Abstract
Objectives
The aim of the present study was to describe and evaluate the results of a new technique in endolymphatic sac decompression surgery.
Methods
Forty‐three patients with intractable unilateral Meniere's disease were selected. Endolymphatic sac was identified after simple mastoidectomy, and its lateral layer was incised, using a sickle knife. Outer layer of the sac was turned around and placed under the anterior bony border.
Results
Mean duration of the follow‐up was 24 months. Mean tinnitus handicap index, pure tone average (PTA) on thresholds at 500, 1000, 2000, and 4000 Hz, mean speech reception threshold, mean speech discrimination score, hearing stage, and mean vertigo score before and after surgery were evaluated.
Conclusion
The new marsupialization technique with anterior bony border is a safe and effective way to improve tinnitus, vertigo, and ear fullness among these patients. According to PTA and hearing stage, this surgery can control progressive hearing loss.
Level of Evidence
3
Keywords: endolymphatic sac decompression, marsupialization technique, Meniere's disease
1. INTRODUCTION
Meniere's disease (MD) is a common inner ear disease, characterized by vertigo, hearing loss, and tinnitus. It occurs at an incidence rate of 15 to 50 per 100 000 individuals. 1 MD is difficult to diagnose, especially in its early stages. Frequently, initial vertigo attacks are frequently diagnosed as viral labyrinthitis. 2 Histopathological findings of temporal bone studies in patients with MD include loss of epithelial integrity of endolymphatic sac, 3 perisacular fibrosis, hypoplasia of vestibular aqueduct, and atrophy of the sac. 4 Medical management in MD consists of conservative treatment and lifestyle modifications, such as low salt diet, avoiding caffeine, alcohol, and tobacco, in addition to diuretics and betahistine prescriptions.
About 10% of patients are refractory to medical management. 5 Ultimately, when conservative treatments fail, surgical management should be considered. There are different surgical approaches to treat patients with MD. Some interventions include transtympanic steroids or gentamicin, endolymphatic sac surgery, ventilation tube placement, vestibular neurectomy, and labyrinthectomy. 6 Nowadays, endolymphatic sac surgery is less invasive and easier to perform in comparison with labyrinthectomy or vestibular neurectomy. 7 Recently, Derebery et al presented encouraging results for endolymphatic sac shunt surgery in comparison with intratympanic gentamicin treatment to control vertigo and to preserve hearing. 8 Although most of the endolymphatic sac techniques report acceptable vertigo control, but preservation of hearing level, ear fullness, suturing in previous techniques using silastic shunt and ventilation tube, surgical site infection, more dissection, and intraop complication in the previous studies were the reasons that we intended to perform a modification of endolymphatic sac decompression surgery with a new marsupialization technique to minimizing the surgical complications without using silastic shunt, ventilation tube, and suturing to reduce the cost of surgery, preserving hearing level with less fibrosis and infection. In addition, with limited dissection, fewer intraop complications are expected.
2. MATERIALS AND METHODS
In this retrospective study, all patients underwent endolymphatic sac decompression surgery in Rasool‐e‐Akram teaching hospital from 2012 to 2017. Forty‐three adult patients were enrolled in this study with unilateral diagnosed intractable MDs, who had fulfilled the criteria of the 1995 American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS) guideline on MD. The individuals were diagnosed according to their clinical symptoms like progressive or fluctuating hearing loss and recurrent vertigo. All patients underwent medical treatment for about 1 year. Intractable MD is defined as progressive hearing deterioration more than 10dB with or without recurrent vertigo at least once a month for about 3 to 6 months which is outlined during the 2008 Lancet seminar. 9
Patients with bilateral MD, neurology disorders, noninteractable MD, peripheral, or central vestibular disorders were excluded from this study. Complete neurological examination was done and any other pathology in the cerebellopontine angle was ruled out by magnetic resonance imaging. Hearing impairment was considered with pure tone average (PTA) with four frequency average (500, 1000, 2000, and 4000 Hz), speech discrimination score (SDS), and speech reception threshold (SRT). The Functional Level Score, hearing stage, and vertigo control were computed according to the 1995 AAO‐HNS guidelines. 10 Hearing stage is classified by the four‐tone average with stage 1 being less than 25 dB, stage 2 between 25 and 40 dB, stage 3 between 41 and 70 dB, and stage 4 more than 70 dB. 11
All these measures were obtained immediately before the treatment and 6 months after the surgery. The proportion of patients with reduction of tinnitus was measured, using patient‐reported questionnaire scores, such the tinnitus handicap index (THI) score. 12
All surgeries were performed by the first author (A. D.), using similar surgical technique. All patients were operated under general anesthesia. All the patients were followed up for at least 24 months.
All patients were informed about the risk of surgical procedures, and they signed a written informed consent. Ethical approval for this study was obtained from local research Ethics Committee of Iran University of Medical Sciences.
2.1. Surgical technique
Under general anesthesia, intact canal mastoidectomy was performed. The incus, lateral and posterior semicircular canal (SCC), and sigmoid sinus were identified. If the sigmoid sinus was anterior, unroofing of the sinus was performed with its posterior decompression. The bone over the dura of the posterior fossa was removed including from the sigmoid sinus posteriorly and up to the posterior SCC anteriorly. In few patients, endolymphatic sac is smaller, making it more difficult for surgeons to identify it. Therefore, Donaldson's line can be used as surgical landmark for the endolymphatic sac. It passes through horizontal SSC bisecting posterior SSC. The endolymphatic sac that appears as thickening of the posterior cranial fossa dura is situated inferior to the Donaldson's line (Figure 1). The endolymphatic sac usually seems whiter than the posterior fossa dura. When the sac is identified, its lateral layer incised by a sickle knife via Y‐shaped incision (Figure 2). This layer was turned around as an anterior based flap and placed under accompanying anterior bony border (Figure 3). Thereafter, the sac was marsupialized. No tube or any other external material was required to preserve the sac orifice (Figure 4).
FIGURE 1.
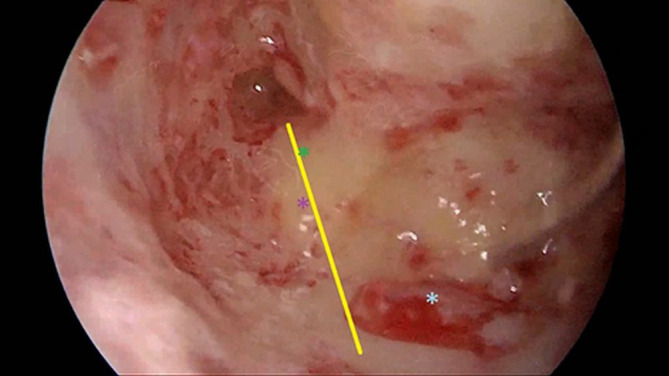
Yellow line: Donaldson's line; green asterisk: lateral semicircular canal; purple asterisk: posterior semicircular canal; blue asterisk: endolymphatic sac
FIGURE 2.
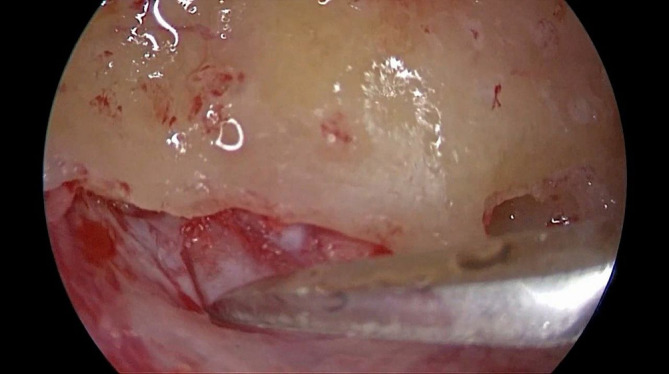
Lateral layer of endolymphatic sac is incised by a sickle knife via Y‐shaped incision
FIGURE 3.

The lateral layer of endolymphatic sac is turned around as an anterior based flap and is placed under accompanying anterior bony border
FIGURE 4.
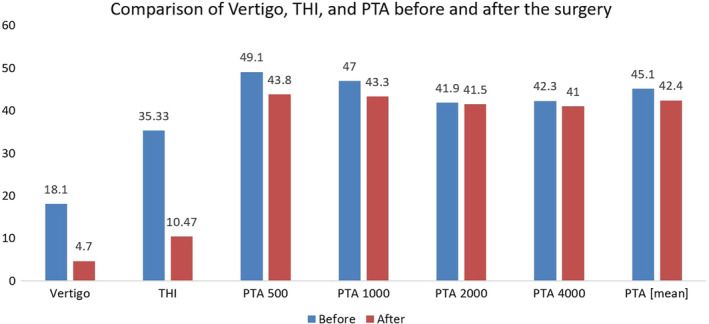
Comparison of vertigo, THI, and PTA before and after the surgery. PTA, pure tone average; THI, tinnitus handicap index
2.2. Statistical analyses
Mean and SD of continuous variables were determined. For continuous variables, normality of data was determined by Kolmogorov‐Smirnov test. If the data were normally distributed, comparison of the results before and after surgery was done by paired t test, unless the results were compared by Wilcoxon signed rank test. For nominal data, comparison of the results before and after the study was done by McNemar test. P values less than .05 were considered to be statistically significant. SPSS version 18 (PASW, IBM corp., Armonk, New York) was used for statistical analyses.
3. RESULTS
In total, 43 patients were enrolled in this study. Their mean age was 38.2 ± 9.3 years old (24‐55) and 29 patients were female (67.4%). Also, 23 surgeries were performed on the left side (53.5%). Mean duration of follow‐up after surgery was 24 months (18‐28).
Ear fullness was seen in 37 patients before the surgery (88.1%), and only in 8 patients after the surgery (19%) (P < .001). All patients without fullness before surgery did not have fullness after surgery (Table 1).
TABLE 1.
Demographic data
| Gender (male:female) (%) | 14:29 (32.6:67.4) | |
| Age (range) | 38.16 (24‐55) | |
| Laterality (Rt:Lt) (%) | (20:23) (46.5:53.5) | |
| Tinnitus (Yes:No) (%) | (32:11) (74.4:25.6) | |
| Ear fullness | Before surgery (Yes:No) (%) | (38:5) (88.4:11.6) |
| After surgery (Yes:No) (%) | (9:34) (20.9:79.1) | |
In total, 11 patients did not complain of tinnitus before the surgery. Among others with some degrees of tinnitus (32 patients, 74.4%), mean THI before the surgery was 47.5 ± 13.8 while it was 14.1 ± 8.3 after the surgery (P < .001). Before surgery and based on THI, 10 cases were severe (23.3%), 18 moderate (41.9%), and 4 mild (9.3%) while after the surgery, among the 29 patients suffering from tinnitus (67.4%), 28 were mild (65.1%) and only one patient complained of moderate tinnitus (2.3%) (P < .001).
Mean PTA (500, 1000, 2000, and 4000 Hz) was 45.1 ± 8 before the surgery while it was 42.4 ± 9.5 after the surgery (P = .04). Comparing PTA before and after surgery, the score increased in 13 patients (30.2%), remained unchanged in 6 patients (14%), and decreased in 24 patients (51.2%).
PTA 500 Hz was 49.1 ± 8.1 before the surgery while it was 43.8 ± 11.4 after the surgery (P = .008). Comparing PTA 500 Hz before and after surgery, the score increased in 13 patients (30.2%), remained unchanged in 2 patients (4.7%), and decreased in 28 patients (65.1%).
PTA 1000 Hz was 47 ± 9.6 before surgery, while it was 43.3 ± 11.7 after the surgery (P = .045). Comparing PTA 1000 Hz before and after the surgery, the score increased in 11 patients (25.6%) (in which it was equal to 10 in nine patients and equal to 20 in two patients), remained unchanged in 8 patients (18.6%), and decreased in the other 24 patients (55.8%) (in which it was equal to 5 in 2 patients, equal to 10 in 16 patients, and equal to 20 in 6 patients).
PTA 2000 Hz was 41.9 ± 10.7 before the surgery, while it was 41.5 ± 12.6 after the surgery (P = .84). Comparing PTA 2000 Hz before and after the surgery, the score increased in 17 patients (39.5%), remained unchanged in 5 patients (11.6%), and decreased in 21 patients (48.8%).
PTA 4000 Hz was 42.3 ± 9.2 before the surgery and it was 41 ± 11.1 after the surgery (P = .52). Comparing PTA 4000 Hz before and after the surgery, the score increased in 17 patients (39.5%), remained unchanged in 7 patients (16.3%), and decreased in 19 patients (44.2%) (Figure 4).
Considering the Hearing stage before the surgery, 12 were stage II (27.9%) and 31 were stage III (72.1%) while after the surgery, 23 were stage II (53.5%) and 20 were stage III (46.5%) (P = .003). In fact, after surgery, Hearing stage increased in 1 patient (2.3%), remained unchanged in 30 patients (69.8%), and decreased in 12 patients (27.9%).
Mean SRT was 44.5 ± 7.7 before the surgery, while it was 42.3 ± 10 after the surgery (P = .11). It increased after the surgery in 16 patients (37.2%) (equal to 15 in 1 patient, equal to 10 in 6 patients, and equal to 5 in 9 patients), remained unchanged in 6 patients (14%), and decreased in 21 patients (equal to 5 in 5 patients, equal to 10 in 10 patients, and equal to 15 in 6 patients).
Mean SDS was 94 ± 5.2 before the surgery, while it was 94.6 ± 4.9 after the surgery (P = .96). It was increased after the surgery in 15 patients (34.9%), remained unchanged in 13 patients (30.2%), and decreased in 15 patients (34.9%).
Mean vertigo score was 18.1 ± 3.9 before the surgery while it was 4.7 ± 3.1 after the surgery (P < .001). It was increased after the surgery in only one patient (2.3%) (equal to 11 points) and decreased in 42 patients (97.7%) (Figure 4).
4. DISCUSSION
Although important progress has been made in treatment of MD, it is still challenging for most clinicians. Portmann in 1927 described the endolymphatic sac decompression for the first time by a small incision to open the endolymphatic sac with the aim to reduce the endolymphatic pressure. 13
In 1976, Paparella introduced the endolymphatic duct valve technique with T‐tube insertion. Vertigo was controlled in 94% of patients, and resulted in 30% improvement in hearing level. 14 In 2006, Convert et al 15 reported a 10‐year follow‐up of patients operated for the endolymphatic sac decompression, and vertigo was controlled in 64.5% and their hearing level improved in 14.8% of the cases, which was in line with Kim et al's report. 16 A systematic review and meta‐analysis from 2014 by Sood et al showed that the endolymphatic sac decompression with or without shunt is excellent for controlling vertigo and hearing level in 75% of the patients with resistant MD. There was a trend toward sac decompression alone over shunting procedures to provide better vertigo control, and the data showed hearing level in patients without Silastic shunt was significantly better in comparison with patients with Silastic. 17
Similar to previous data, many studies have considered endolymphatic sac surgery as an effective treatment of intractable MD. Huang in 2002 reported his experience after more than 3000 endolymphatic sac decompression surgeries, he stated that although it seems unlikely to obtain a short‐term rate of vertigo control above 90%, improvements could be made to the long‐term control of MD symptoms, by modifying the surgical procedure in the future or performing intervention in early stages of the MD. 18 Saliba et al in 2015 introduced endolymphatic duct blockage technique showed that good vertigo control as well as a significantly increased quality of life. 19 According to the systematic review meta‐analysis in 2017 by Volkenstein et al comparing the endolymphatic sac decompression and endolymphatic duct blockage, more difficult technique and intraoperation cerebrospinal fluid (CSF) leak were seen in endolymphatic duct blockage surgery due to the more dissection of the bone adjacent to the dura matter to identify the endolymphatic duct in its superior and inferior part to clip the duct. 20 In 2019, Naples et al considered intratympanic gentamicin as initial therapy for early and long‐term control of MD, and showed that this procedure is a safe and effective way for controlling vertigo without risk of hearing loss, but there are still concerns regarding bilateral MD. 21
However, endolymphatic sac decompression is an excellent nondestructive surgical option for patients with resistant or bilateral MD; therefore, we attempted to find a new way for endolymphatic sac decompression, to minimize the damage to hearing level and reduce surgical site infection. Using silastic shunt could damage the surrounding tissue due to the fibroproliferative response. 22 Owing to more hearing level impairment in patients using Silastic in comparison to group without Silastic, we decided to use a new technique to minimize the risk of surgical interventions. To do so, we did not use silastic, any tube, or suturing during our surgeries, and for making a permanent shunt, we used adjacent anterior bony border. The main advantage of this procedure is its simplicity and fewer complications due to limited dissection in comparison with endolymphatic duct blockage. Besides, anterior bony border helps the flap to stay in place, make a permanent shunt, and minimize the recurrence rate. In this study, we showed significant improvement in ear fullness after surgery (88.1% before surgery in comparison with 19% after surgery). According to THI score, tinnitus decreased significantly (before surgery, it was 47.5 ± 13.8, while it was 14.1 ± 8.3 after surgery; P < .001). Mean PTA, mean SRT, and SDS did not significantly change before and after surgery (44.5 ± 7.7 before surgery, while it was 42.3 ± 10 after surgery [P = .11]; 94 ± 5.2 before surgery, while it was 94.6 ± 4.9 after surgery [P = .96], respectively). Mean vertigo score showed 97.7% improvement after surgery and follow‐up (18.1 ± 3.9 before surgery, while it was 4.7 ± 3.1 after surgery; P < .001). We only had one patient with refractory vertigo after surgery who required secondary treatment. In this case, we chose vestibular neurectomy and achieved symptoms relief. No symptom recurrence or any infection was seen in these patients. During our follow‐up, three patients had MD symptom in contralateral ear, which was due to the nature of MD; 10% to 40% of cases of unilateral MD progress to bilateral bypassing time. None of the patients had intraoperative CSF leak, posterior SSC injury, facial nerve injury, or sigmoid sinus injury. There was also no problem regarding preservation of the bony border or flap during surgery. However, this is a delicate procedure and the surgeon should be very careful not to tear the flap or damage the surrounding bony border. No patient required revision surgery due to foreign material; hence, the risk of more fibrosis as a result of revision surgery was reduced. Therefore, we hope that this technique could help MD patients to improve their quality of life and reduce the cost of the health insurance.
Not having a control group and small number of patients could be mentioned as limitations of this study. There is no modality to confirm that the marsupialized sac is still open except by clinical responses in the follow‐ups. A multicenter, prospective study with more patients and longer follow‐up period would provide higher level of evidence.
5. CONCLUSION
Endolymphatic sac decompression surgery and new marsupialization technique under anterior bony border is safe and an excellent nondestructive surgery for patients with resistant MD. Significant vertigo and hearing level control were observed in these patients with no significant complication.
Surgical treatment is indicated in some patients with intractable MD. Nowadays, endolymphatic sac surgery is known as a safe procedure. Endolymphatic sac decompression surgery is one of the best options to control MD. Use of marsupialization technique under anterior bony border is a novel method for endolymphatic sac decompression without the need for suturing with low recurrence rate. Also, vertigo and hearing level were controlled in these patients with no significant complication.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Farideh Hosseinzadeh and Ahmad Daneshi had full access to all study data and take responsibility for the study integrity, the accuracy of data analysis, and conceived and designed the study. Ahmad Daneshi took responsibility for funding and supervised the study. Farideh Hosseinzadeh, Alimohamad Asghari, Ahmad Daneshi, Mohammd Mohseni, Saleh Mohebbi, and S. Saeed Mohammadi interpreted the data. Farideh Hosseinzadeh analyzed the data. Farideh Hosseinzadeh was responsible for study coordination and recruitment. Farideh Hosseinzadeh, Alimohamad Asghari, Ahmad Daneshi, Mohammd Mohseni, Saleh Mohebbi, and S. Saeed Mohammadi drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final manuscript.
ACKNOWLEDGMENTS
The authors acknowledge ENT Head and Neck Research Center, Iran University of Medical Sciences for financial support. The authors wish to thank Mr. H. Argasi at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for his invaluable assistance in editing this article.
Daneshi A, Hosseinzadeh F, Mohebbi S, Mohseni M, Mohammadi SS, Asghari A. New marsupialization technique in endolymphatic sac surgery. Laryngoscope Investigative Otolaryngology. 2020;5:546–551. 10.1002/lio2.403
Funding information ENT Head and Neck Research Center, Iran University of Medical Sciences, Grant/Award Number: 12354
REFERENCES
- 1. Stahle J, Stahle C, Arenberg IK. Incidence of Meniere's disease. Arch Otolaryngol. 1978;104(2):99‐102. [PubMed] [Google Scholar]
- 2. da Costa SS, de Sousa LC, Piza MR. Meniere's disease: overview, epidemiology, and natural history. Otolaryngol Clin North Am. 2002;35(3):455‐495. 10.1016/s0030-6665(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 3. Sando I, Ikeda M. The vestibular aqueduct in patients with Meniere's disease. A temporal bone histopathological investigation. Acta Otolaryngol. 1984;97(5‐6):558‐570. 10.3109/00016488409132934. [DOI] [PubMed] [Google Scholar]
- 4. Brinson GM, Chen DA, Arriaga MA. Endolymphatic mastoid shunt versus endolymphatic sac decompression for Meniere's disease. Otolaryngol Head Neck Surg. 2007;136(3):415‐421. 10.1016/j.otohns.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 5. Wetmore S. Endolymphatic sac surgery for Ménière's disease: long‐term results after primary and revision surgery. Arch Otolaryngol Head Neck Surg. 2008;134(11):1144‐1148. [DOI] [PubMed] [Google Scholar]
- 6. van Benthem PPG. Surgery for Menière's disease. Curr Otorhinolaryngol Rep. 2014;2(3):162‐166. 10.1007/s40136-014-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ostrowski VB, Kartush JM. Endolymphatic sac‐vein decompression for intractable Meniere's disease: long term treatment results. Otolaryngol Head Neck Surg. 2003;128(4):550‐559. 10.1016/s0194-5998(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 8. Derebery MJ, Fisher LM, Berliner K, Chung J, Green K. Outcomes of endolymphatic shunt surgery for Meniere's disease: comparison with intratympanic gentamicin on vertigo control and hearing loss. Otol Neurotol. 2010;31(4):649‐655. 10.1097/MAO.0b013e3181dd13ac. [DOI] [PubMed] [Google Scholar]
- 9. Sajjadi H, Paparella MM. Meniere's disease. Lancet. 2008;372(9636):406‐414. 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 10. Committee on Hearing and Equilibrium . Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. Otolaryngol Head Neck Surg. 1995;113(3):181‐185. [DOI] [PubMed] [Google Scholar]
- 11. Wick CC, Manzoor NF, McKenna C, Semaan MT, Megerian CA. Long‐term outcomes of endolymphatic sac shunting with local steroids for Meniere's disease. Am J Otolaryngol. 2017;38(3):285‐290. 10.1016/j.amjoto.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 12. Kleinstäuber M, Frank I, Weise C. A confirmatory factor analytic validation of the tinnitus handicap inventory. J Psychosom Res. 2015;78(3):277‐284. [DOI] [PubMed] [Google Scholar]
- 13. Portmann G. Surgical treatment of vertigo by opening of the saccus endolymphaticus. Arch Otolaryngol. 1969;89(6):809‐815. 10.1001/archotol.1969.00770020811005. [DOI] [PubMed] [Google Scholar]
- 14. Paparella MM, Hanson DG. Endolymphatic sac drainage for intractable vertigo (method and experiences). Laryngoscope. 1976;86(5):697‐703. 10.1288/00005537-197605000-00010. [DOI] [PubMed] [Google Scholar]
- 15. Convert C, Franco‐Vidal V, Bebear JP, Darrouzet V. Outcome‐based assessment of endolymphatic sac decompression for Meniere's disease using the Meniere's disease outcome questionnaire: a review of 90 patients. Otol Neurotol. 2006;27(5):687‐696. 10.1097/01.mao.0000227661.52760.f1. [DOI] [PubMed] [Google Scholar]
- 16. Kim SH, Ko SH, Ahn SH, Hong JM, Lee WS. Significance of the development of the inner ear third window effect after endolymphatic sac surgery in Meniere disease patients. Laryngoscope. 2012;122(8):1838‐1843. 10.1002/lary.23332. [DOI] [PubMed] [Google Scholar]
- 17. Sood AJ, Lambert PR, Nguyen SA, Meyer TA. Endolymphatic sac surgery for Meniere's disease: a systematic review and meta‐analysis. Otol Neurotol. 2014;35(6):1033‐1045. 10.1097/MAO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 18. de Lourdes Flores García M, de la Llata Segura C, Lesser JCC, Pianese CP. Endolymphatic sac surgery for Ménière's disease ‐ current opinion and literature review. Int Arch Otorhinolaryngol. 2017;21:179‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saliba I, Gabra N, Alzahrani M, Berbiche D. Endolymphatic duct blockage: a randomized controlled trial of a novel surgical technique for Meniere's disease treatment. Otolaryngol Head Neck Surg. 2015;152(1):122‐129. 10.1177/0194599814555840. [DOI] [PubMed] [Google Scholar]
- 20. Volkenstein S, Dazert S. Recent surgical options for vestibular vertigo. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2017;16:Doc01 10.3205/cto000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naples JG, Henry L, Brant JA, Eliades SJ, Ruckenstein MJ. Intratympanic therapies in Meniere disease: evaluation of outcomes and early vertigo control. Laryngoscope. 2019;129(1):216‐221. 10.1002/lary.27392. [DOI] [PubMed] [Google Scholar]
- 22. Flores Garcia ML, Llata Segura C, Cisneros Lesser JC, Pane CP. Endolymphatic sac surgery for Meniere's disease ‐ current opinion and literature review. Int Arch Otorhinolaryngol. 2017;21(2):179‐183. 10.1055/s-0037-1599276. [DOI] [PMC free article] [PubMed] [Google Scholar]


