Hunters exhibit high skill across the life span, individuals vary substantially in skill, and age-related peaks vary across sites.
Abstract
Human adaptation depends on the integration of slow life history, complex production skills, and extensive sociality. Refining and testing models of the evolution of human life history and cultural learning benefit from increasingly accurate measurement of knowledge, skills, and rates of production with age. We pursue this goal by inferring hunters’ increases and declines of skill from approximately 23,000 hunting records generated by more than 1800 individuals at 40 locations. The data reveal an average age of peak productivity between 30 and 35 years of age, although high skill is maintained throughout much of adulthood. In addition, there is substantial variation both among individuals and sites. Within study sites, variation among individuals depends more on heterogeneity in rates of decline than in rates of increase. This analysis sharpens questions about the coevolution of human life history and cultural adaptation.
INTRODUCTION
Among hominoids, humans are distinguished by a suite of life history traits that includes a prolonged juvenile and adolescent period, short interbirth intervals, and an extended postreproductive life span (1). Multiple conceptual models have been advanced to explain the evolution of these traits, focusing on distinctive human behaviors such as pair bonding and alloparental care from grandparents and others (2–4). Any satisfactory model of human life history evolution must simultaneously account for the large brains that characterize our species. Foraging complexity and competitive social challenges have been alternately championed as the evolutionary prime mover of encephalization, while others combine perspectives by citing the advantages of flexible cultural learning among juveniles as a solution to social and ecological challenges (5).
Progress is made in these debates via models that integrate growth, reproduction, cognitive development, skill development, sociality, and cultural evolution. The most advanced attempt that we know is the optimal control model of González-Forero and Gardner (6). Drawing on observed rates of brain and somatic growth, their model estimates the relative importance of different ecological and social challenges to the evolution of intelligence. In comparison to alternatives, the ecological challenge of acquiring food emerges as the strongest predictor of the observed pattern of human growth. In this model, brains develop first followed by the body because this sequence allows a longer period of learning and ultimately higher adult productivity. This finding complements recent comparative work on the prominence of foraging complexity as a predictor of primate brain sizes (7). These findings and predictions direct our attention to age-related variation in foraging skill in human societies. To the extent that foraging complexity underlies the evolution of human life history traits, we anticipate protracted mastery of foraging tasks across the life span.
Here, we focus on age as a predictor of harvests in the largest yet assembled database of hunting returns (Fig. 1). We focus on subsistence hunting for multiple reasons. First, because hunting returns are evident at the conclusion of an excursion; the modeling of hunting inputs and outputs generally requires fewer assumptions than the analogous analysis of agricultural or pastoralist returns. Second, increased hunting is often cited as an adaptive shift that coincides with other distinguishing features of the genus Homo (8). However, we note that none of the societies in our sample rely exclusively on hunting (or foraging more generally). Contemporary hunting is not a primitive economy, but rather a recurring one that integrates with other means of production. The data should be evaluated accordingly.
Fig. 1. Distribution of study sites.
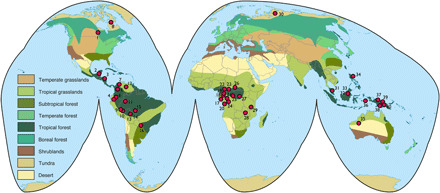
For the key, see Table 1.
Table 1. Study sites and their numerical and text codes.
See the help file of the cchunts package for related citations.
| Number | Code | Country | Group |
Dataset in cchunts package |
| 1 | CRE | Canada | Cree | Winterhalder |
| 2 | MYA | Belize | Maya | Pacheco |
| 3 | MYN | Nicaragua | Mayangna | Koster |
| 4 | QUI | Ecuador | Quichua | Siren |
| 5 | ECH | Colombia | Embera Chami | Ross |
| 6 | WAO | Ecuador | Waorani | Franzen |
| 7 | BAR | Venezuela | Bari | Beckerman |
| 8 | INU | Canada | Inuit | Ready |
| 9 | MTS | Peru | Matsigenka | Yu_et_al |
| 10 | PIR | Peru | Piro | Alvard |
| 11 | CLB | Colombia | Van_Vliet_et_al_ South_America_sites |
|
| 12 | PME | Venezuela | Pume | Kramer_Greaves |
| 13 | TS1 | Bolivia | Tsimane | Fernandez_ Llamazares |
| 14 | TS2 | Bolivia | Tsimane | Reyes-Garcia |
| 15 | TS3 | Bolivia | Tsimane | Trumble_Gurven |
| 16 | ACH | Paraguay | Aché | Hill_Kintigh |
| 17 | GB1 | Gabon | Coad | |
| 18 | GB2 | Gabon | Van_Vliet_et_al_Gabon | |
| 19 | GB3 | Gabon | Van_Vliet_et_al_Ovan | |
| 20 | CN1 | DR Congo | Van_Vliet_et_al_ Phalanga |
|
| 21 | GB4 | Gabon | Van_Vliet_et_al_ Djoutou |
|
| 22 | BK1 | Cameroon | Baka | Gallois |
| 23 | BK2 | Cameroon | Baka | Duda |
| 24 | CN2 | Congo | Van_Vliet_et_al_Ingolo | |
| 25 | CN3 | Congo | Van_Vliet_et_al_ Ngombe |
|
| 26 | BFA | Central African Republic |
Bofi and Aka | Lupo_Schmitt |
| 27 | CN4 | DR Congo | Van_Vliet_et_al_Baego | |
| 28 | BIS | Zambia | Valley Bisa | Marks |
| 29 | HEH | Tanzania | Nielsen | |
| 30 | DLG | Russia | Dolgan | Ziker |
| 31 | BTK | Malaysia | Batek | Venkataraman_et_al |
| 32 | PN1 | Indonesia | Punan | Gueze |
| 33 | PN2 | Indonesia | Punan | Napitupulu |
| 34 | AGT | Philippines | Agta | Headland |
| 35 | MRT | Australia | Martu | Bird_Bird_Codding |
| 36 | NUA | Indonesia | Nuaulu | Ellen |
| 37 | NIM | Indonesia | Nimboran | Pangau_Adam |
| 38 | NEN | Papua New Guinea |
Nen | Healey_Nen_PNG |
| 39 | MAR | Papua New Guinea |
Maring | Healey |
| 40 | WOL | Papua New Guinea |
Wola | Sillitoe |
RESULTS
There are many ways to summarize the model inferences. We focus on three foundational issues that motivated the analysis.
1) What is the overall pattern of skill development?
2) How variable is this pattern within and between societies?
3) Which components of the model—increases early in life or declines later in life—describe variation?
At the highest level of pooling, the model provides a statistical answer to the question, “What is a typical human life history of foraging skill?” This is very much an abstraction, one that attempts to factor away all the variation in production functions and associated elasticities to reveal an underlying, dimensionless life history. It cannot say much about absolute levels of production, either within or between societies. However, it can inform comparisons of relative skill at different stages of life.
The statistically average hunter in this sample peaks at 33 years of age (top left plot, Fig. 2). However, this peak is not sharp. At age 18, this fictional average hunter has 89% of maximum skill. In addition, skill declines slowly, such that skill falls below 89% of maximum only after age 56. The blue shading around the posterior mean in this plot shows the entire posterior distribution, fading out to transparent as probability declines. There is correspondingly a lot of information in this sample about the global mean.
Fig. 2. Skill functions.
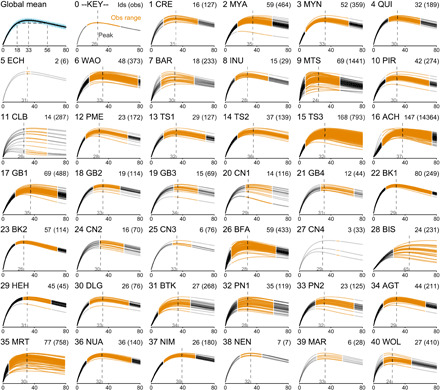
The figure depicts the global average of skill (top left plot) and skill at the respective study sites. Within study sites, each curve is the posterior mean skill for an individual hunter, standardized to the maximum within each site. In the header of each plot, the site number and three-letter code are shown along with the number of individual hunters in each sample, followed by the number of observed harvests in parentheses. The orange span of ages corresponds to ages observed within each site, while the gray ranges were unobserved and are instead implied by the underlying model. The vertical dashed lines show the average ages at peak within sites.
While the overall pattern is clear, not every site nor individual forager exhibits the same pattern. The site-level plots in Fig. 2 illustrate this variation. Each site displays the mean skill function for each hunter in the sample. While there is substantial uncertainty about individual skill curves, there is good evidence of individual-level variation at some sites, such as the Matsigenka (9 MTS), the Colombian site (11 CLB), the Aché (16 ACH), and the Martu (35 MRT). Differences among individuals can be quite large. Some individuals have half the adult skill of others in the same community.
For each site, the figure also shows the age of peak skill for a statistically average hunter at that site, as indicated by the vertical dashed lines. While these peak ages cluster around 30 years of age, there is noteworthy variation. On the low end, the Matsigenka (9 MTS) and Wola (40 WOL) peak early, near 24 years of age. Note that the best hunters at these sites tend to peak even earlier, a trend that is also evident among the Barí (7 BAR). On the higher end, the Aché (16 ACH) and Valley Bisa (28 BIS) peak at 37 and 45, respectively, but with relatively slow declines.
These skill functions are inputs into site-specific production functions that also include labor and technology inputs that vary in importance across sites. This means that the relationship between production and age can be different from the relationship between skill and age. In the Supplementary Materials, we therefore present alternative versions of the plot that incorporate the other components of production (figs. S5 to S7). One feature of the production components is that variation can arise from different sources, which, in turn, have different implications for age-related variation in harvests at the individual level. Furthermore, at some sites (e.g., the Dolgan, site 30), the skill functions for individual hunters cluster around a central mean. However, this does not necessarily support inferences that hunters at these sites have equal skill because there may be insufficient evidence to distinguish them. For an alternative perspective on the anticipated variation among hunters within sites, we simulate variation from the posterior samples of the model (fig. S8).
Last, skill functions vary both within sites and between sites. Which components of skill contribute to this variation? To address this question, we examine the model parameters that measure variation in the components k (rate of increase) and m (rate of decline) of the skill function. Since this is a nonlinear model, we cannot exactly partition total variance. The impact of variation in a component of skill depends on the values of all the other components. We can, however, consider relative sizes of components of variation on the latent scale.
First, we find moderately greater variation in m than k within sites (note the cyan curve in the top left plot of Fig. 3). By contrast, between-site heterogeneity in age-related skill is divided roughly evenly between variation in m and k (see the orange curve in the top left plot in Fig. 3). Some caution is necessary in these comparisons because the relationship between m and k is not additive. However, the implication is that among individuals within a given ecology, skill varies more later in life than earlier in life.
Fig. 3. Variation in components of skill.
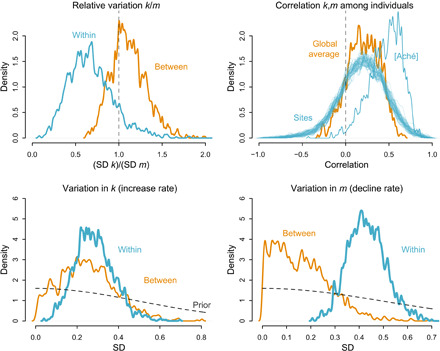
(Top left) Relative variation in k and m. The horizontal axis is the ratio of the SD of k to the SD of m. The vertical dashed line at 1 indicates equality of variances. The orange density is between-site variation. The cyan density is within-site variation. There is more variation in declines (m) than increases (k) in skill within sites, whereas the ratio is roughly equivalent across sites. (Top right) Correlation between k and m among individuals within sites. The orange density is the global average. Each cyan density represents a single site. The Aché stand out and are labeled separately. (Bottom left and bottom right) Variation in k (left) and m (right) comparing variation within and between sites.
We also find a modest positive correlation between k and m (top right plot), suggesting that hunters who develop skill relatively quickly also show advanced skill later in life. Each cyan density in the top right plot is the posterior correlation between hunters’ k and m parameters at a given site. This correlation is particularly pronounced for the Aché and modest otherwise. This may reflect the lack of longitudinal data on individual hunters at most study sites, limiting what can be learned about this correlation. In contrast, the Aché site contains enough longitudinal data on individuals to make stronger inferences about the correlation.
Last, relative variation in m and k can be decomposed within and between sites. We show the posterior distributions of the SDs in both k (bottom left) and m (bottom right) in Fig. 3. The cyan densities are the SDs within sites. This corresponds to the plausible values for variation among individuals. The orange densities are the SDs among sites, corresponding to the plausible values for variation in site-level averages. The dashed curves in both plots show the prior distributions, which were the same for both within and between components. For both k and m, there is relatively less information about variation among sites. As a result, the orange curves remain flatter than the cyan curves. There is substantially more information about variation within sites, and so the cyan curves are rather peaked in both cases. While there is a hint that variation between sites contributes more to variation in k, while variation within sites contributes more to variation in m, strong inferences cannot be drawn until more information is available for inferring the between-site variance.
DISCUSSION
Overall, these results provide an empirical counterpoint to computational models of life history evolution [e.g., (6, 9)]. On the one hand, there is agreement among models about the central tendencies for the ontogeny of skill, which accelerates most rapidly during childhood and adolescence before reaching a plateau during adulthood. In all study sites, skill peaks after physical and reproductive maturity. This result is largely consistent with predictions of embodied capital theory (4), although, on average, the empirical data do not reveal the distinct peaks in skill during middle age that are evident in previous studies of the Aché (10, 11). Instead, the empirical model suggests that skill starts to plateau by early adulthood and that only moderate increases are evident subsequently. Declines are typically slow, such that an 80-year-old may retain two-thirds of maximum skill.
Another noteworthy result is the extent of variability in skill, both among and within sites. Cross-cultural variation is evident in the rate at which hunters develop peak skill. Within sites, the rate at which hunters develop skill is relatively homogeneous compared to the variation that distinguishes young hunters in different study sites. To explain cross-cultural variation in the development of foraging proficiency, it is common and reasonable for anthropologists to emphasize ecological predictors, such as extrinsic mortality risks [e.g., (12)]. However, varying rates of skill development may stem as well from mediating social factors that relate only indirectly to ecological differences. Additional theorizing is needed to generate hypotheses about the cross-cultural ontogeny of foraging skill in response to variables such as experience, motivation, opportunities for social learning, and the physical and cognitive demands of hunting in different socioecological environments. As opposed to a canalized human life history strategy, this study suggests potential developmental plasticity in traits associated with hunting skill, which manifest not only in contemporary settings but also potentially in ancestral settings. These results further imply that singular study sites can rarely be viewed as straightforward analogs for evolutionarily relevant environments (13).
Within the respective study sites, the model brings new attention to the variation in skill among individual hunters. What explains this variation? Data and theory suggest that physical strength and stamina, accumulated knowledge, and motivation all plausibly contribute to age-related variation (14, 15). In most empirical datasets, however, only data on the hunters’ ages are available as predictors, not other attributes of the individuals. The peak in the average skill function at approximately 33 years old is also near the age when physical strength and ecological knowledge plateau (15–18). From a theoretical perspective, an optimal life history should develop these components together, with the important caveat that brain growth may need to precede body growth, to enable learning (9). To distinguish the relative importance of phenotypic traits to variation in hunting skill across the life span, data are needed that link measurements of these attributes to individual-level hunting returns, ideally longitudinally. In the interim, the currently available data suggest that individual hunters develop physical and cognitive abilities in concert, resulting in high hunting success by their late 20s and early 30s.
Estimates from empirical studies provide inputs to the parameterization of computational life history models. Our analysis of hunting by age provides refined estimates of the average skill function, a target of inference for recent theoretical work (6, 9). However, the added clarity about average skill is belied by the substantial heterogeneity that is evident among individual hunters. For future theoretical developments about the unique human life history pattern, this variation in skill merits careful attention. Prevailing hypotheses about the adaptive shift to hunting by human ancestors assert that reciprocal food sharing in small bands was necessary to smooth variance in consumption, given that daily harvests by hunters are unpredictable (19–21). In this literature, variability in the skill of individual hunters has received relatively little consideration (22). Our analysis suggests that this variability typifies communities of human foragers while concomitantly altering the effectiveness of food sharing for buffering risk. That is, when hunters vary substantially in their skill and productivity, there are asymmetric benefits to participation in risk-pooling distribution systems. To the extent that prosociality and other traits in the human lineage stem from the cooperative challenges posed by this asymmetry, the high variation in hunting skill across the life span merits further attention.
MATERIALS AND METHODS
The total sample contains 23,747 observations of 1821 individual foragers across 40 study sites (Fig. 1). There is substantial imbalance in sample size across units. One site contributes only six trips from two individuals. Another contributes more than 14,000 trips from 147 individuals. Some individuals contribute only a single outcome, while others contribute dozens. The majority of the sample comprises male hunters, with too little data on female hunters to infer generalizable sex differences. Most sites contribute primarily cross-sectional data, while a few others exhibit impressive time series.
Because skill cannot be directly observed, what is required is a model with latent age-varying skill, which informs a production function for observable foraging returns. The model is described in detail in the Supplementary Materials. Building on earlier research (10), our modeling framework was developed in a grant proposal and reviewed before seeing the assembled sample. Using a Cobb-Douglas production function common to economic research, we model hunting returns (harvest) on excursions as a standard log-linear function of skill, labor inputs, and auxiliary inputs from technology and cooperation
| (1) |
where S represents the hunter’s skill with its elasticity, η, the labor input and its elasticity are represented by Lβ, and α is a linear model for covariates such as group size, the number of assistants, the use of dogs, and the use of firearms. These latter variables have been shown to influence hunting returns (23–26). Note that production requires both skill and labor; if either is zero, then there is no harvest. The respective elasticities reflect the proportional effects that skill and labor have on harvests. That is, increasing either skill or labor results in increased harvests, but the scale of the increase is reflected in the elasticities. Our parameterization of the function does not impose constant returns to scale [cf. (27)].
Data on hunting returns pose the particular challenge of including many zeroes (for hunting trips in which nothing was acquired), and the harvests on successful hunting trips exhibit positive skew. We therefore adapt the Cobb-Douglas function in Eq. 1 to a zero-augmented model in which the zeroes and nonzero harvests are modeled via separate functions, as detailed in the Supplementary Materials. As in previous work (10), we use a Bernoulli distribution to model the probability of success versus failure, and the distribution of nonzero harvests is assumed to follow a gamma distribution.
To model latent skill across the life span, we adapt the von Bertalanffy growth model (28). The benefit of the model is that individuals’ skill is assumed to be lowest at birth with eventual declines due to senescence. Within these constraints, the functional form of the model potentially exhibits considerable diversity depending on the empirical data. Age-related variation in skill is determined by a rate of growth, k, and a rate of decline, m. The growth component, k, potentially includes ecological knowledge, strength, cognitive function, and other traits that underlie foraging success but that exhibit reduced acceleration with age. For simplicity, the composite of these attributes can be dubbed knowledge. For a hunter of age x, the growth function, K, is represented by
| (2) |
where k is a parameter greater than zero that reflects the rate of increase.
The declining component, m, reflects the senescence of traits related to hunting skill. For a forager of age x, the decline in productive ability, M, decreases at a constant rate given by
| (3) |
where m is a parameter greater than zero that reflects the rate of decline.
Age-specific skill is then represented by the weighted product of the two preceding functions
| (4) |
where b is represents the elasticity for knowledge in the skill function. As detailed in the Supplementary Materials, the von Bertalanffy model permits diverse functional forms of skill across the life span, ranging from approximately sigmoidal to roughly quadratic shapes. Although we assume that the growth and senescence components of skill relate to proximate mechanisms, such as age-related variation in ecological knowledge and physical abilities, the available data do not allow us to examine those proximate mechanisms [cf. (15, 18)]. As a result, age-related variation in skill must be inferred from its effects on the observed productivity of hunters of heterogeneous ages.
The statistical model allows the von Bertalanffy parameters to vary across individuals, reflecting different rates of increasing skill or senescence among hunters. The parameters also vary across study sites, allowing for different rates of increase and decline in heterogeneous environments. As noted previously, we model hunting returns using a zero-augmented gamma model, and the respective skill functions of hunters and societies are estimated jointly from the Bernoulli and gamma functions. It would have been possible to estimate separate, correlated individual-level and site-level skill functions for the Bernoulli and gamma functions, respectively. Our modeling approach instead assumes that increases in skill have comparable effects in terms of reducing the probability of unsuccessful excursions and increases in the amount of meat acquired on successful outings. This assumption receives support from an earlier analysis of the Aché dataset, which suggests that there is a positive correlation (and no evident tradeoff) between hunters’ probabilities of acquiring something and the amounts that are harvested on successful trips (10).
In addition to the varying effects on the skill parameters, the model also allows the parameters for labor inputs and covariates in the linear model to vary across study sites. Missing data, particularly for hunt duration and technological variables, are common and addressed using Bayesian imputation and averaging methods.
At some study sites, hunters work cooperatively to harvest prey, and the data on these excursions assign the hunting returns to the group, not individual hunters. In those cases, we replace individual hunter skill in the production equation with the weighted average of the skill of the group members. The statistical model follows the principles of a multiple membership model (29, 30). When hunters are observed in different combinations of groups, it is possible to distinguish differences in skill between them.
We cannot rule out selection biases that complicate inferences. For instance, if there were a study site where highly skilled hunters are active regardless of environmental conditions and the relatively unskilled hunters are active only when returns are expected to be particularly favorable, then the estimated variation in hunters’ skill would likely be lower than a site where hunting activity occurs independently of skill.
We fit our model using a Hamiltonian Monte Carlo sampling algorithm in the Rstan package, version 2.16.1 (31). We implemented the model both as a forward simulation and as a statistical model. The forward simulation validates that the statistical model can recover parameters from data with known values. The statistical model, estimated from 10 chains of 500 iterations, exhibits efficient mixing and adequate diagnostics. The data and convenience functions are included as part of the cchunts R package, which is available alongside the model posterior, coding scripts, and accompanying information about the Open Science Framework (https://osf.io/2kzb6/).
Supplementary Material
Acknowledgments
Audiences in London, Lausanne, Nijmegen, Aarhus, UCLA, and the University of Utah contributed useful feedback on draft analyses and interpretations, as did the anonymous reviewers. The map was created was the multimedia department at the Max Planck Institute for Evolutionary Anthropology. Funding: This study was funded by the NSF (#1534548). Author contributions: J.K. and R.M. conceived the study, analyzed the data, and wrote the paper. All other authors contributed hard-earned field data. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Supplementary data and code are available at https://osf.io/2kzb6/. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/26/eaax9070/DC1
REFERENCES AND NOTES
- 1.Jones J. H., Primates and the evolution of long, slow life histories. Curr. Biol. 21, R708–R717 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovejoy C. O., The origin of man. Science 211, 341–350 (1981). [DOI] [PubMed] [Google Scholar]
- 3.Hawkes K., O’Connell J. F., Jones N. B., Alvarez H., Charnov E. L., Grandmothering, menopause, and the evolution of human life histories. Proc. Natl. Acad. Sci. U.S.A. 95, 1336–1339 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan H., Hill K., Lancaster J., Hurtado A. M., A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (2000). [Google Scholar]
- 5.Herrmann E., Call J., Hernández-Lloreda M. V., Hare B., Tomasello M., Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science 317, 1360–1366 (2007). [DOI] [PubMed] [Google Scholar]
- 6.González-Forero M., Gardner A., Inference of ecological and social drivers of human brain-size evolution. Nature 557, 554–557 (2018). [DOI] [PubMed] [Google Scholar]
- 7.DeCasien A. R., Williams S. A., Higham J. P., Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 1, 0112 (2017). [DOI] [PubMed] [Google Scholar]
- 8.R. A. Foley, The evolutionary consequences of increased carnivory in hominids, in The Early Human Diet: The Role of Meat; Meat-Eating and Human Evolution, C. B. Stanford, H. T. Bunn, Eds. (Oxford Univ. Press, 2001), pp. 305–331. [Google Scholar]
- 9.González-Forero M., Faulwasser T., Lehmann L., A model for brain life history evolution. PLOS Comput. Biol. 13, e1005380 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElreath R., Koster J., Using multilevel models to estimate variation in foraging returns. Effects of failure rate, harvest size, age, and individual heterogeneity. Hum. Nat. 25, 100–120 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Walker R., Hill K., Kaplan H., McMillan G., Age-dependency in hunting ability among the Ache of Eastern Paraguay. J. Hum. Evol. 42, 639–657 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Blurton Jones N., Hawkes K., Draper P., Foraging returns of !Kung adults and children: Why didn’t !Kung children forage? J. Anthropol. Res. 50, 217–248 (1994). [Google Scholar]
- 13.Irons W., Adaptively relevant environments versus the environment of evolutionary adaptedness. Evol. Anthropol. 6, 194–204 (1998). [Google Scholar]
- 14.Blurton Jones N., Marlowe F. W., Selection for delayed maturity : Does it take 20 years to learn to hunt and gather? Hum. Nat. 13, 199–238 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Gurven M., Kaplan H., Gutierrez M., How long does it take to become a proficient hunter? Implications for the evolution of extended development and long life span. J. Hum. Evol. 51, 454–470 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Zent S., López-Zent E., Ethnobotanical convergence, divergence, and change among the Hoti of the Venezuelan Guayana. Adv. Econ. Bot. 15, 37–78 (2004). [Google Scholar]
- 17.Demps K., Zorondo-Rodŕıguez F., Garćıa C., Reyes-Garćıa V., Social learning across the life cycle: Cultural knowledge acquisition for honey collection among the Jenu Kuruba, India. Evol. Hum. Behav. 33, 460–470 (2012). [Google Scholar]
- 18.Koster J., Bruno O., Burns J. L., Wisdom of the elders? Ethnobiological knowledge across the lifespan. Curr. Anthropol. 57, 113–121 (2016). [Google Scholar]
- 19.Isaac G., The food-sharing behavior of protohuman hominids. Sci. Am. 238, 90–108 (1978). [DOI] [PubMed] [Google Scholar]
- 20.Winterhalder B., Diet choice, risk, and food sharing in a stochastic environment. J. Anthropol. Archaeol. 5, 369–392 (1986). [Google Scholar]
- 21.Kaplan H. S., Schniter E., Smith V. L., Wilson B. J., Risk and the evolution of human exchange. Proc. Biol. Sci. 279, 2930–2935 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R. Boyd, The evolution of reciprocity when conditions vary, in Coalitions and Alliances in Humans and Other Animals, A. Harcourt, F. DeWaal, Eds. (Oxford Univ. Press, 1992), pp. 473–489. [Google Scholar]
- 23.Hames R. B., A comparison of the efficiencies of the shotgun and the bow in neotropical forest hunting. Hum. Ecol. 7, 219–252 (1979). [Google Scholar]
- 24.Smith E. A., Inuit foraging groups: Some simple models incorporating conflicts of interest, relatedness, and central-place sharing. Ethol. Sociobiol. 6, 27–47 (1985). [Google Scholar]
- 25.Alvard M., Shotguns and sustainable hunting in the Neotropics. Oryx 29, 58–66 (1995). [Google Scholar]
- 26.Koster J. M., Hunting with dogs in Nicaragua: An optimal foraging approach. Curr. Anthropol. 49, 935–944 (2008). [Google Scholar]
- 27.Arrow K. J., Chenery H. B., Minhas B. S., Solow R. M., Capital-labor substitution and economic efficiency. Rev. Econ. Stat. 43, 225–250 (1961). [Google Scholar]
- 28.von Bertalanffy L., Untersuchungen Über Die Gesetzlichkeit Des Wachstums : I. Teil: Allgemeine Grundlagen Der Theorie; Mathematische Und Physiologische Gesetzlichkeiten Des Wachstums Bei Wassertieren. Wilhelm Roux Arch. Entwickl. Mech. Org. 131, 613–652 (1934). [DOI] [PubMed] [Google Scholar]
- 29.Browne W. J., Goldstein H., Rasbash J., Multiple membership multiple classification (MMMC) models. Stat. Model. 1, 103–124 (2001). [Google Scholar]
- 30.Aven B., Hillmann H., Structural role complementarity in entrepreneurial teams. Manage. Sci. 64, 5688–5704 (2018). [Google Scholar]
- 31.Stan Development Team, RStan: The R interface to Stan (2016). R package version 2.14.1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/26/eaax9070/DC1


