenous and subcutaneous tocilizumab administration might be capable of reducing the risk of invasive mechanical ventilation or death in patients with severe COVID-19 pneumonia. Although these results are encouraging, they should be confirmed in ongoing randomised studies.
This online publication has been corrected. The corrected version first appeared at thelancet.com/rheumatology on September 3, 2020
Acknowledgments
Acknowledgments
We thank Barbara Beghetto, Giulia Nardini, and Enrica Roncaglia at the Office of Clinical Protocols and Data Management in Modena, Maria Grazia Bitonti on behalf of Modena nursing team, Tommaso Trenti at the Labaratory Policlinico of Modena, Alessandra Melegari, Rossella Fogliani, Grazia Righini, Roberto Savigni, and Mario Lugli at the Office of Information and Communication Technologies of the Policlinico di Modena, Mirko Orsini and Enrico Calanchi from Data River, and Nilla Viani, Marianna Rivasi, Lisa Daya, Laura Cancian, and Cinzia Barberini from the Pharmacy of Modena.
Contributors
GG, MMes, AC-L, JM, RT, CS, MMas, PLV, and CM conceptualised and designed the study. GG, MMes, AC-L, JM, RT, and CM wrote and revised the manuscript. GG, MMes, AC-L, JM, and CM supervised the final version of the manuscript. AC-L did the statistical analysis. GG, ST, and GD were in charge of the database of the three centres. MMen did the figures. All authors contributed to data collection, clinical management of the patients, and data interpretation.
Modena COVID-19 Working Group
Erica Bacca, Giulia Burastero, Giacomo Ciusa, Matteo Faltoni, Giacomo Franceschi, Vittorio Iadisernia, Damiano Larné, Francesco Pellegrino, Alessandro Raimondi, Carlotta Rogati, Marco Tutone, Sara Volpi, Dina Yaacoub, Alberto Andreotti, Emanuela Biagioni, Filippo Bondi, Stefano Busani, Giovanni Chierego, Marzia Scotti, Lucia Serio, Caterina Bellinazzi, Rebecca Borella, Sara De Biasi, Anna De Gaetano, Lucia Fidanza, Lara Gibellini, Anna Iannone, Domenico Lo Tartaro, Marco Mattioli, and Annamaria Paolini.
Declaration of interests
We declare no competing interests.
Collaborative Research Group of Reggio Emilia
Fabrizio Boni, Elisabetta Teopompi, Enrico Barchi, Monica Cocchi, Giada Chiara Contardi, Romina Corsini, Claudia Lazzaretti, Giulia Marini, Guido Menozzi, Sergio Mezzadri, Paolo Pavone, Francesca Prati, Nicoletta Riva, Fabio Sampaolesi, Matteo Seligardi, Giuliana Zoboli, Anaflorina Matei, Pierpaolo Salsi, Giovanni Bettelli, Nicola Facciolongo, Matteo Fontana, Giulia Ghidoni, Emanuele Alberto Negri, Rosaria Santi, Chiara Trenti, Enrica Minelli, Luca Boracchia, Andrea Caruso, Giuseppe Germanò, Giulia Pazzola, Francesco Muratore, Gianluigi Bajocchi, Manuela Castagnetti, Massimo Costantini, Marco Foracchia, Alessandro Zerbini, Laura Albertazzi, Stefania Croci, and Lucia Belloni.
Supplementary Material
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. published online Feb 24. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. published online March 23. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-J, Ni Z-Y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicastri E, D'Abramo A, Faggioni G. Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.11.2000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77:1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stebbing J, Phelan A, Griffin I. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2008;4:767–775. doi: 10.2147/tcrm.s3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerdlow DI, Holmes MV, Kuchenbaecker KB. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214–1224. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norelli M, Camisa B, Barbiera G. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 15.Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JH, Tuckwell K, Dimonaco S. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 17.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. published online April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: a cautionary case report. Chest. 2020 doi: 10.1016/j.chest.2020.04.024. published online April 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sciascia S, Aprà F, Baffa A. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 20.Klopfenstein T, Zayet S, Lohse A. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.05.001. published online May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toniati P, Piva S, Cattalini M. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102568. published online May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Commission of the People's Republic of China Chinese management guideline for COVID-19 (version 6.0) Feb 19, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf
- 23.Italian National Society of Infectious Diseases Guidelines for the managment of COVID-19 infection. https://www.eahp.eu/sites/default/files/covid19_vademecum_2.0_13_marzo_2020.03_11.pdf
- 24.Yao X, Ye F, Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le RQ, Li L, Yuan W. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor t cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter-subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55:789–805. doi: 10.1007/s40262-015-0361-4. [DOI] [PubMed] [Google Scholar]
- 27.Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52:83–124. doi: 10.1007/s40262-012-0027-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013;51:443–455. doi: 10.5414/CP201819. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Palta S, Saroa R, Palta A, Sama S, Gombar S. Sequential organ failure assessment scoring and prediction of patient's outcome in Intensive Care Unit of a tertiary care hospital. J Anaesthesiol Clin Pharmacol. 2016;32:364–368. doi: 10.4103/0970-9185.168165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgs A, McGrath BA, Goddard C. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120:323–352. doi: 10.1016/j.bja.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Davidson AC, Banham S, Elliott M. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(suppl 2):ii1–ii35. doi: 10.1136/thoraxjnl-2015-208209. [DOI] [PubMed] [Google Scholar]
- 33.Lodi S, Phillips A, Lundgren J. Effect estimates in randomized trials and observational studies: comparing apples with apples. Am J Epidemiol. 2019;188:1569–1577. doi: 10.1093/aje/kwz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pipeline Review Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID-19 pneumonia. April 28, 2020. https://pipelinereview.com/index.php/2020042874458/Antibodies/Tocilizumab-improves-significantly-clinical-outcomes-of-patients-with-moderate-or-severe-COVID-19-pneumonia.html
- 35.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;195:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavalli G, De Luca G, Campochiaro C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng Y, Wu P, Lu W. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16:1–13. doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


