Supplemental Digital Content is available in the text.
Keywords: anticoagulants, coronary artery disease, diabetes mellitus, peripheral artery disease, platelet aggregation inhibitors
Abstract
Background:
Patients with established coronary artery disease or peripheral artery disease often have diabetes mellitus. These patients are at high risk of future vascular events.
Methods:
In a prespecified analysis of the COMPASS trial (Cardiovascular Outcomes for People Using Anticoagulation Strategies), we compared the effects of rivaroxaban (2.5 mg twice daily) plus aspirin (100 mg daily) versus placebo plus aspirin in patients with diabetes mellitus versus without diabetes mellitus in preventing major vascular events. The primary efficacy end point was the composite of cardiovascular death, myocardial infarction, or stroke. Secondary end points included all-cause mortality and all major vascular events (cardiovascular death, myocardial infarction, stroke, or major adverse limb events, including amputation). The primary safety end point was a modification of the International Society on Thrombosis and Haemostasis criteria for major bleeding.
Results:
There were 10 341 patients with diabetes mellitus and 17 054 without diabetes mellitus in the overall trial. A consistent and similar relative risk reduction was seen for benefit of rivaroxaban plus aspirin (n=9152) versus placebo plus aspirin (n=9126) in patients both with (n=6922) and without (n=11 356) diabetes mellitus for the primary efficacy end point (hazard ratio, 0.74, P=0.002; and hazard ratio, 0.77, P=0.005, respectively, Pinteraction=0.77) and all-cause mortality (hazard ratio, 0.81, P=0.05; and hazard ratio, 0.84, P=0.09, respectively; Pinteraction=0.82). However, although the absolute risk reductions appeared numerically larger in patients with versus without diabetes mellitus, both subgroups derived similar benefit (2.3% versus 1.4% for the primary efficacy end point at 3 years, Gail-Simon qualitative Pinteraction<0.0001; 1.9% versus 0.6% for all-cause mortality, Pinteraction=0.02; 2.7% versus 1.7% for major vascular events, Pinteraction<0.0001). Because the bleeding hazards were similar among patients with and without diabetes mellitus, the prespecified net benefit for rivaroxaban appeared particularly favorable in the patients with diabetes mellitus (2.7% versus 1.0%; Gail-Simon qualitative Pinteraction=0.001).
Conclusions:
In stable atherosclerosis, the combination of aspirin plus rivaroxaban 2.5 mg twice daily provided a similar relative degree of benefit on coronary, cerebrovascular, and peripheral end points in patients with and without diabetes mellitus. Given their higher baseline risk, the absolute benefits appeared larger in those with diabetes mellitus, including a 3-fold greater reduction in all-cause mortality.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01776424.
Clinical Perspective.
What Is New?
In a prespecified analysis, COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) Diabetes compared low-dose rivaroxaban (2.5 mg twice daily) plus aspirin versus placebo plus aspirin in 6922 patients with stable coronary or peripheral artery disease and diabetes mellitus.
Although there was a consistent and similar relative risk reduction with rivaroxaban plus aspirin versus placebo plus aspirin in patients both with and without diabetes mellitus for the primary efficacy end point and all-cause mortality, notably, the absolute risk reductions appeared larger in patients with diabetes mellitus, including a 3-fold greater reduction in mortality.
There appeared to be a larger absolute net clinical benefit in those with diabetes mellitus.
What Are the Clinical Implications?
In patients with stable atherosclerosis and diabetes mellitus without an indication for dual antiplatelet therapy such as recent stenting or recent acute coronary syndromes, the addition of low-dose rivaroxaban to aspirin provides substantial reductions in ischemic events, including a significant reduction in all-cause mortality, with absolute risk reductions that appeared larger in those with versus without diabetes mellitus.
Non-fatal major bleeding was increased similarly in those with versus without diabetes mellitus.
In patients at acceptable bleeding risk, the addition of low-dose rivaroxaban to aspirin should be considered in the secondary prevention regimen of patients with atherosclerosis and diabetes mellitus.
Editorial, see p 1855
Diabetes mellitus is a commonly occurring major risk amplifier in patients with established atherosclerosis.1–4 In particular, those with polyvascular disease, a marker of significant clinical atherosclerotic burden, and concomitant diabetes mellitus, which frequently coexist, constitute a very high-risk group of patients subject to coronary, cerebral, and peripheral ischemic events.1,5,6 Lipid-lowering therapies and glycemia-modifying drugs can help attenuate this risk.7–18 Despite effective control of other risk factors, diabetes mellitus still contributes to a prothrombotic state and residual cardiovascular risk.19 Antiplatelet therapy, including dual antiplatelet therapy, has been established as effective across a wide variety of stable atherosclerotic patients, with some suggestion of heightened benefit in those with diabetes mellitus at baseline.20–29
More recently, a strategy of dual pathway antithrombotic therapy with an antiplatelet and a reduced-dose anticoagulant has been tested and shown to be effective.30–38 The COMPASS trial (Cardiovascular Outcomes for People Using Anticoagulation Strategies) demonstrated that aspirin plus rivaroxaban 2.5 mg twice daily was superior to aspirin plus rivaroxaban placebo for the reduction of ischemic events in 27 395 patients with coronary artery disease or peripheral artery disease. A significant reduction in cardiovascular death was seen with dual pathway inhibition, as well as lower all-cause mortality.
In the present prespecified analysis of COMPASS, we analyzed the results of rivaroxaban plus aspirin versus aspirin alone in the subgroups of patients with or without diabetes mellitus at baseline.
Methods
The data that support the findings of this study may be made available from the corresponding author on reasonable request. The design and results of the overall COMPASS trial have been previously published. In brief, COMPASS was a multicenter, double-blind, randomized, placebo-controlled trial of 27 395 patients with a history of coronary artery disease or peripheral artery disease. Patients were randomized to aspirin plus rivaroxaban placebo, rivaroxaban (5 mg twice daily) plus aspirin placebo, or double antithrombotic therapy with aspirin plus rivaroxaban 2.5 mg twice daily. The primary outcome was cardiovascular death, myocardial infarction (MI), or stroke. Secondary end points included all-cause mortality and major adverse limb events. We also analyzed all major ischemic vascular events (cardiovascular death, MI, stroke, and major adverse limb events, including amputation). The primary safety end point was a modification of the International Society on Thrombosis and Haemostasis criteria for major bleeding. The prespecified net clinical benefit was defined as MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ. The protocol was approved by the relevant health authorities and institutional review boards. Written informed consent was required from all participants.
The trial was stopped early at the recommendation of the independent data and safety monitoring board because of the overwhelming efficacy of the rivaroxaban plus aspirin arm versus aspirin alone. This analysis focuses on the 18 278 patients in those 2 study groups and compares the outcomes in those with and those without diabetes mellitus according to the case history at baseline.
Statistical Analysis
Analyses were conducted according to the intention-to-treat principle. We compared baseline characteristics of patients with and without diabetes mellitus at baseline using Wilcoxon 2-sample tests for continuous variables and Pearson χ2 tests for categorical variables. Survival analyses were based on the time to a first event. Kaplan-Meier risks at 36 months were calculated. We used stratified Cox proportional hazards regression models to estimate hazard ratios (HRs) and corresponding 95% CIs to compare the effects of antithrombotic regimens in patients with and without diabetes mellitus. Significance was tested with the use of stratified log-rank tests. The assumption of the proportional hazards was verified by use of the plots of the log of the negative log of survival function against the log of time. Interaction between the effect of treatment with rivaroxaban/aspirin and diabetes mellitus status was tested in a stratified Cox model fitted to all patients. The Gail-Simon test for qualitative interactions was used to test for interaction of absolute risk reduction, with the null hypothesis that not all of the subgroup reductions favored rivaroxaban plus aspirin. All reported P values are 2 sided. No adjustments were made for multiple subgroup or end-point comparisons; therefore, all results presented herein should be viewed as hypothesis generating. Analyses were performed with SAS software for Linux, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Of the 27 395 randomized patients with stable atherosclerosis in COMPASS, 10 341 had diabetes mellitus at enrollment and 17 054 did not. A total of 18 278 patients were randomized to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11 356 did not have diabetes mellitus. Baseline characteristics of those with and without diabetes mellitus from the entire trial are shown in Table I in the Data Supplement, and those from the rivaroxaban plus aspirin and placebo plus aspirin arms are shown in Table 1. Those with diabetes mellitus were significantly younger and more likely female; it is not surprising that there were several other significant differences between the 2 groups. Table II in the Data Supplement shows the baseline characteristics in the rivaroxaban plus aspirin and rivaroxaban plus placebo arms in those with diabetes mellitus, and Table III in the Data Supplement provides this information for those without diabetes mellitus.
Table 1.
Baseline Characteristics of Patients With and Without Diabetes Mellitus at Baseline Randomized to Rivaroxaban Plus Aspirin or to Placebo Plus Aspirin
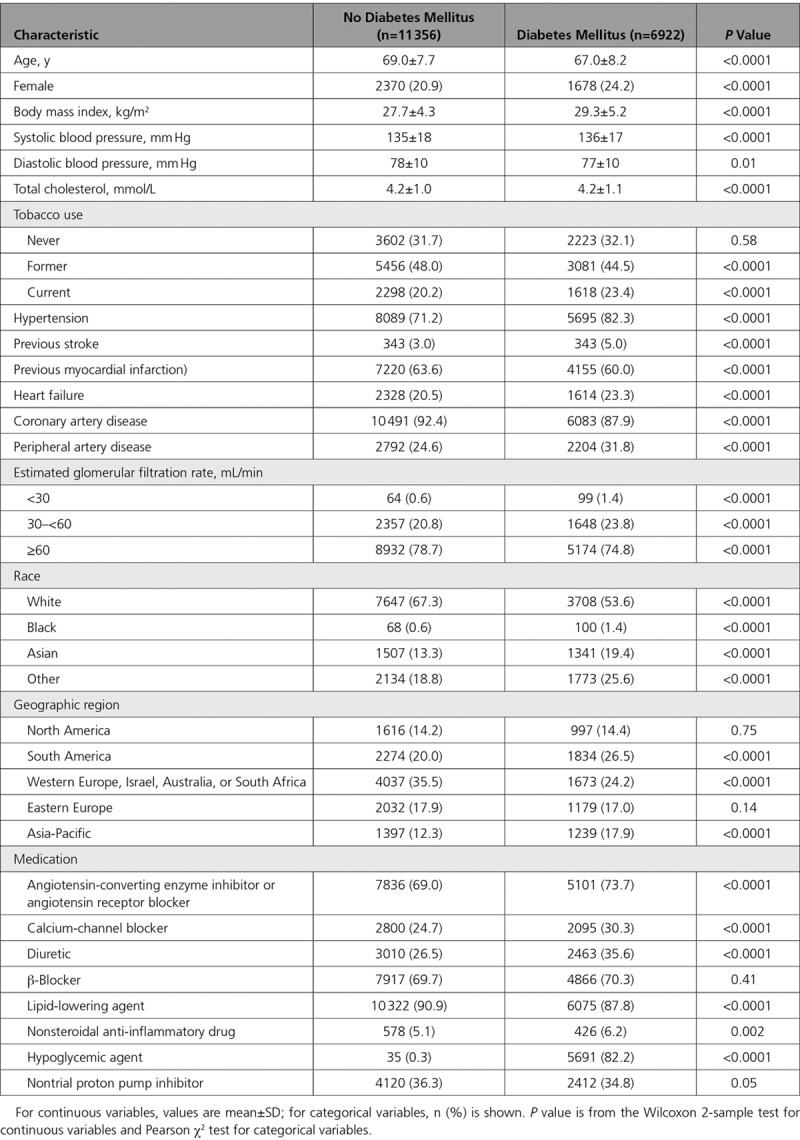
The primary efficacy end point for aspirin plus low-dose rivaroxaban versus aspirin plus rivaroxaban placebo in those with and without diabetes mellitus is shown in Figure 1. Table 2 provides several efficacy and safety comparisons. There was a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin versus aspirin alone in patients with and without diabetes mellitus for the primary efficacy end point and the secondary end points, including mortality (Figure 2). However, because of their higher baseline risk, although the absolute risk reductions appeared larger in patients with versus without diabetes mellitus, both subgroups derived similar benefit (Kaplan-Meier event rates, 2.3% versus 1.4% for the primary end point at 3 years, Gail-Simon qualitative Pinteraction<0.0001; 1.9% versus 0.6% for all-cause mortality, Pinteraction= 0.02); the respective number needed to treat for 3 years was 44 versus 73 and 54 versus 167.
Figure 1.
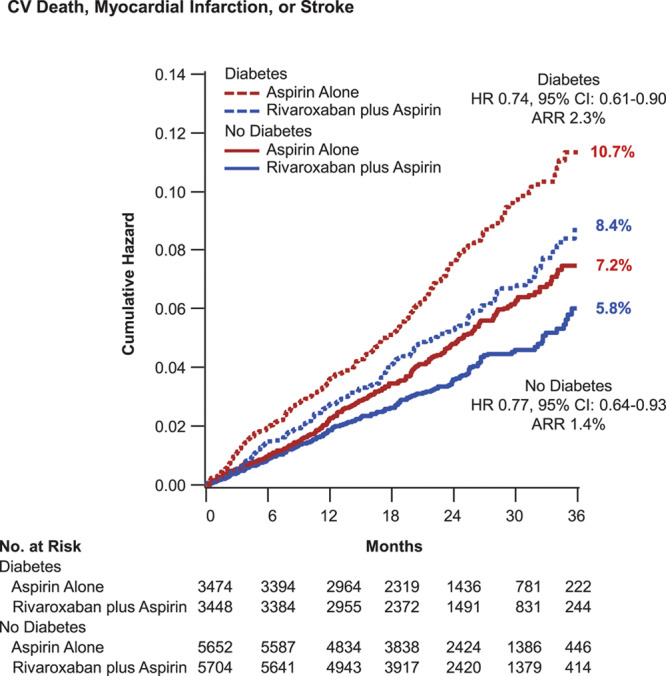
Cardiovascular death, myocardial infarction, or stroke. Kaplan-Meier event curves for patients with and without diabetes mellitus randomized to aspirin plus placebo or aspirin plus low-dose rivaroxaban. The primary end point of cardiovascular death, myocardial infarction, or stroke is shown. Percentages are Kaplan-Meier risks at 3 years. ARR indicates absolute risk reduction; and HR, hazard ratio.
Table 2.
Outcomes in Patients With and Without Diabetes Mellitus for Rivaroxaban Plus Aspirin Versus Placebo Plus Aspirin
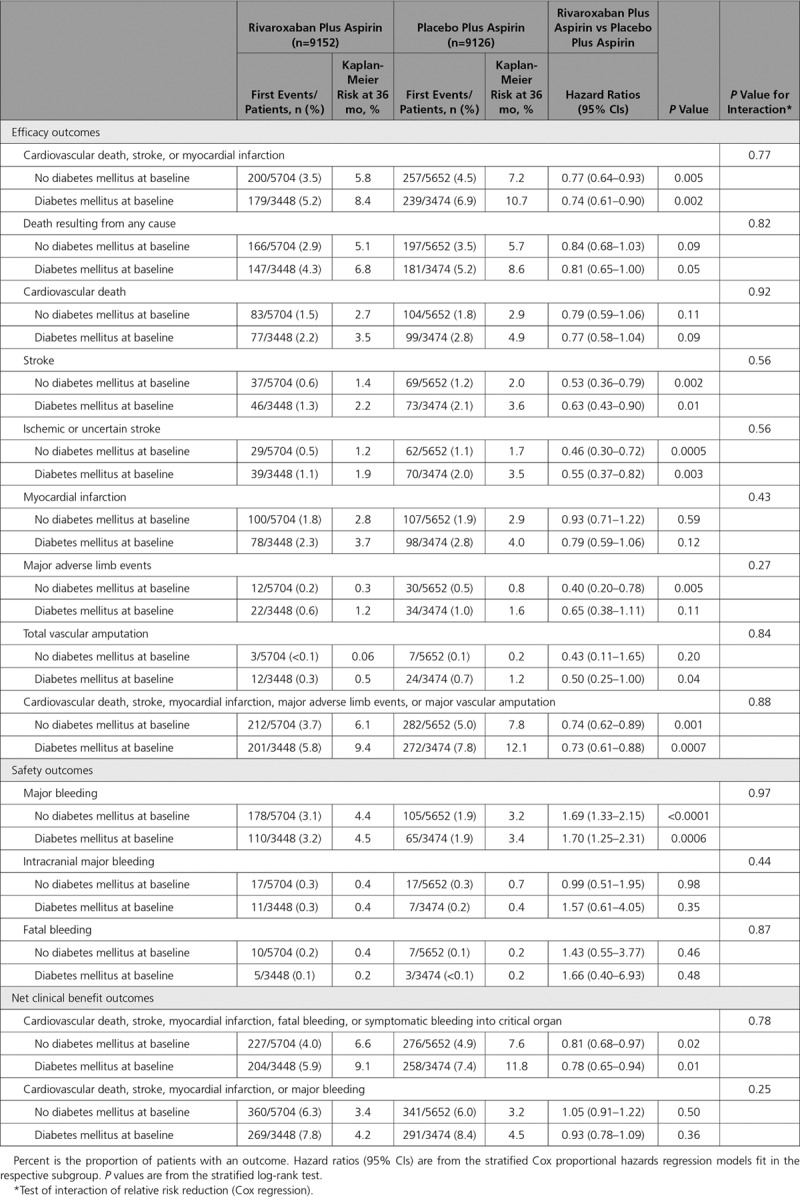
Figure 2.
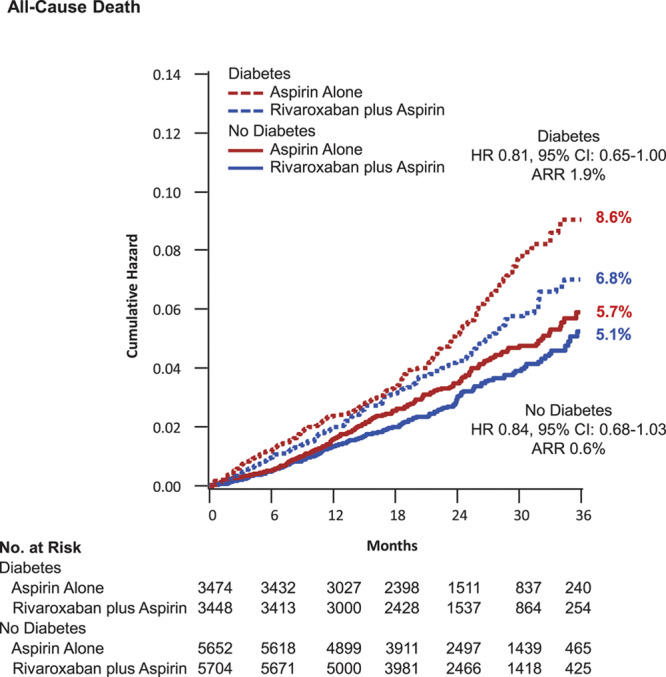
All-cause death. Kaplan-Meier event curves for patients with and without diabetes mellitus randomized to aspirin plus placebo or aspirin plus low-dose rivaroxaban. The secondary end point of all-cause death is shown. Percentages are Kaplan-Meier risks at 3 years. ARR indicates absolute risk reduction; and HR, hazard ratio.
In an evaluation of the totality of ischemic events (cardiovascular death, stroke, MI, major adverse limb events, or major vascular amputation) at 3 years, those without diabetes mellitus at baseline had a significant reduction to 6.1% from 7.8% (HR, 0.74 [95% CI, 0.62–0.89]; P=0.001) with dual pathway antithrombotic therapy; in those with diabetes mellitus, the corresponding rates were 9.4% and 12.1% (HR, 0.73 [95% CI, 0.61–0.88]; P=0.0007; Table 2). Although the HRs were similar, the absolute risk reductions were 1.7% and 2.7%, respectively (Gail-Simon qualitative Pinteraction<0.0001; Figure 3). The respective number needed to treat for 3 years was 60 versus 38.
Figure 3.
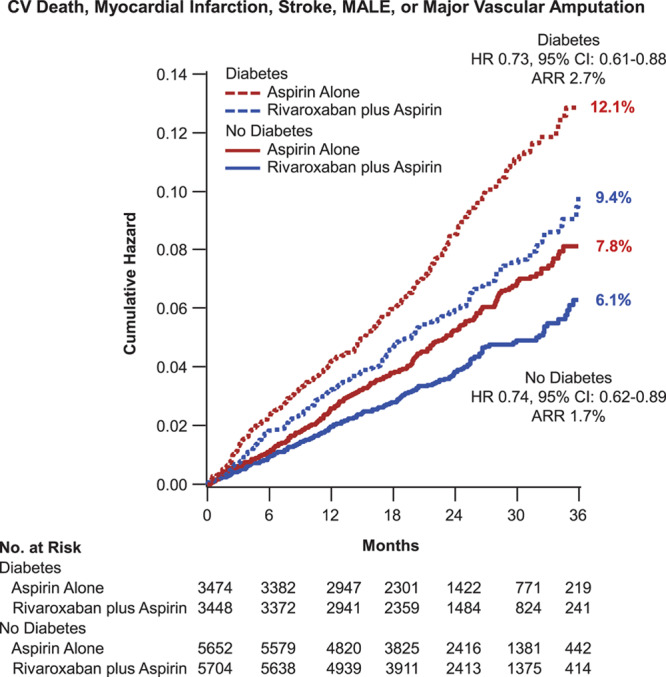
Major vascular events. Kaplan-Meier event curves for patients with and without diabetes mellitus randomized to aspirin plus placebo or aspirin plus low-dose rivaroxaban. The expanded end point of all major vascular events (cardiovascular death, myocardial infarction, stroke, or major adverse limb events [MALEs], including amputation) is shown. Percentages are Kaplan-Meier risks at 3 years. ARR indicates absolute risk reduction; and HR, hazard ratio.
As in the trial overall, there was a significant increase in major bleeding with the dual pathway regimen in the subgroups with and without diabetes mellitus, with a similar degree of risk increase. In those without diabetes mellitus, major bleeding was increased at 3 years to 4.4% from 3.2% (HR 1.69 [95% CI, 1.33–2.15]; P<0.0001). In those with diabetes mellitus, major bleeding was increased at 3 years to 4.5% from 3.4% (HR, 1.69 [95% CI, 1.33–2.15]; P=0.0006, Pinteraction=0.97). There were no significant increases in intracranial or fatal bleeding. The absolute net clinical benefit for dual pathway inhibition with our prespecified definition was numerically greater (2.7% versus 1.0%) in those with versus those without diabetes mellitus, although both subgroups derived similar benefit (Gail-Simon qualitative Pinteraction=0.001; Figure 4). In a nonprespecified post hoc analysis, major bleeding was combined with the primary efficacy end point, and this resulted in no significant difference between treatment arms in either those with or without diabetes mellitus (Table 2). There was no significant interaction with randomization to proton pump inhibitor versus placebo on the increased risk of major bleeding with rivaroxaban in the patients with diabetes mellitus (Table IV in the Data Supplement).
Figure 4.
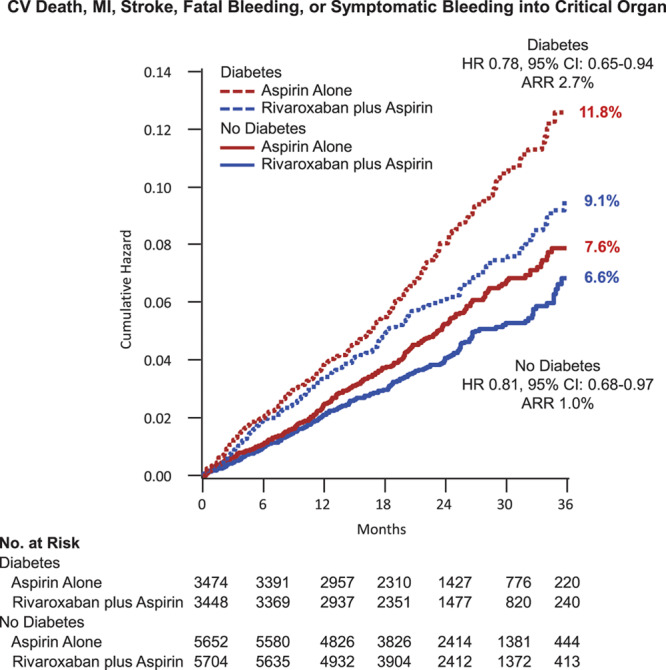
Net clinical benefit. Kaplan-Meier event curves for patients with and without diabetes mellitus randomized to aspirin plus placebo or aspirin plus low-dose rivaroxaban. The net clinical benefit outcome (cardiovascular death, myocardial infarction [MI], stroke, fatal bleeding, or symptomatic bleeding into a critical organ) is shown. Percentages are Kaplan-Meier risks at 3 years. ARR indicates absolute risk reduction; and HR, hazard ratio.
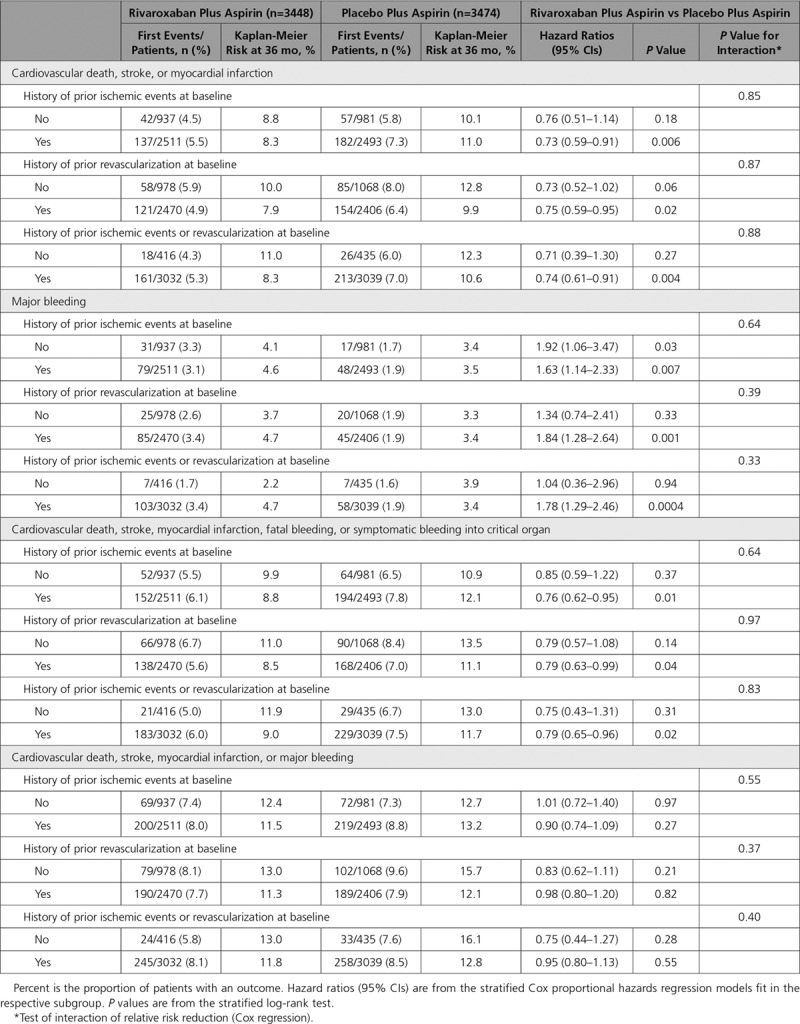
Results were similar in those with diabetes mellitus treated with medications versus those with diabetes mellitus but not receiving diabetes mellitus medications at baseline (Table 3). Consistent results were also seen in the patients with diabetes mellitus with or without a history of ischemic events (MI, unstable angina, stroke, transient ischemic attack) and with or without a history of revascularization (percutaneous coronary intervention, coronary artery bypass grafting, peripheral artery intervention, peripheral artery bypass surgery; Table 4).
Table 3.
Outcomes in Patients With Diabetes Mellitus (Untreated and Treated With Hypoglycemic Agents) and Without Diabetes Mellitus for Rivaroxaban Plus Aspirin Versus Placebo Plus Aspirin
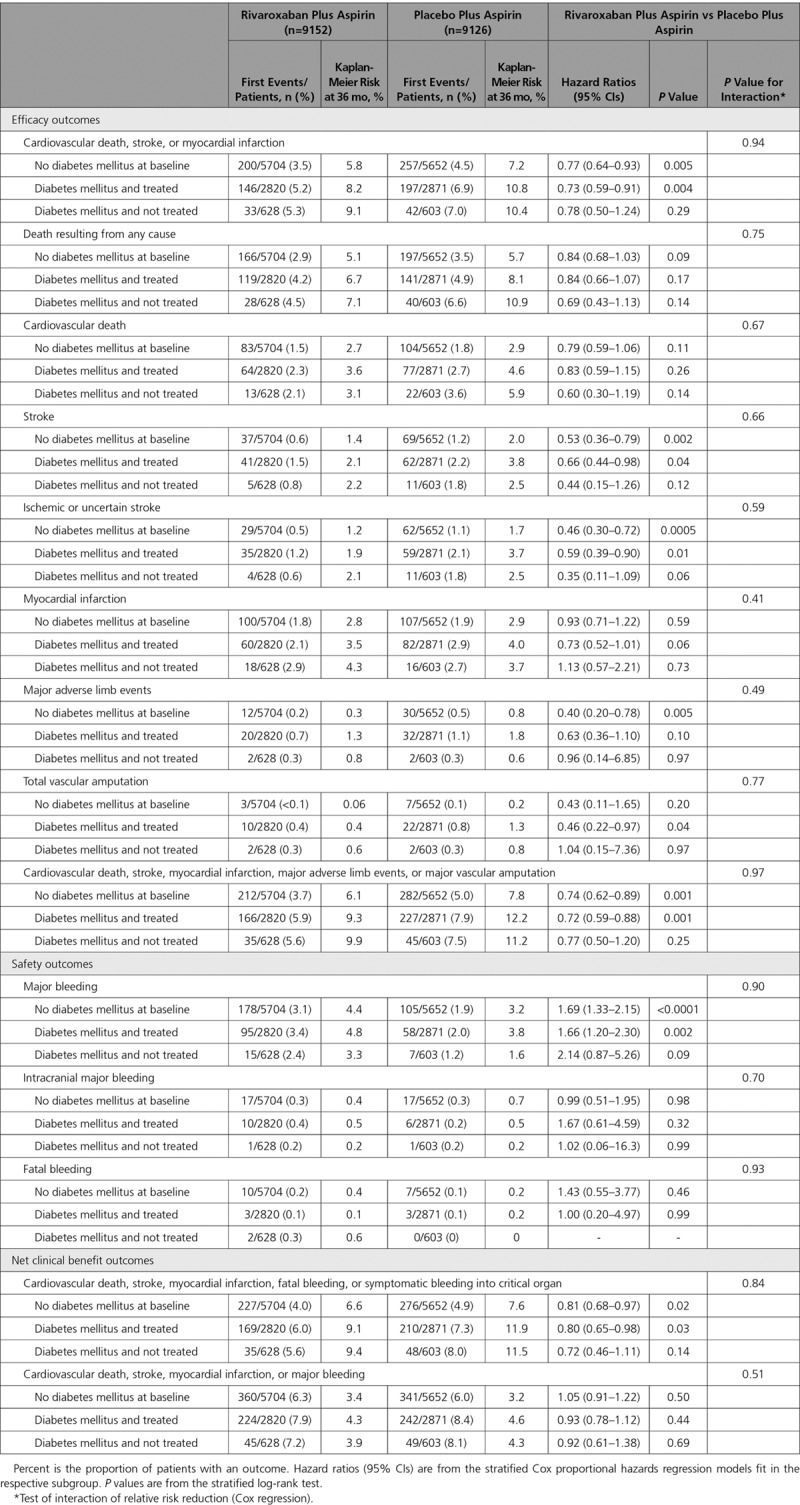
Table 4.
Effect of Antithrombotic Therapies in Subgroups of Patients With Diabetes Mellitus
Discussion
This prespecified analysis of COMPASS shows that patients with stable atherosclerosis with concomitant diabetes mellitus have similar relative but, because of their more dismal prognosis, numerically greater absolute risk reductions in ischemic events than those without diabetes mellitus. This greater absolute efficacy occurs without any incremental increase in major bleeding complications in those with versus those without diabetes mellitus. Thus, the net clinical benefit for irreversible outcomes appears greater in those with versus those without diabetes mellitus. This finding makes the use of dual pathway inhibition with aspirin plus low-dose rivaroxaban particularly attractive in this high-risk population.
Patients with atherosclerosis and diabetes mellitus are a very high-risk group. Despite several advances in different therapeutic areas such as lipid, blood pressure, and glycemic control, patients with diabetes mellitus continue to have high rates of recurrent ischemic events. The population of patients with diabetes mellitus studied in COMPASS represents a very broad representation of secondary prevention, including patients with coronary artery disease, peripheral artery disease, and carotid disease. Patients had prior ischemic events or stable atherosclerosis without such a history. Patients with a history of revascularization and those without prior revascularization were enrolled in COMPASS, and all these subgroups appeared to have a consistent benefit in the overall trial and in the patients with diabetes mellitus. This latter observation does distinguish these results from the multiple trials of dual antiplatelet therapy that also show significant benefit and suggest greater absolute risk reductions in those with diabetes mellitus but that have not demonstrated convincing benefit in as diverse a group of patients with atherosclerosis outside of those with prior ischemic events or prior stenting. It is worth noting, however, that ischemic event rates in patients with diabetes mellitus in COMPASS treated with aspirin plus low-dose rivaroxaban were still higher than the rate in those without diabetes mellitus treated with placebo. Thus, there is further room for residual risk reduction.
In the setting of diabetic primary prevention, aspirin has been found to be superior to placebo, even in the contemporary era, although predictably bleeding was increased.39 However, with careful patient selection, there are patients with diabetes mellitus without evident atherosclerosis who have a favorable net clinical benefit.40–42 Now, in the secondary prevention of patients with diabetes mellitus, it is also clear that intensifying the antithrombotic regimen beyond aspirin alone is warranted in patients who are at an acceptable risk of bleeding. Examination of the prespecified definition of net clinical benefit in COMPASS, consisting of irreversible harms, demonstrated significant benefit for dual pathway inhibition, whereas a post hoc definition of net clinical benefit incorporating all major bleeding did not demonstrate significant benefit. However, although major bleeding is important, it is not appropriate to weight it equivalently to MI, ischemic stroke, amputations, or certainly all-cause mortality.42
Limitations of this analysis include that it is a subgroup not specifically powered for efficacy or safety assessments, although the analysis was prespecified. The early stopping of the trial further limits the power of subgroup analysis, although the independent data and safety monitoring board felt that the trial needed to be stopped as a result of overwhelming efficacy, including a reduction in all-cause mortality that echoed a prior trial with this double antithrombotic regimen.43,44 Nevertheless, sufficient statistical power was present to demonstrate a significant reduction in the primary end point in the overall trial and in those with and without diabetes mellitus, increasing confidence in the subgroup analyses presented herein. Another limitation is that diabetes mellitus was defined only by case history, and duration of diabetes mellitus was not captured in the case report form. Some prior studies of antiplatelet agents have shown a gradient of benefit among those treated with insulin versus oral medications versus diet only; however, insulin treatment was not captured.45,46
Conclusions
Aspirin plus low-dose rivaroxaban reduces major cardiovascular events versus aspirin alone in patients with stable atherosclerosis, regardless of the presence or absence of diabetes mellitus, although the absolute risk reductions are numerically larger in those with diabetes mellitus.
Sources of Funding
The COMPASS study was funded by Bayer AG.
Disclosures
Dr Bhatt discloses the following relationships: Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, PLx Pharma, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), and Population Health Research Institute; honoraria: American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; vice chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (editor in chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (editor in chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), Medtelligence/ReachMD (Continuing Medical Education steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national coleader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (Continuing Medical Education steering committees); other: Clinical Cardiology (deputy editor), NCDR-ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); research funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; royalties: Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site coinvestigator: Biotronik, Boston Scientific, CSI, St Jude Medical (now Abbott), and Svelte; trustee: American College of Cardiology; and unfunded research: FlowCo, Merck, Novo Nordisk, and Takeda. Dr Eikelboom reports consulting fees and/or honoraria from AstraZeneca, Bayer Boehringer-Ingelheim, Bristol-Myer-Squibb, Daiichi-Sankyo, Eli Lilly, Glaxo-Smith-Kline, Pfizer, Janssen, and Sanofi-Aventis and grant support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Glaxo-Smith-Kline, Pfizer, Janssen, and Sanofi-Aventis. Dr Connolly reports lecture fees and consulting fees from Bristol-Myers Squibb, Pfizer, Portola Pharmaceuticals, Boehringer Ingelheim, Servier, Daiichi Sankyo, and Medtronic. Dr Steg discloses the following relationships: research grant from Amarin, Bayer, Sanofi, and Servier, as well as speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer-Ingelheim, Bristol-Myers-Squibb, Idorsia, Novartis, Novo-Nordisk, Pfizer, Regeneron, Sanofi, and Servier. Dr Anand has received speaking and consulting fees from Bayer. Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery and reports receiving research grants and/or speaking honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. He is also president of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. Dr Branch has received consulting fees from Bayer, Janssen, and AstraZeneca and research support from Bayer, Astellas, and the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr Bosch received fees for advisory board work for Bayer. Dr Maggioni reports receiving fees for serving as a study committee member from Novartis, Bayer, Fresenius Medical Care, and Cardiorentis. Dr Szarek reports receiving consulting fees from CiVi and Esperion, consulting fees and fees for serving on a data and safety monitoring board from Resverlogix and Baxter, and fees for serving on a steering committee from Regeneron and Sanofi. Dr Widimský reports honoraria and/or advisory board fees from Bayer, AstraZeneca, Phizer, Servier, Medtronic, and Novartis and fees for the COMPASS trial national coordinator role from Bayer. Dr Avezum discloses the following relationships: Population Health Research Institute (for the COMPASS Operations Committee, Publications Committee, Steering Committee, and Brazil national coleader, funded by Bayer), lecture fees from Bayer and Boheringer-Ingelheim, and research funding from Sanofi Aventis. Dr Diaz has received research grants from Sanofi, Eli Lilly, Amgen, Population Heart Research Institute, Duke Clinical Research Institute, Montreal Health Research Coordinating Center, Lepetit Sa, Dalcor, Cirius Therapeutics, and Heart Initiative and speaker fees from Sanofi, AstraZeneca, Eli Lilly, and Amgen. Dr Lewis reports research funding from Bayer Healthcare, MSD, AstraZeneca, Pfizer, and Kowa Pharmaceuticals, as well as consultant fees and honoraria from MSD and Pfizer. Dr Berkowitz is employed as a clinical research physician by Bayer US, LLC. Dr Fox received grants from Bayer/Janssen and AstraZeneca and consulting and honoraria from Bayer/Janssen, Sanofi/Regeneron, and Verseon. Dr Ryden reports research grants from the Swedish Heart-Lung Foundation, The Familjen Erling-Perssons Foundation, private foundations, Amgen, Bayer AG, Boehringer Ingelheim, MSD, and Novo Nordisk, as well as personal fees (consulting) from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi. Dr Yusuf has received grants and honoraria from Bayer, BI, Astra, BMS, and Cadila. The other authors report no conflicts.
Supplementary Material
Appendix
Steering Committee: S. Yusuf, K.A.A. Fox, S. Connolly, J.W. Eikelboom, J. Bosch, V. Aboyans, M. Alings, S. Anand, A. Avezum, D.L. Bhatt, K. Branch, P. Commerford, N. Cook-Bruns, G. Dagenais, A. Dans, R. Diaz, G. Ertl, C. Felix, T. Guzik, R. Hart, M. Hori, A. Kakkar, K. Keltai, M. Keltai, J. Kim, A. Lamy, F. Lanas, B. Lewis, Y. Liang, L. Liu, E. Lonn, P. Lopez-Jaramillo, A. Maggioni, K. Metsarinne, P. Moayyedi, M. O’Donnell, A. Parkhomenko, L. Piegas, N. Pogosova, J. Probstfield, L. Ryden, M. Sharma, P.G. Steg, S. Stoerk, A. Tonkin, C. Torp-Pedersen, J. Varigos, P. Verhamme, D. Vinereanu, P. Widimsky, K. Yusoff, and J. Zhu.
Footnotes
A complete list of COMPASS Steering Committee and Investigators is provided in the Appendix.
Sources of Funding, see page 1852
Guest Editor for this article was Gregory Lip, MD.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.046448.
References
- 1.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Röther J, et al. REACH Registry Investigators. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, et al. REACH Registry Investigators. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D’Agostino R, Liau CS, et al. REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. doi: 10.1001/jama.2010.1322. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 4.Cavender MA, Steg PG, Smith SC, Jr, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL REACH Registry Investigators. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Circulation. 2015;132:923–931. doi: 10.1161/CIRCULATIONAHA.114.014796. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 5.Alberts MJ, Bhatt DL, Mas JL, Ohman EM, Hirsch AT, Röther J, Salette G, Goto S, Smith SC, Jr, Liau CS, et al. REduction of Atherothrombosis for Continued Health Registry Investigators. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur Heart J. 2009;30:2318–2326. doi: 10.1093/eurheartj/ehp355. doi: 10.1093/eurheartj/ehp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez JA, Scirica BM, Bonaca MP, Steg PG, Mosenzon O, Hirshberg B, Im K, Raz I, Braunwald E, Bhatt DL. Prevalence and outcomes of polyvascular (coronary, peripheral, or cerebrovascular) disease in patients with diabetes mellitus (from the SAVOR-TIMI 53 trial). Am J Cardiol. 2019;123:145–152. doi: 10.1016/j.amjcard.2018.09.014. doi: 10.1016/j.amjcard.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 8.Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni-Berthold I, Lewis BS, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950. doi: 10.1016/S2213-8587(17)30313-3. doi: 10.1016/S2213-8587(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 9.Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, Budaj AJ, Diaz R, Goodman SG, Hanotin C, et al. ODYSSEY OUTCOMES Committees and Investigators. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:618–628. doi: 10.1016/S2213-8587(19)30158-5. doi: 10.1016/S2213-8587(19)30158-5. [DOI] [PubMed] [Google Scholar]
- 10.Verma S, Bhatt DL. More CREDENCE for SGLT2 Inhibition. Circulation. 2019;140:1448–1450. doi: 10.1161/CIRCULATIONAHA.119.041181. doi: 10.1161/CIRCULATIONAHA.119.041181. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Poulter NR, Bhatt DL, Bain SC, Buse JB, Leiter LA, Nauck MA, Pratley RE, Zinman B, Ørsted DD, et al. Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes mellitus with or without history of myocardial infarction or stroke. Circulation. 2018;138:2884–2894. doi: 10.1161/CIRCULATIONAHA.118.034516. doi: 10.1161/CIRCULATIONAHA.118.034516. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Mazer CD, Bhatt DL. The perils of polyvascular disease in type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6:914–916. doi: 10.1016/S2213-8587(18)30311-5. doi: 10.1016/S2213-8587(18)30311-5. [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Bhatt DL, Bain SC, Buse JB, Mann JFE, Marso SP, Nauck MA, Poulter NR, Pratley RE, Zinman B, et al. LEADER Publication Committee on behalf of the LEADER Trial Investigators. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137:2179–2183. doi: 10.1161/CIRCULATIONAHA.118.033898. doi: 10.1161/CIRCULATIONAHA.118.033898. [DOI] [PubMed] [Google Scholar]
- 14.Connelly KA, Bhatt DL, Verma S. Can we DECLARE a victory against cardio-renal disease in diabetes? Cell Metab. 2018;28:813–815. doi: 10.1016/j.cmet.2018.11.010. doi: 10.1016/j.cmet.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Verma S, Leiter LA, Latter DA, Bhatt DL. A LEADER in the management of type 2 diabetes and cardiorenal disease. J Thorac Cardiovasc Surg. 2020;159:978–984. doi: 10.1016/j.jtcvs.2019.03.134. doi: 10.1016/j.jtcvs.2019.03.134. [DOI] [PubMed] [Google Scholar]
- 16.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 17.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 18.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 19.Bhatt DL. Antiplatelet therapy following myocardial infarction in patients with diabetes. JAMA. 2012;308:921–922. doi: 10.1001/2012.jama.11467. doi: 10.1001/2012.jama.11467. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, et al. CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, et al. CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988. doi: 10.1016/j.jacc.2007.03.025. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF, Im K, Murphy SA, Held P, Braunwald E, et al. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol. 2016;67:2732–2740. doi: 10.1016/j.jacc.2016.03.529. doi: 10.1016/j.jacc.2016.03.529. [DOI] [PubMed] [Google Scholar]
- 24.Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, Goodrich E, Nicolau JC, Parkhomenko A, López-Sendón J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67:2719–2728. doi: 10.1016/j.jacc.2016.03.524. doi: 10.1016/j.jacc.2016.03.524. [DOI] [PubMed] [Google Scholar]
- 25.Dalby AJ, Gottlieb S, Cyr DD, Magnus Ohman E, McGuire DK, Ruzyllo W, Bhatt DL, Wiviott SD, Winters KJ, Fox KAA, et al. TRILOGY ACS Investigators. Dual antiplatelet therapy in patients with diabetes and acute coronary syndromes managed without revascularization. Am Heart J. 2017;188:156–166. doi: 10.1016/j.ahj.2017.03.015. doi: 10.1016/j.ahj.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park SJ, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 2016;37:390–399. doi: 10.1093/eurheartj/ehv443. doi: 10.1093/eurheartj/ehv443. [DOI] [PubMed] [Google Scholar]
- 27.Bhatt DL, Fox K, Harrington RA, Leiter LA, Mehta SR, Simon T, Andersson M, Himmelmann A, Ridderstråle W, Held C, et al. THEMIS Steering Committee. Rationale, design and baseline characteristics of the effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients intervention study. Clin Cardiol. 2019;42:498–505. doi: 10.1002/clc.23164. doi: 10.1002/clc.23164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, Held C, Andersson M, Himmelmann A, Ridderstråle W, et al. THEMIS Steering Committee and Investigators. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381:1309–1320. doi: 10.1056/NEJMoa1908077. doi: 10.1056/NEJMoa1908077. [DOI] [PubMed] [Google Scholar]
- 29.Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, Held C, Andersson M, Himmelmann A, Ridderstråle W, et al. THEMIS Steering Committee and Investigators. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394:1169–1180. doi: 10.1016/S0140-6736(19)31887-2. doi: 10.1016/S0140-6736(19)31887-2. [DOI] [PubMed] [Google Scholar]
- 30.Bosch J, Eikelboom JW, Connolly SJ, Bruns NC, Lanius V, Yuan F, Misselwitz F, Chen E, Diaz R, Alings M, et al. Rationale, design and baseline characteristics of participants in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) Trial. Can J Cardiol. 2017;33:1027–1035. doi: 10.1016/j.cjca.2017.06.001. doi: 10.1016/j.cjca.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. COMPASS Investigators. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 32.Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, Metsarinne K, O’Donnell M, Dans AL, Ha JW, et al. COMPASS investigators. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:205–218. doi: 10.1016/S0140-6736(17)32458-3. doi: 10.1016/S0140-6736(17)32458-3. [DOI] [PubMed] [Google Scholar]
- 33.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, Aboyans V, Alings M, Kakkar AK, Keltai K, et al. COMPASS Investigators. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391:219–229. doi: 10.1016/S0140-6736(17)32409-1. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 34.Anand SS, Eikelboom JW, Dyal L, Bosch J, Neumann C, Widimsky P, Avezum AA, Probstfield J, Cook Bruns N, Fox KAA, et al. COMPASS Trial Investigators. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol. 2019;73:3271–3280. doi: 10.1016/j.jacc.2019.02.079. doi: 10.1016/j.jacc.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 35.Darmon A, Bhatt DL, Elbez Y, Aboyans V, Anand S, Bosch J, Branch KR, Connolly SJ, Dyal L, Eikelboom JW, et al. External applicability of the COMPASS trial: an analysis of the Reduction of Atherothrombosis for Continued Health (REACH) Registry. Eur Heart J. 2018;39:750–757a. doi: 10.1093/eurheartj/ehx658. doi: 10.1093/eurheartj/ehx658. [DOI] [PubMed] [Google Scholar]
- 36.Darmon A, Sorbets E, Ducrocq G, Elbez Y, Abtan J, Popovic B, Ohman EM, Röther J, Wilson PF, Montalescot G, et al. REACH Registry Investigators. Association of multiple enrichment criteria with ischemic and bleeding risks among COMPASS-eligible patients. J Am Coll Cardiol. 2019;73:3281–3291. doi: 10.1016/j.jacc.2019.04.046. doi: 10.1016/j.jacc.2019.04.046. [DOI] [PubMed] [Google Scholar]
- 37.Fox KAA, Eikelboom JW, Anand SS, Bhatt DL, Bosch J, Connolly SJ, Harrington RA, Steg PG, Yusuf S. Anti-thrombotic options for secondary prevention in patients with chronic atherosclerotic vascular disease: what does COMPASS add? Eur Heart J. 2019;40:1466–1471. doi: 10.1093/eurheartj/ehy347. doi: 10.1093/eurheartj/ehy347. [DOI] [PubMed] [Google Scholar]
- 38.Boden WE, Bhatt DL. Will COMPASS point to a new direction in thrombotic risk reduction in patients with stable cardiovascular disease? Circulation. 2018;138:858–860. doi: 10.1161/CIRCULATIONAHA.118.035405. doi: 10.1161/CIRCULATIONAHA.118.035405. [DOI] [PubMed] [Google Scholar]
- 39.Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. doi: 10.1056/NEJMoa1804988. [DOI] [PubMed] [Google Scholar]
- 40.Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, Blumenthal RS, McEvoy JW. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393:2155–2167. doi: 10.1016/S0140-6736(19)30541-0. doi: 10.1016/S0140-6736(19)30541-0. [DOI] [PubMed] [Google Scholar]
- 41.Abdelaziz HK, Saad M, Pothineni NVK, Megaly M, Potluri R, Saleh M, Kon DLC, Roberts DH, Bhatt DL, Aronow HD, et al. Aspirin for primary prevention of cardiovascular events. J Am Coll Cardiol. 2019;73:2915–2929. doi: 10.1016/j.jacc.2019.03.501. doi: 10.1016/j.jacc.2019.03.501. [DOI] [PubMed] [Google Scholar]
- 42.Steg PG, Bhatt DL. Is there really a benefit to net clinical benefit in testing antithrombotics? Circulation. 2018;137:1429–1431. doi: 10.1161/CIRCULATIONAHA.117.033442. doi: 10.1161/CIRCULATIONAHA.117.033442. [DOI] [PubMed] [Google Scholar]
- 43.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, et al. ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 44.Gibson CM, Chakrabarti AK, Mega J, Bode C, Bassand JP, Verheugt FW, Bhatt DL, Goto S, Cohen M, Mohanavelu S, et al. ATLAS-ACS 2 TIMI 51 Investigators. Reduction of stent thrombosis in patients with acute coronary syndromes treated with rivaroxaban in ATLAS-ACS 2 TIMI 51. J Am Coll Cardiol. 2013;62:286–290. doi: 10.1016/j.jacc.2013.03.041. doi: 10.1016/j.jacc.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90:625–628. doi: 10.1016/s0002-9149(02)02567-5. doi: 10.1016/s0002-9149(02)02567-5. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt DL, Marso SP, Lincoff AM, Wolski KE, Ellis SG, Topol EJ. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol. 2000;35:922–928. doi: 10.1016/s0735-1097(99)00650-6. doi: 10.1016/s0735-1097(99)00650-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


