A new oxidative-stress sensor, MTK1, regulates the duration and magnitude of SAPK signaling to dictate cell fate decisions.
Abstract
Cells respond to oxidative stress by inducing intracellular signaling, including stress-activated p38 and JNK MAPK (SAPK) pathways, but the underlying mechanisms remain unclear. Here, we report that the MAP three kinase 1 (MTK1) SAPK kinase kinase (SAPKKK) functions as an oxidative-stress sensor that perceives the cellular redox state and transduces it into SAPK signaling. Following oxidative stress, MTK1 is rapidly oxidized and gradually reduced at evolutionarily conserved cysteine residues. These coupled oxidation-reduction modifications of MTK1 elicit its catalytic activity. Gene knockout experiments showed that oxidative stress–induced SAPK signaling is mediated by coordinated activation of the two SAPKKKs, MTK1 and apoptosis signal–regulating kinase 1 (ASK1), which have different time and dose-response characteristics. The MTK1-mediated redox sensing system is crucial for delayed and sustained SAPK activity and dictates cell fate decisions including cell death and interleukin-6 production. Our results delineate a molecular mechanism by which cells generate optimal biological responses under fluctuating redox environments.
INTRODUCTION
Living organisms are frequently exposed to a wide array of cellular stresses, which are denoted as environmental (extrinsic) or intrinsic conditions that are deleterious to normal cell growth and survival. Typical cellular stresses include physical, chemical, and biological insults, such as ultraviolet (UV) and ionizing radiation, genotoxins, heat shock, high osmolarity, accumulation of misfolded proteins, and oxidative stress. Of these stressors, oxidative stress is an inevitable consequence of aerobic life and arises because of an imbalance between reactive oxygen species (ROS) generation and the extent of antioxidant defenses (1, 2). The most common forms of ROS in cells and tissues are hydrogen peroxide (H2O2), superoxide (O2−), and hydroxyl radicals (•OH), whereas the defense system against ROS involves nonenzymatic and enzymatic antioxidants, such as catalase, and glutathione- or thioredoxin (Trx)–dependent reduction systems (3, 4). When the excessive ROS production overwhelms the defense system in vivo, vital biological macromolecules (e.g., proteins, lipids, and nucleic acids) undergo ROS-mediated oxidative damage, which eventually leads to the perturbation of cellular homeostasis and functions (1). Such oxidative damage is considered to be a major cause of cell injury and death under various pathological conditions including aging, cancer, neurodegenerative disorders, diabetes, atherosclerosis, and inflammatory diseases (5).
Besides these etiological aspects, ROS also serve as second messengers in physiological signaling events that regulate key biological processes such as cell proliferation, differentiation, apoptosis, and innate immunity (3, 6). For instance, upon contact with microorganisms, phagocytic cells [e.g., macrophages (MFs) and neutrophils] consume oxygen and generate large amounts of ROS by activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (7). This process, termed the respiratory (or oxidative) burst, initiates the killing of infected bacterial and fungal pathogens and promotes the production of proinflammatory cytokines including tumor necrosis factor–α (TNFα) and interleukin-6 (IL-6), and is thus vital for the host defense against such pathogens (8). Therefore, elucidation of the effects of ROS on cellular homeostasis is of paramount importance to understand these critical biological processes and to develop therapeutic interventions for many chronic and degenerative diseases. Nevertheless, the molecular mechanisms underlying the cellular responses to ROS remain ill defined.
In mammalian cells, various stressors including oxidative stress elicit activation of specific intracellular signaling pathways that dictate cell fate and behavior. Perhaps the most prominent signaling systems that control the cellular responses to these stressors are the stress-activated p38 and JNK MAPK (SAPK) cascades (9). Each of these cascades consists of three tiers of protein kinases: SAPK kinase kinase (SAPKKK), SAPKK, and SAPK. Upon stress stimuli, SAPKKKs directly phosphorylate and activate SAPKKs, which, in turn, activate their cognate SAPKs. Once activated, SAPKs phosphorylate various substrates in the cytoplasm and the nucleus to bring about changes in protein function and gene expression, thereby provoking appropriate cellular responses to stress, such as cell cycle arrest, apoptosis, and/or cytokine production (9). While there are a low number of SAPKKs (MKK3/4/6/7), more than a dozen structurally diverse SAPKKKs exist for cells to respond to a wide array of stress stimuli. These SAPKKKs include apoptosis signal–regulating kinase 1/2/3 (ASK1/2/3), transforming growth factor β–activated kinase 1 (TAK1), MAPK/ERK kinase kinase 1/2/3 (MEKK1/2/3), MAP three kinase 1 (MTK1) (also known as MEKK4/MAP3K4), mixed lineage kinase 1/2/3 (MLK1/2/3), and thousand and one amino acid protein kinase 1/2/3 (TAO1/2/3). Although it is believed that different SAPKKKs are activated in response to distinct sets of stress stimuli via disparate mechanisms, the precise roles and activation mechanisms of individual SAPKKKs are either unknown or are ill defined. These issues therefore need to be clarified to better understand the physiological and pathological significance of individual SAPKKKs and the molecular mechanisms underlying cell sensing and responses to stress stimuli.
We previously identified the human MTK1 SAPKKK and its activators, the growth arrest and DNA damage-inducible 45 (GADD45) family proteins (GADD45α/β/γ) (10–12). All GADD45 family genes are inducible at the level of transcription by various cellular stresses, although the optimal stimuli for each gene are different. Upon stress, the synthesized GADD45 proteins bind to the MTK1 regulatory domain, thereby inducing MTK1 activation through its autophosphorylation at Thr1493 (13). Thus, under certain types of stress, SAPK signaling is mediated, at least in part, by GADD45-induced MTK1 activation. This GADD45-MTK1-SAPK signaling axis is involved in various biological phenomena including stress-induced apoptosis, transforming growth factor β–mediated gene expression, hematopoiesis, T helper type I (TH1) activation, neocortex development, male sex differentiation, somite segmentation, and skeletal muscle atrophy (14–20). However, other potential mechanisms for MTK1 activation under stress remain unexplored. Furthermore, the role, if any, of MTK1 in regulation of the oxidative stress response is totally unknown.
Here, we report a novel, GADD45-independent mechanism of MTK1 activation, which is essential for delayed and sustained SAPK activity under oxidative stress. In this mechanism, MTK1 itself functions as an oxidative stress sensor that perceives changes in the intracellular redox state and transduces them into SAPK signaling. Analyses of the cells deficient for MTK1 and/or ASK1, another SAPKKK that participates in oxidative stress, showed that these two SAPKKKs cooperatively but differentially regulate oxidative stress–induced SAPK activity. We further show that the MTK1-mediated redox sensing and response system is crucial for cell fate decisions, including oxidative stress–induced cell death, and IL-6 production during the respiratory burst in MFs. Our results delineate a molecular mechanism by which cells sense and respond to oxidative stress to generate optimal biological responses.
RESULTS
MTK1 is activated under specific stress conditions
We previously showed that MTK1 is activated by the DNA alkylating agent, methyl methanesulfonate (MMS), which is a potent inducer of the GADD45 family genes (11, 13). To more comprehensively identify stress stimuli that elicit MTK1 activation, we treated human embryonic kidney (HEK) 293 cells stably expressing Myc-tagged MTK1 (termed M57 cells) with various stresses that induce SAPK activation (9, 21) and assessed the activation status of immunoprecipitated Myc-MTK1 by immunoblotting using an anti-phospho MTK1(T1493) antibody that recognizes only an active form of MTK1 (Fig. 1A) (13, 22). As expected, MMS treatment gradually increased MTK1 activity, which reached close to maximum at 4 hours, and remained high for at least 8 hours (Fig. 1A, top). Other DNA damage inducers such as irinotecan (CPT-11; a topoisomerase I inhibitor that induces single-strand DNA breaks), cisplatin (CDDP; a DNA-cross-linking agent), and etoposide (a topoisomerase II inhibitor that induces double-strand DNA breaks) also elicited MTK1 activation. Furthermore, other types of stresses such as microtubule destabilizing agents (vinblastine and nocodazole), endoplasmic reticulum stress inducers (thapsigargin and tunicamycin), heat shock (44°C), and oxidative stress (H2O2) efficiently induced MTK1 activity. Thus, MTK1 is markedly activated under these stress conditions. In addition, the mitogen 12-O-tetradecanoylphorbol 13-acetate (TPA), which activates both SAPK and extracellular signal–regulated kinase pathways (23), also induced MTK1 activation (Fig. 1B). Following TPA treatment, MTK1 activity was substantially elevated at 90 min and persisted for at least 480 min. We confirmed that, under these conditions, the activating T1493 phosphorylation of MTK1 resulted from autophosphorylation, rather than phosphorylation by an upstream kinase, as no T1493 phosphorylation occurred when the kinase defective Myc-MTK1(K/R) mutant was expressed, instead of wild-type (WT) MTK1, in cells (Fig. 1, A and B, right). In contrast to the above stimuli, no MTK1 activation was detected when the cells were treated with UV irradiation, high osmolarity stress (sorbitol), a ribotoxic stressor (anisomycin), or a calcium ionophore (ionomycin) (Fig. 1C), indicating that MTK1 activation is restricted to specific stressors at least in this cell line.
Fig. 1. Oxidative stress activates MTK1 in a GADD45-independent manner.
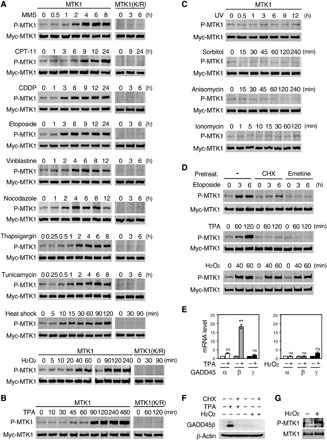
(A to C) HEK293 cells stably expressing Myc-MTK1 (M57 cells) or kinase-defective Myc-MTK1(K/R) were treated with MMS, CPT-11, CDDP, etoposide, vinblastine, nocodazole, thapsigargin, tunicamycin, heat shock, or H2O2 (A), with TPA (B), or with UV, sorbitol, anisomycin, or ionomycin (C) for the indicated times. (D) M57 cells were pretreated with cycloheximide (CHX) (15 μg/ml for 30 min) or emetine (25 μg/ml for 15 min), followed by treatment with etoposide, TPA, or H2O2. (A to D) Myc-MTK1 or Myc-MTK1(K/R) was immunoprecipitated from cell extracts, and phosphorylation of its activation site was detected by immunoblotting using an anti–phospho-MTK1(T1493) antibody (top rows). The same filters were reprobed with an anti-Myc antibody (second rows). (E and F) HEK293 cells were treated with TPA (for 120 min) or H2O2 (for 60 min). In (E), total RNA was extracted and analyzed for GADD45α, β, or γ mRNA expression (fold change) using quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Data are means ± SEM (n = 3). **P < 0.02; ns, not significant. In (F), cell extracts were probed for GADD45β or β-actin (loading control). Where indicated, the cells were pretreated for 30 min with CHX. (G) HEK293 cells were stimulated with H2O2 (for 60 min). Immunoprecipitated endogenous MTK1 was probed with anti–P-MTK1 or anti-MTK1 antibodies.
Oxidative stress activates MTK1 in a GADD45-independent manner
We next investigated whether the observed MTK1 activation occurred through stress-induced production of the GADD45 family proteins (GADD45α/β/γ), which are specific activators of MTK1 (11–13). For this purpose, we pretreated M57 cells with one of two different protein synthesis inhibitors (PSIs), cycloheximide or emetine, before stress and examined whether suppression of de novo synthesis of the GADD45 proteins impaired the observed MTK1 activation. As anticipated, pretreatment with these PSIs profoundly suppressed MTK1 activation induced by most of the stimuli tested: etoposide (Fig. 1D, top), MMS, heat shock, cisplatin, vinblastine, thapsigargin, tunicamycin (fig. S1, A and B), and TPA (Fig. 1D, middle), suggesting that these stimuli likely activate MTK1 through the induction of one (or more) of the GADD45 proteins. Notably, however, MTK1 activation was not decreased by these PSIs when the cells were exposed to oxidative stress (H2O2) (Fig. 1D, bottom). We confirmed that TPA stimulation selectively and strongly up-regulated GADD45β mRNA expression (Fig. 1E, left), whereas H2O2 did not significantly induce the expression of any of the GADD45 family mRNAs (Fig. 1E, right). Moreover, TPA, but not H2O2, induced the production of GADD45β protein in the absence of cycloheximide (Fig. 1F). Thus, these data indicate that oxidative stress–induced MTK1 activation occurs independently of the GADD45 proteins via a hitherto unidentified mechanism. We further confirmed that H2O2 activated Myc-MTK1 in M57 cells in a dose-dependent manner (fig. S1C) and induced the activation of endogenous MTK1 in HEK293 cells (Fig. 1G).
Oxidative stress–induced MTK1 activation requires its initial oxidation and subsequent reduction
We next asked how oxidative stress activates MTK1. Since the oxidation of Cys residues is a major mechanism regulating cellular responses to oxidative stress, we initially investigated whether Cys residues of MTK1 could be oxidized by H2O2 in cells. For this purpose, HEK293 or M57 cells were treated with various concentrations of H2O2 for 10 min. Cell lysates were then separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) in the absence or presence of a reducing agent, 2-mercaptoethanol (2ME), and immunoblotted to detect endogenous (HEK293) or Myc-tagged (M57) MTK1 (Fig. 2, A and B). Under nonreducing conditions (without 2ME), a marked shift in the electrophoretic mobility of endogenous or Myc-MTK1 to high–molecular weight species was observed in H2O2-treated cells in a dose-dependent manner. Since incubation of the lysates with 2ME completely abolished this mobility shift (Fig. 2, A and B), we concluded that MTK1 is rapidly oxidized in response to H2O2 exposure, resulting in the formation of its covalent complex with (an)other protein(s) through disulfide bonds.
Fig. 2. Oxidative stress–induced MTK1 activation requires both oxidation and reduction of MTK1.
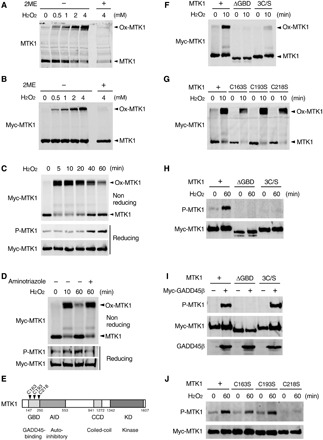
(A and B) HEK293 (A) and M57 cells (B) were stimulated with various concentrations of H2O2 for 10 min. Cell extracts were electrophoresed under nonreducing (−2ME) or reducing (+2ME) conditions, and probed for endogenous MTK1 (A) and Myc-MTK1 (B) by immunoblotting. Ox-MTK1, oxidized MTK1. (C) M57 cells were treated with H2O2 (1 mM). Cell extracts were electrophoresed under nonreducing conditions and immunoblotted with anti-Myc antibody (top). Immunoprecipitated Myc-MTK1 was electrophoresed under reducing conditions and probed with anti–P-MTK1 or anti-Myc antibodies (middle and bottom). (D) M57 cells were pretreated with the catalase inhibitor aminotriazol and stimulated with H2O2 (0.5 mM). Oxidation (top), phosphorylation (middle), and the expression level (bottom) of Myc-MTK1 were assessed as in (C). (E) Schematic diagram of MTK1 structure. GBD, GADD45-binding domain; AID, autoinhibitory domain; CCD, coiled-coil domain; KD, kinase domain. (F to H and J) HEK293 cells expressing Myc-MTK1 or its mutant derivatives (ΔGBD, 3C/S, C163S, C193S, or C218S) were treated with H2O2. Oxidation (F and G) and phosphorylation (H and J) of Myc-MTK1 were monitored as in (C). (I) Cos7 cells were cotransfected with Myc-MTK1 (WT, ΔGBD, or 3C/S) and GADD45β. Immunoprecipitated Myc-MTK1 was probed with anti–P-MTK1 or anti-Myc antibodies.
To examine whether the oxidation status of MTK1 correlates with its activation status, we next analyzed the time course of the oxidation and activation of MTK1 following H2O2 treatment (Fig. 2C). Application of H2O2 (1 mM) rapidly (within 5 min) induced oxidation of the majority of MTK1. Oxidized MTK1 was maintained for up to 20 min and then gradually returned to the reduced form (Fig. 2C, top). In contrast, H2O2 treatment induced relatively slow activation of MTK1 (Fig. 2C, middle), which occurred concurrently with the reduction of oxidized MTK1. This correlation between the decreased oxidation and the increased kinase activity of MTK1 suggested that the reduction of oxidized MTK1 is a prerequisite for its activation. MTK1 activation was not elicited following H2O2 treatment when MTK1 reduction was suppressed by cell treatment with the catalase inhibitor aminotriazole (Fig. 2D). Thus, the redox regulation of MTK1, i.e., its initial oxidation and subsequent reduction, mediates its activation under oxidative stress.
Redox regulation of Cys218 in MTK1 is required for oxidative stress–induced MTK1 activation
To identify the Cys residue(s) of MTK1 that is/are oxidized, we first transfected HEK293 cells with various deletion mutants of MTK1 and monitored their oxidation states by Western blot analysis under nonreducing conditions. MTK1 deleted in the GADD45-binding domain (MTK1-ΔGBD) showed no band shift following H2O2 treatment (Fig. 2, E and F), suggesting that the three Cys residues (C163/C193/C218) within the GBD are potential oxidation sites. Cys-to-Ser mutations of all three Cys residues (3C/S), but not single or double C/S mutations, profoundly inhibited the oxidation-induced band shift (Fig. 2, F and G, and fig. S2, A to C). These combined findings indicate that all three Cys residues in the GBD are oxidation targets.
Next, to determine whether the oxidation of these Cys residues in the GBD is relevant to its kinase activity, we examined whether H2O2 could evoke the activation of the oxidation-defective MTK1 mutants. As shown in Fig. 2H, neither MTK1-ΔGBD nor MTK1-3C/S were activated by H2O2 in HEK293 cells. However, the MTK1-3C/S mutant was robustly activated when GADD45β was coexpressed (Fig. 2I), indicating that the GBD Cys residues are essential for oxidative stress–induced, but not GADD45-mediated, MTK1 activation. Furthermore, while MTK1-C163S or MTK1-C193S single mutation resulted in little inhibition of H2O2-induced MTK1 activation, the MTK1-C218S mutant could not be activated by H2O2 (Fig. 2J). We therefore concluded that the MTK1 GBD functions not only as the binding domain for GADD45 but also as a redox sensor domain to perceive oxidative stress, and that redox regulation at C218 is essential for oxidative stress–induced MTK1 activation. C218 is highly conserved among MTK1 orthologs in diverse organisms (fig. S2D), suggesting that this redox-mediated MTK1 regulation might be similarly conserved.
Reduction of oxidized MTK1 elicits conformational changes critical for its activation
We next considered how the redox state of MTK1 regulates its kinase activity. We previously demonstrated the molecular mechanism of GADD45-mediated MTK1 activation as follows (see fig. S3A) (13): Under specific stress conditions, GADD45 proteins are induced and bind to the MTK1 GBD. This binding causes dissociation of the N-terminal autoinhibitory domain (AID) from the C-terminal kinase domain (KD) and leads to homodimerization of MTK1 via the coiled-coil dimerization domain (CCD). Dimerized MTK1 then becomes fully active by intermolecular autophosphorylation at T1493. Thus, N-C dissociation and homodimerization of MTK1 are the two critical steps of its activation. We therefore examined whether the oxidation and subsequent reduction of MTK1 could also elicit these activation steps. First, we tested whether oxidative stress disrupted the inhibitory N-C interaction. The MTK1 N-terminal (MTK1-N) and C-terminal (MTK-C) fragments, expressed as two separate molecules, can interact with each other, mimicking the N-C interaction, and this interaction can be disrupted by coexpression of GADD45β as we previously reported (Fig. 3A, lanes 2 and 3) (13). Following H2O2 treatment, MTK1 N-C interaction was not affected at 10 min, when MTK1 is highly oxidized and remains inactive (Fig. 2C and fig. S3B), but was markedly attenuated at 90 min, when oxidized MTK1 is converted to the reduced and active form. In contrast, the oxidation-defective MTK1-N(3C/S) remained associated with MTK1-C at 90 min following H2O2 treatment (Fig. 3B, left). Thus, the reduction of oxidized MTK1 is indispensable for the disruption of the inhibitory N-C interaction, explaining why MTK1 activation occurs concomitantly with its deoxidation. We next examined whether dimerization of MTK1 via the CCD, which is essential for the trans-autophosphorylation of MTK1 at T1493, is also required for its oxidative stress–induced activation. For this purpose, we used a dimerization-defective MTK1-3P mutant in which three hydrophobic residues (L997/I1001/V1008) critical for the coiled-coil–mediated helix bundle formation were substituted by a helix-disrupting proline (fig. S3C) (13). Although MTK1-3P was robustly oxidized (fig. S3D), it was not activated following H2O2 treatment (Fig. 3C). These findings indicate that, similar to the GADD45-mediated activation, disruption of the N-C interaction and subsequent CCD-mediated dimerization are both essential for oxidative stress–induced MTK1 activation and that oxidative stress can elicit these two sequential MTK1 activation steps in the absence of GADD45.
Fig. 3. Reduction of oxidized MTK1 induces its conformational change.
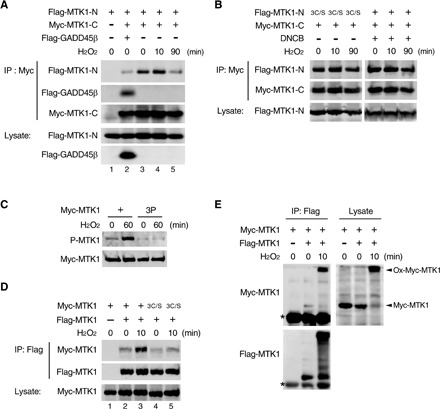
(A and B) Reduction of MTK1 disrupts the inhibitory N-C domain interaction. Cos7 cells were transfected with Myc-MTK1-C, Flag-MTK1-N (WT or its 3C/S mutant), or Flag-GADD45β as indicated and were stimulated with H2O2 for the indicated times. Myc-MTK1-C was then immunoprecipitated (IP), and coprecipitating Flag-MTK1-N was detected by immunoblotting (top). In (A), coprecipitating Flag-GADD45β is also shown (second). In (B), where indicated, the transfected cells were pretreated with the Trx-reductase inhibitor 2,4-dinitro-1-chlorobenzene (DNCB) (100 μM) before H2O2 stimulation. (C) HEK293 cells stably expressing Myc-MTK1 or its 3P (L997P/I1001P/V1008P) mutant were stimulated with H2O2. Immunoprecipitated Myc-MTK1 was probed with anti–P-MTK1 (top). (D and E) HEK293 cells were transfected with Flag-MTK1 together with Myc-MTK1 or its 3C/S mutant as indicated and were treated with or without H2O2. Immunoprecipitated Flag-MTK1 was then probed for coprecipitating Myc-MTK1 by Western blotting (top rows) under reducing (D) or nonreducing (E) conditions. In (E), asterisks indicate the immunoglobulin G (IgG) heavy chain. (A to E) The levels of protein expression are shown in the lower rows.
Last, since oxidized MTK1 forms a covalent complex with (an)other protein(s) through disulfide bonds, we investigated whether oxidized MTK1 molecules could be linked to each other via disulfide bridges between the Cys residues on GBDs. To test this possibility, we coexpressed Flag-tagged MTK1 (Flag-MTK1) and Myc-MTK1 in HEK293 cells, and Flag-MTK1 was immunoprecipitated before or after a short (10 min) exposure to H2O2. Coprecipitated Myc-MTK1 was then detected by immunoblotting. In unstimulated cells, Flag-MTK1 and Myc-MTK1 only weakly coprecipitated (Fig. 3D, lane 2). However, H2O2 treatment greatly enhanced the interaction between Flag-MTK1 and Myc-MTK1 (Fig. 3D, lane 3), although it did not augment the interaction between Flag-MTK1 and oxidation-defective Myc-MTK1-3C/S (Fig. 3D, lane 5). Western blot analysis under nonreducing conditions confirmed that oxidized Flag-MTK1 coprecipitated an oxidized form of Myc-MTK1 (Fig. 3E). These data showed that MTK1 proteins are covalently linked to each other via disulfide bonds between the Cys residues. Together, these findings indicate that, under oxidative stress, MTK1 is rapidly oxidized, resulting in dimer formation via disulfide bridges between the GBD Cys residues, but at this stage, MTK1 remains inactive. Oxidized MTK1 is then gradually reduced in cells, which leads to conformational changes of MTK1, i.e., the N-C dissociation, CCD-mediated dimerization, and activating autophosphorylation of MTK1, to induce its kinase activity. Since the activating autophosphorylation of MTK1 occurs intermolecularly, the initial disulfide bond–mediated formation of an inactive dimer may facilitate the subsequent, reduction-induced, MTK1 activation.
Trx activates MTK1 under oxidative stress by reducing oxidized MTK1
Since the reduction of MTK1 is essential for its activation, we next sought to determine how the oxidized MTK1 is converted to a reduced form. In general, the disulfide bonds formed by oxidized Cys residues are reduced to thiols by the Trx family proteins (24). We therefore determined whether Trx family proteins such as Trx, NRX, and TRP32 play a role in MTK1 reduction. Trx family proteins can be trapped with their specific substrates by Ser mutation of the second redox-active Cys residue in their conserved catalytic-site sequence (CxxC) (25, 26). In vivo coimmunoprecipitation experiments using these mutants showed that Myc-MTK1 interacted tightly with the substrate-trapping Flag-Trx(C35S) and weakly with WT Flag-Trx in response to H2O2 treatment (Fig. 4A). In contrast, MTK1 did not bind to Trx(C32S/C35S), a mutant defective in both substrate binding and enzymatic activity (Fig. 4A), to trapping mutants of other members of the Trx family (i.e., NRX and TRP32) (Fig. 4, B and C), or to another antioxidant enzyme, glutathione S-transferase P 1 (GSTP1) (fig. S4A). Furthermore, Trx (WT or the C35S mutant) did not interact with the oxidation-defective MTK1-3C/S even in the presence of H2O2 (Fig. 4D). Thus, Trx only associated with an oxidized form of MTK1 through its catalytic Cys residues. Moreover, we confirmed that Myc-MTK1 coprecipitated endogenous Trx from H2O2-treated cells (Fig. 4E). Inhibition of the endogenous Trx activity in cells using the Trx-reductase inhibitor 2,4-dinitro-1-chlorobenzene (27) strongly suppressed MTK1 reduction (Fig. 4F), thereby impeding the disruption of the inhibitory N-C interaction of MTK1 (Fig. 3B, right). Incubation of purified, oxidized Myc-MTK1 with recombinant Trx, but not with catalytically inactive Trx(C32S/C35S), induced substantial reduction of MTK1 in vitro (fig. S4B). On the basis of these combined findings, we concluded that MTK1 serves as a physiological substrate for Trx.
Fig. 4. Trx-mediated reduction of oxidized MTK1 elicits its kinase activity.
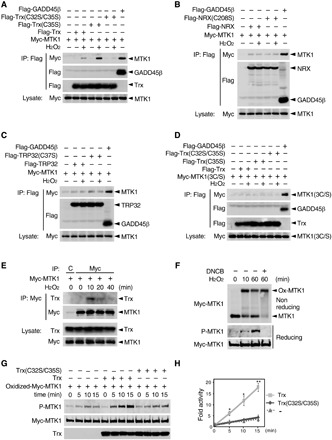
(A to D) HEK293 cells stably expressing Myc-MTK1 (A to C) or its 3C/S mutant (D) were transfected with Flag-GADD45β, Flag-Trx (WT, C35S, or C32S/C35S) (A), Flag-NRX (WT or C208S) (B), or Flag-TRP32 (WT or C37S) (C) as indicated, and treated with H2O2 for 20 min. Immunoprecipitated Flag-tagged proteins were probed for coprecipitating Myc-MTK1 (top). The levels of protein expression are shown in the lower rows. Flag-GADD45β served as a positive control for MTK1 binding. (E) M57 cells were treated with H2O2. Immunoprecipitated Myc-MTK1 was probed for coprecipitating endogenous Trx (top). C, control IgG. (F) M57 cells were pretreated with or without the Trx-reductase inhibitor 2,4-dinitro-1-chlorobenzene (DNCB) (100 μM) and stimulated with H2O2. Oxidation (top), phosphorylation (middle), and the expression level (bottom) of Myc-MTK1 were assessed as in Fig. 2C. (G) Oxidized Myc-MTK1 was immunopurified from H2O2-treated M57 cells, and incubated with recombinant Trx or Trx(C32S/C35S) for 30 min in vitro. Adenosine triphosphate was then added to initiate the kinase reaction and incubated for the indicated times. The reaction mixture was probed with anti–P-MTK1 (top) and anti-Myc (middle) antibodies. The total amounts of Trx and Trx(C32S/C35S) were also probed (bottom). (H) The intensity of the P-MTK1 bands in (G) was quantified. Error bars, SEM (n = 3). *P < 0.05; **P < 0.02.
We next tested whether Trx-mediated reduction of oxidized MTK1 would directly trigger MTK1 activation, using purified Trx and MTK1 proteins in an in vitro kinase activation assay. Oxidized Myc-MTK1 was immunopurified from H2O2-treated M57 cells, incubated with recombinant Trx (WT or its mutant derivatives), and then the kinase activity of MTK1 was assessed by its autophosphorylation at T1493 in an in vitro kinase assay. Incubation with purified recombinant Trx induced the reduction of oxidized MTK1 (fig. S4B) and stimulated its kinase activity (Fig. 4, G and H). In contrast, Trx(C32S/C35S) and Trx(C35S), both of which failed to reduce oxidized MTK1 (fig. S4B), had no stimulatory effect (Fig. 4, G and H, and fig. S4, C and D). Thus, the Trx-mediated reduction of oxidized MTK1 directly activates its kinase activity.
MTK1 and ASK1 cooperate to regulate oxidative stress–induced SAPK activation, but with different response characteristics
Next, to clarify the role of MTK1 in the regulation of oxidative stress–induced SAPK activation, we generated MTK1-null HEK293 cells (MTK1KO) using CRISPR-Cas9 technology and examined whether the activities of SAPKs (p38 and JNK) were affected in these cells. In WT HEK293 cells, p38 and JNK activities were rapidly up-regulated in response to H2O2 (1 mM) stimulation and subsequently gradually decreased (Fig. 5A). Compared to WT cells, the early phase (at 30 min after stimulation) of p38 and JNK activation was only marginally reduced in MTK1KO cells, whereas this activation was more profoundly reduced in MTK1KO cells at later time points (with both p38 and JNK activities almost undetectable at 120 min). Reintroduction of Myc-MTK1 into MTK1KO cells restored H2O2-induced p38 and JNK activities. Similar results were obtained at the level of the SAPKKs (MKK3, MKK6, and MKK4) that are the direct substrates of MTK1 and directly upstream of p38 and JNK activation (Fig. 5A), although H2O2 did not induce MKK7 activation in these and other cells at least under our experimental conditions (fig. S5, A and B). Thus, MTK1 plays an essential role in the induction of delayed and sustained activation of the p38 and JNK pathways following oxidative stress exposure.
Fig. 5. MTK1 mediates delayed and sustained activation of SAPKs by oxidative stress.
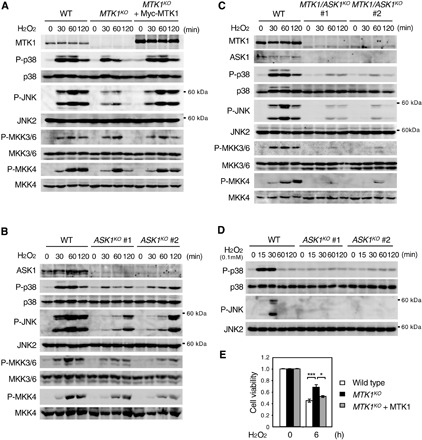
(A) Parental HEK293 cells (WT), MTK1 knock-out cells (MTK1KO), and MTK1KO cells reexpressing Myc-MTK (MTK1KO + Myc-MTK1) were treated with H2O2 (1 mM) for the indicated times. Phosphorylation levels of p38, JNK, MKK3/6, and MKK4 were analyzed by immunoblotting using appropriate phospho-specific antibodies (second, fourth, sixth, and eighth rows). The expression levels of MTK1, p38, JNK, MKK3/6, and MKK4 in cell lysates are also shown (top, third, fifth, seventh, and bottom). (B and D) ASK1 mediates early activation of SAPKs by oxidative stress. HEK293 cells and ASK1-KO cells (clones #1 and #2) were stimulated with H2O2 [1 mM for (B) and 0.1 mM for (D)]. Cell lysates were analyzed by immunoblotting for the expression levels and phosphorylation states of the indicated proteins as in (A). (C) MTK1 and ASK1 are major mediators of SAPK activation by oxidative stress. HEK293 and MTK1/ASK1 double-KO cells (clones #1 and #2) were stimulated with H2O2 (1 mM). The expression levels and phosphorylation states of the indicated proteins were analyzed by immunoblotting. (E) MTK1 promotes H2O2-induced cell death. The indicated cells were treated with H2O2 (0.5 mM) for 6 hours. Cell viability was assessed using the CCK8 assay. Data are means ± SEM (n = 3). *P < 0.05; ***P < 0.01.
Previous studies have shown that another SAPKKK, ASK1, is also involved in oxidative stress–induced SAPK activation (28). To examine whether there is any functional difference between MTK1 and ASK1 in the regulation of the oxidative stress response, we used CRISPR-Cas9 to generate ASK1-knockout (ASK1KO) and MTK1/ASK1–double knockout (MTK1/ASK1KO) cells. In each knockout cell line, we established two independent cell clones using two distinct guide RNAs targeting different sequences of the ASK1 gene to avoid the possibility of off-target effects. In contrast to MTK1KO cells, ASK1KO cells exhibited decreased p38 and JNK activities versus WT cells in the early period but not in the late phase (at 120 min) of p38 and JNK activation after H2O2 exposure (Fig. 5B). Similar time-dependent inhibitory effects were observed at the level of the SAPKKs. Furthermore, in MTK1/ASK1KO cells, both early and delayed p38 and JNK activation were markedly inhibited (Fig. 5C). Moreover, since MTK1 is activated by H2O2 in a dose-dependent manner (fig. S1C), we next analyzed the kinetics of p38 and JNK activation at a lower concentration of H2O2 (0.1 mM), which only weakly activates MTK1. Stimulation of parental HEK293 cells with 0.1 mM H2O2 induced only short-term (less than 60 min) activation of p38 and JNK, and, interestingly, this activation was suppressed in ASK1KO cells (Fig. 5D). These combined data indicate that, although MTK1 and ASK1 both regulate oxidative stress–induced SAPK activation, they have different response characteristics. When cells are exposed to relatively high oxidative stress, ASK1 mediates the early activation of SAPK signaling, while MTK1 mediates its delayed and prolonged activation. However, under weak–oxidative stress conditions, SAPK activation is mainly mediated by ASK1 to restrict the duration and amplitude of the stress signaling. These two SAPKKKs with different time and dose-response characteristics under oxidative stress may both be needed to elicit optimal biological responses to a rapidly fluctuating redox environment under physiological and pathological conditions. We found that H2O2 (0.5 mM)–induced cell death was significantly suppressed in MTK1KO cells (Fig. 5E), consistent with previous reports that prolonged activation of the SAPK pathways is crucial for induction of apoptotic cell death (29, 30).
MTK1-SAPK signaling is critical for IL-6 production following respiratory burst in MFs
To gain more insight into the physiological relevance of oxidative stress–induced MTK1 activation, we next investigated its role in the regulation of cytokine production, in which the SAPK pathways play a pivotal role (31, 32), following respiratory burst in MFs. Upon contact with microorganisms, MFs generate high levels of ROS and produce several proinflammatory cytokines (7, 8). To investigate whether oxidative stress–induced MTK1 activation contributes to MF function, we differentiated the human monocyte cell line THP-1 into MFs and used these MFs as a model (33). Initially, we confirmed that endogenous MTK1 was readily oxidized in these MFs immediately after H2O2 stimulation (5 min) and that it had returned to a reduced form within 90 min (Fig. 6A). Depletion of MTK1 by two distinct small hairpin RNAs (shRNAs) in the cells did not affect p38 and JNK activation at 30 min following H2O2 stimulation but significantly suppressed their activation at a later time point (120 min) (Fig. 6, B and C). Thus, the redox regulation of MTK1 and the resulting delayed activation of MTK1-SAPK signaling are also conserved in MFs. We then treated the MFs with zymosan, a yeast cell wall component that mimics fungal infection, to induce endogenous ROS production (Fig. 6D). Following zymosan exposure, oxidation of MTK1 was induced and maintained at high levels for approximately 120 min and then gradually returned to the reduced form by 360 min (Fig. 6E). Compared with control MFs, depletion of MTK1 by shRNA markedly suppressed zymosan-induced p38 and JNK activities at the late time point (4 hours), whereas ASK1 knockdown by small interfering RNA (siRNA) inhibited those preferentially at the earlier time point (2 hours) (Fig. 6F). Furthermore, simultaneous depletion of ASK1 and MTK1 resulted in decreased p38 and JNK activities at both early and late time points. We next quantified production of the major MF-derived proinflammatory cytokines, TNFα and IL-6, at the mRNA level using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (Fig. 6G). Consistent with a previous report (34), in WT MFs, TNFα mRNA rapidly increased (within 2 hours) following zymosan addition and then immediately declined, whereas IL-6 mRNA induction was delayed relative to that of TNFα. Only a slight increase in IL-6 mRNA was detected at 2 hours after zymosan addition, but a strong induction was evident at 6 hours. These findings suggest that these two cytokines are differentially regulated following respiratory burst and that IL-6 expression correlates with delayed activation of SAPK signaling. Depletion of MTK1 by the shRNAs, which selectively inhibits the late phase of SAPK activation, profoundly suppressed the zymosan-induced, delayed IL-6 expression but did not significantly affect the rapid TNFα expression (Fig. 6G). We confirmed that the zymosan-induced IL-6 production was dependent on ROS production and SAPK signaling, as pretreatment of the cells with an antioxidant N-acetyl cysteine or with a combination of p38 and JNK inhibitors (SB239063 and SP600125, respectively) markedly suppressed this IL-6 production (Fig. 6H and fig. S6A). Furthermore, zymosan did not trigger the expression of the GADD45 family genes in MFs (Fig. 6I and fig. S6B). Thus, ROS-induced MTK1 activation and the resulting sustained SAPK signaling are critical for IL-6 production induced by the respiratory burst in MFs. In contrast to MTK1 knockdown, depletion of ASK1 by siRNA, which selectively inhibits the early SAPK activation, significantly suppressed the rapid TNFα expression (fig. S6C).
Fig. 6. MTK1 is critical for respiratory burst-induced IL-6 production in MFs.
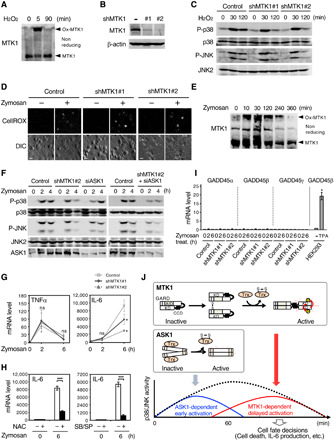
(A and E) MFs were stimulated with H2O2 (1 mM) (A) or zymosan (0.2 mg/ml) (E). Cell lysates were electrophoresed under nonreducing conditions and probed for endogenous MTK1. (B) Immunoblot analysis of MTK1 expression in THP-1 cells transduced with two different shRNAs against MTK1 (#1 or #2). (C) Immunoblot analyses of phosphorylated or total p38 and JNK in H2O2-stimulated MFs. (D) ROS production in MFs treated with (+) or without (−) zymosan was visualized using the fluorescent ROS probe CellROX Green. DIC, differential interference contrast. Scale bar, 30 μm. (F) Control MFs and MFs silenced for MTK1 (shMTK1#2) or/and ASK1 (siASK1) were stimulated with zymosan. Phosphorylation states of p38 and JNK were analyzed by immunoblotting. (G) Expression levels of TNFα (left) and IL-6 (right) mRNAs in zymosan-treated MFs were analyzed using qRT-PCR. (H) MFs were pretreated with N-acetyl cysteine (NAC) (left) or p38 and JNK inhibitors (SB/SP) (right), followed by stimulation with zymosan. IL-6 mRNA expression was then quantified. (I) qRT-PCR analyses of GADD45α, β, and γ expression in zymosan-treated MFs. TPA-treated HEK293 cells served as a positive control. All data were normalized by β-actin mRNA expression. (G to I) Error bars, SEM (n = 3). *P < 0.05; ***P < 0.01. (J) A schematic model of oxidative stress–induced SAPK activation. MTK1 and ASK1 are major mediators of oxidative stress–induced SAPK signaling. However, they have different time and dose-response characteristics.
DISCUSSION
In this study, we provide the first evidence that the MTK1 SAPKKK serves as an oxidative stress sensor that perceives cellular redox states and converts them into intracellular signaling to regulate cell fate decisions such as cell death and cytokine production. This stress sensor is unique in that it does not simply detect intracellular oxidation events but it responds to coupled oxidation and reduction reactions, and it also acts as an effector to induce delayed and sustained activation of SAPK signaling through its C-terminal KD. We found that the three Cys residues (C163/C193/C218) in its N-terminal GBD were critical for the redox sensor function of MTK1. These residues are rapidly oxidized and then gradually reduced following oxidative stress exposure, and, of these residues, the redox regulation of C218 is essential for the activation of the KD. Therefore, the GBD of MTK1 not only binds to the GADD45 proteins to respond to GADD45-inducing stresses (e.g., DNA damage) but also perceives intracellular redox environments to evoke cellular responses to oxidative stress. On the basis of this bifunctionality of the GBD, we propose renaming this domain as the GADD45-binding and redox-sensor domain (GARD). Since the amino-acid sequence of GARD and the redox-sensing C218 residue are highly conserved among vertebrates, the GADD45- and redox-mediated dual regulation of MTK1 might be similarly conserved across species.
We further showed here that the antioxidant protein Trx is a critical cofactor for oxidative stress–induced MTK1 activation, as it is responsible for the deoxidation of MTK1 in cells. We therefore propose the following mechanism for oxidative stress–induced MTK1 activation (Fig. 6J, upper inset). Upon oxidative stress, ROS quickly oxidizes the three Cys residues in the GARD of MTK1, resulting in the formation of the disulfide bonds between two MTK1 molecules. At this stage, MTK1 still remains in a closed (inhibited) conformation in which the N-terminal AID blocks the C-terminal KD and CCD. Subsequently, Trx gradually reacts with and reduces the disulfide bonds of oxidized MTK1 via its catalytic C32 and C35 residues. Trx-mediated reduction of MTK1, particularly at C218, causes dissociation of the AID from the KD and leads to the CCD-mediated homodimerization of the KD. Dimerized MTK1 is then activated by trans-autophosphorylation at T1493 in the kinase activation loop. During these sequential activation processes, reduction of oxidized MTK1 by Trx is relatively slow in cells and therefore serves as the rate-limiting step, explaining why MTK1 activation occurs only in the late phase following oxidative stress exposure.
Another interesting finding of the present study is that the two SAPKKKs, MTK1 and ASK1, cooperatively, but differentially, regulate SAPK signaling under oxidative stress conditions. By analysis of MTK1 or/and ASK1-KO cells, we showed that, although both of these SAPKKKs are major mediators of oxidative stress–induced SAPK activation, they have different response characteristics (Fig. 6J). Under relatively high oxidative stress conditions (which induce strong and long-term SAPK activation), ASK1 primarily mediates the early activation of SAPKs, while MTK1 mediates the delayed and prolonged SAPK activity. However, under weak oxidative stress (which produces weak and short-term SAPK activation), MTK1 is only slightly activated, and therefore, SAPK activation is mainly mediated by ASK1 to limit the duration and magnitude of SAPK activity. Thus, whereas ASK1 rapidly and sensitively responds to oxidative stress, and induces early and transient activation of SAPKs, MTK1 is relatively less sensitive to oxidative stress and slowly activated. However, once MTK1 is activated, it can elicit prolonged SAPK activation to dictate cell fate decisions.
Such different properties of the two SAPKKKs may be due to their distinct activation mechanisms. In this respect, previous studies showed that Trx also controls oxidative stress–induced ASK1 activation (28), but in a manner different from its control of MTK1 (Fig. 6J, middle inset). Under steady-state conditions, a reduced form of Trx interacts with ASK1 and inhibits its kinase activity. However, upon oxidative stress, Trx is rapidly oxidized at the catalytic C32 and C35 and forms an intramolecular disulfide bond between these two Cys residues. This conformational change of Trx leads to its dissociation from ASK1, allowing ASK1 activation. Therefore, in contrast to its positive regulatory role in MTK1 activation, Trx acts as an inhibitor for ASK1 activation. Since the catalytic Cys residues of Trx are highly sensitive to oxidation (24), even weak oxidative stress can efficiently and quickly oxidize Trx to induce the rapid activation of ASK1. ASK1 can be activated within 10 min following H2O2 treatment (35). In contrast, oxidation of MTK1 occurs less efficiently, and its activation requires the initial oxidation and subsequent slow Trx-mediated reduction. Thus, these different modes of action of Trx in ASK1 and MTK1 regulation may confer the distinct time and dose-response characteristics to oxidative stress on these two SAPKKKs.
Since the magnitude and duration of SAPK activities are critical determinants of cell fates and functions, including cell death and the immune response, the MTK1-induced prolonged SAPK activities would play a key role in dictating cellular responses to oxidative insults. Consistent with previous findings that a delayed and sustained phase of SAPK activation preferentially mediates cell death signaling (29), depletion of MTK1 in HEK293 cells significantly suppressed oxidative stress–induced cell death. Even more importantly, we showed here that MTK1 is essential for the respiratory burst-induced production of IL-6, but not of TNFα, in MFs. Although the precise mechanism by which MTK1 selectively regulates IL-6 production remains to be elucidated, previous studies have shown that IL-6 transcription induced by TLR ligands such as zymosan occurs slowly in innate immune cells including MFs, as it requires de novo synthesis of inhibitor of nuclear factor κB ζ (IκBζ), an inducible transcription factor essential for IL-6 gene expression (36). Following TLR stimulation, IκBζ mRNA is gradually increased, reaches a maximum as late as 2 hours after stimulation, and remains high for several hours. In contrast, TNFα can be rapidly transcribed without requirement of de novo protein synthesis (37). Therefore, to robustly induce IL-6 production following the respiratory burst, SAPK activity must persist via the MTK1-dependent mechanism for at least a few hours until IκBζ is sufficiently expressed, as SAPK activities are vital for the expression of various cytokines, including IL-6, at multiple levels such as chromatin modification, transcription, and mRNA stabilization (32, 38). Since IL-6 production from innate immune cells promotes inflammation as well as the development of acquired immunity by stimulating effector T cell development and antibody production (39), MTK1-dependent IL-6 biogenesis would play an important role in these processes. A detailed exploration of the response characteristics of individual SAPKKKs would greatly improve our understanding of the molecular basis of biological stress responses and human diseases. Given that oxidative stress–induced cell death and IL-6 production are both involved in various pathological conditions including chronic inflammatory diseases, metabolic disorders, cancer, and neurodegenerative diseases and that anti–IL-6 therapies are highly effective against certain diseases (e.g., rheumatoid arthritis, Takayasu arteritis, and Castleman diseases) (40), MTK1 could be a potential target for the development of novel therapeutic interventions for these and other diseases in which oxidative insults are involved.
MATERIALS AND METHODS
Cell culture
Cos7 and HEK293 cells and their derivatives were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), l-glutamate, penicillin, and streptomycin. THP-1 cells (provided by RIKEN BRC) and their derivatives were maintained in RPMI 1640 medium with 20% FBS and l-glutamate. To differentiate THP-1 cells to MFs, THP-1 cells were treated with TPA (80 nM) for 24 hours in RPMI 1640 medium without FBS and then incubated in RPMI 1640 medium with 10% FBS and l-glutamate for 24 hours. In Fig. 1, to activate SAPK pathways, the cells were treated with MMS (100 μg/ml), CPT-11 (10 μM), CDDP (100 μM), etoposide (50 μM), vinblastine (1 μM), nocodazole (2 μg/ml), thapsigargin (5 μM), tunicamycin (10 μg/ml), heat shock (44°C), H2O2 (1 mM), TPA (80 nM), UV (40 J/m2), sorbitol (0.4 M), anisomycin (10 μg/ml), or ionomycin (2 μM) for the indicated times.
Media and buffers
All lysis buffers (A to E) contained 20 mM tris-HCl (pH 7.5), 137 mM NaCl, 2 mM EDTA, 10% glycerol, 50 mM β-gycerophosphate, 1 mM sodium vanadate (Na3VO4), 10 mM sodium fluoride (NaF), 1 mM phenylmethylsufonyl fluoride (PMSF), aprotinin (10 μg ml−1), and leupeptin (10 μg ml−1). Triton X-100 (TX-100) (1%), 0.5% deoxycholate (DOC), and 1 mM dithiothreitol (DTT) were added to lysis buffer A. TX-100 (1%), 0.5% DOC, 25 mM N-ethylmaleimide (NEM), and 1.5 mM iodoacetic acid (IAA) were added to lysis buffer B. TX-100 (1%), 25 mM NEM, and 1.5 mM IAA were added to lysis buffer C. Nonidet P-40 (NP-40) (0.5%), 25 mM NEM, and 1.5 mM IAA were added to lysis buffer D. TX-100 (1%) was added to lysis buffer E. All immunoprecipitation (IP) wash buffers (A to D) contained 20 mM tris-HCl (pH 7.5), 2 mM EDTA, 10% (v/v) glycerol, 50 mM β-glycerophosphate, 1 mM Na3VO4, 10 mM NaF, and 1 mM PMSF. NaCl (137 mM), 2 mM EDTA, 1% TX-100, and 1 mM DTT were added to IP wash buffer A. NaCl (137 mM) and 1% TX-100 were added to IP wash buffer B. NaCl (137 mM) and 0.5% NP-40 were added to IP wash buffer C. NaCl (0.5 M) and 1% TX-100 were added to IP wash buffer D. Kinase wash buffer contained 25 mM tris-HCl (pH 7.5), 25 mM MgCl2, 25 mM β-glycerophosphate, 0.25 mM Na3VO4, and 4 mM EGTA. Kinase buffer contained 25 mM tris-HCl (pH 7.5), 25 mM MgCl2, 10 mM β-glycerophosphate, and 12.5 mM Na3VO4. SDS-PAGE loading buffer A consisted of 65 mM tris-HCl (pH 6.8), 5% (v/v) 2ME, 2% SDS, 0.1% bromophenol blue, and 10% glycerol. SDS-PAGE loading buffer B: SDS-PAGE loading buffer A without 2ME. Glutathione S-transferase (GST) buffer contained 50 mM tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, and aprotinin (5 μg ml−1). PreScission buffer contained 50 mM tris-HCl (pH 7.5), 150 mM NaCl, and 1 mM EDTA.
Plasmids
Expression plasmids for Myc-MTK1, Flag-MTK1, and their derivative mutants were described previously (13). Flag-GADD45β, Flag-Trx, Flag-NRX, Flag-TRP32, Flag-GSTP1, and their derivative mutants were subcloned into pcDNA3 (Invitrogen). Various point mutants of MTK1, Trx, NRX, and TRP32 were constructed using PCR-based mutagenesis. To generate retroviral expression vectors, Myc-MTK1, Flag-MTK1, or their derivative mutants were subcloned into pDON-AI (TaKaRa), pQCXIH, or pQCXIP (Invitrogen). pSUPER.retro.puro vector (Oligoengine) was used to generate shMTK1 constructs. pGEX-6P-1 (GE Healthcare) was used for bacterial expression of GST-Trx and its derivative mutants.
Transient transfection and retroviral infection
For transient transfection, preseeded cells were transfected with their respective expression plasmids using the X-tremeGENE 9 DNA Transfection Reagent (Sigma-Aldrich) according to the manufacturer’s protocol. Stable human cell lines were generated by retroviral infection and puromycin, hygromycin, or G418 selection. Retroviruses were produced in GP2-293 packaging cells by transient transfection with pVSV and pDON, pQCXIP, pQCXIH, or pSUPER.retro.puro plasmids. The MTK1 shRNA (#1 and #2) expression vectors were constructed by annealing the forward and reverse oligonucleotides [shMTK1#1 (forward), 5′-gatccccGCATGCAGGTGGATAATCTttcaagagaAGATTATCCACCTGCATGCttttta-3′; shMTK1#1 (reverse), 5′-agcttaaaaaGCATGCAGGTGGATAATCTtctcttgaaAGATTATCCACCTGCATGCggg-3′; shMTK1#2 (forward), 5′-gatccccACAACAGAGCGTGATCATAAttcaagagaTTATGATCACGCTCTGTTGTttttta-3; shMTK1#2 (reverse), 5′-agcttaaaaaACAACAGAGCGTGATCATAAtctcttgaaTTATGATCACGCTCTGTTGTggg-3′] and introducing them into the pSUPER.retro.puro vector.
Immunoblotting analyses
Immunoblot analyses were carried out as described previously (13). Digital images were captured using LAS-1000 Plus (Fujifilm). The following primary antibodies were used: monoclonal anti–Myc 9E10 (Santa Cruz Biotechnology, sc-40), anti–p38α F9 (sc-271120), anti–JNK2 D-2 (sc-7345), anti–ASK1 F-9 (sc-5294); anti–β-actin 6D1 (Wako, 010-27841); anti-Flag M2 (Sigma-Aldrich, F1804); anti–phospho-p38 D3F9 (Cell Signaling Technology, 4511), anti–phospho-JNK 81E11 (4668), anti–phospho-JNK 98F2 (4671), anti–phospho-MAPKAPK2 27B7 (3007), anti–phospho–c-Jun 54B3 (2361); polyclonal anti-Flag (Sigma-Aldrich, F7425); anti-JNK (Cell Signaling Technology, 9252), anti–phospho-MKK3/6 (9231), anti–phospho-MKK4 (9156), anti–phospho-MKK7 (4171), anti-MKK7 (4172), anti-MAPKAPK2 (3042); anti-Trx (Santa Cruz Biotechnology, sc-20146), anti-Myc (sc-789), anti-p38α (sc-535), anti-JNK (sc-571), anti-MKK3/6 (sc-13069), anti-MKK4 (sc-13070), anti-MTK1 (sc-28770), anti–c-jun (sc-1694); and anti-GADD45β (Cloud-Clone, PAL535Hu01). Affinity-purified anti-MTK1 and anti–phospho-MTK1(T1493) antibodies were made in-house (13, 15). All antibodies were used at a dilution of 1:1000, except anti-ASK1 (1:500) and anti-GADD45β (1:400).
Coimmunoprecipitation assay
Cell lysates were prepared in lysis buffer C or D and precleared with protein A or G Sepharose beads for 1 hour at 4°C. Precleared lysates were incubated with an appropriate antibody immobilized on protein G/A Sepharose beads at 4°C for 4 hours with gentle rotation. Immunoprecipitates were collected by centrifugation and washed three times with wash buffer B or C. Proteins were separated by SDS-PAGE and immunoblotted with the indicated antibodies.
In vitro kinase assay
Immune-complex kinase assays were performed as described previously (13). Briefly, M57 cells were treated with H2O2 (1 mM) for 10 min and harvested. Cell lysates were immunoprecipitated with an anti-Myc antibody at 4°C for overnight. Immune complexes were recovered with the aid of protein G Sepharose beads, washed twice with IP wash buffer D, twice with IP wash buffer B, and twice with kinase wash buffer. Immunoprecipitates resuspended in 45 μl of kinase buffer were reacted with 0.8 μg of recombinant Trx at 16°C for 30 min. The kinase reaction was initiated by the addition of 200 μM adenosine triphosphate, incubated at 16°C for 0, 5, 10, or 15 min, and terminated with SDS loading buffer.
Purification of recombinant Trx
Escherichia coli strain DH5α, transformed with pGEX6P-Trx (WT or its mutant derivatives), was precultured in 25 ml of LB/Ampicillin (100 μg ml−1) at 37°C for overnight. Each cultured medium was transferred to a fresh LB/ampicillin medium. After 37°C incubation for 3 hours, each cultured medium was treated with isopropyl-β-d(−)-thiogalactopyranoside (0.1 mM) and further incubated at 30°C for 2 hours. DH5α cells were harvested by centrifugation and resuspended in GST buffer. Cells were sonicated and treated with TX-100 (final concentration, 1%), and the cell lysates were incubated with Glutathione Sepharose 4B (GSH) beads (GE Healthcare) at 4°C for overnight. Recombinant protein–conjugated GSH beads were washed once with ice-cold tris-buffered saline, 0.1% tween 20 (TBST) and twice with ice-cold tris-buffered saline. To remove GST-tag from GST-Trx to obtain tag-free Trx, 16 U of PreScission protease (GE Healthcare) was added to GST-Trx–conjugated GSH beads in PreScssion buffer. After overnight incubation at 4°C, the supernatant was incubated with the reducing reagent DTT (2 mM) for 30 min on ice and dialyzed with Micro Float-A-Lyzer (Spectrum) at 4°C for overnight. Dialyzed liquid was then concentrated by Vivaspin 500 (Sartorius). The purified, reduced form of Trx was used for the experiments.
RNA extraction and qRT-PCR analyses
Total RNA was extracted from appropriate cells using TRIzol reagent (Invitrogen), precipitated by the ethanol precipitation method, and was then reverse-transcribed using the Prime Script-RT Master Mix (TaKaRa). Reverse-transcribed complementary DNA was used for real-time PCR quantification. RT-PCR was performed using the Thunderbird SYBR qPCR Mix (TOYOBO) and the following primers: GADD45α (forward), 5′-AGAGCAGAAGACCGAAAGGATGGA-3′; GADD45α (reverse), 5′-GCAGGATGTTGATGTCGTTCTCGC-3′; GADD45β (forward), 5′-ATTGCAACATGACGCTGGAAGAGC-3′; GADD45β (reverse), 5′-GGATGAGCGTGAAGTGGATT-3′; GADD45γ (forward), 5′-GACACAGTTCCGGAAAGCAC-3′; GADD45γ (reverse), 5′-TCAAGACTTTGGCTGACTCG-3′; GAPDH (forward), 5′-ACCCACTCCTCCACCTTTGA-3′; GAPDH (reverse), 5′-CTGTTGCTGTAGCCAAATTCGT-3′; ACTβ (forward), 5′-TCCCTGGAGAAGAGCTACGA-3′; ACTβ (reverse), 5′-TGAAGGTAGTTTCGTGGATGC-3′; IL-6 (forward), 5′-AAAGAGGCACTGGCAGAAAA-3′; IL-6 (reverse), 5′-TTTCACCAGGCAAGTCTCCT-3′; TNFα (forward), 5′-CGAGTGACAAGCCTGTAGC-3′; TNFα (reverse), 5′-GGTGTGGGTGAGGAGCACAT-3′. Each target gene was amplified using a two-step PCR program.
Generation of MTK1-KO and/or ASK1-KO cell lines
To construct CRISPR-Cas9 plasmids targeting human MTK1 or ASK1, target genomic DNA sequences were designed using the online design tool CRISPRdirect (https://crispr.dbcls.jp). The following oligos were then designed according to each specific target genomic DNA sequence: MTK1 KO (forward), 5′-CACCGTCGGGTTCTGACTCGGTCTC-3′; MTK1 KO (reverse), 5′-AAACGAGACCGAGTCAGAACCCGAC-3′; ASK1 KO#1 (forward), 5′-CACCGTGCCCCTGGCATCGGTTGTC-3′; ASK1 KO#1 (reverse), 5′-AAACGACAACCGATGCCAGGGGCAC-3′; ASK1 KO#2 (forward), 5′-CACCGCGGGGGCAGCCGACGGACCA-3′; ASK1 KO#2 (reverse), 5′-AAACTGGTCCGTCGGCTGCCCCCGC-3′. The single-guide RNAs were prepared and inserted into the pSpCas9-Puro expression plasmid (Addgene). HEK293 cells were transfected with a pSpCas9-Puro expression vector carrying MTK1-KO, ASK1-KO #1, or ASK1-KO #2 guide RNA using X-tremeGENE 9 (Sigma-Aldrich). Puromycin-selected cells were diluted and subcloned into 96-well plates to collect single-cell clones.
siRNA knockdown experiment
THP1-derived MFs were transfected with ASK1-siRNA using Lipofectamine RNAiMAX (Invitrogen). The targeting sequence was as follows: siASK1, 5′-GAUGUUCUCUACUAUGUUAdTdT-3′. The cells were subjected to Western blot analysis 70 hours after transfection.
Cell viability assay
Cell viability was monitored using the Cell Counting Kit-8 (CCK-8, DOJINDO) according to the manufacturer’s protocol. Briefly, parental HEK293, MTK1KO, and MTK1KO + Myc-MTK1 cells were seeded in a 96-well plate at a density of 10,000 cells per well. Twenty-four hours after incubation, cells were treated with H2O2 for 3 hours. Four microliters of WST-8 reagent (DOJINDO) was added to each well and incubated for further 3 hours. The percentage of viable cells were determined by measuring absorbance at 450 nm.
Statistics
The statistical significance of the difference between mean values was tested using Student’s t test. Data are means ± SEM of at least three independent experiments.
Supplementary Material
Acknowledgments
We thank P. O’Grady for critical reading of the manuscript. Funding: This work was supported, in part, by Grant-in-Aid for Scientific Research on Innovative Areas and other grants from the Japan Society for the Promotion of Science (JSPS) (15H04703, 16H06574, and 18H02609 to M.T.; 18 K15042 to T.N.) by grants from the Princess Takamatsu Cancer Research Fund, the Tokyo Biochemical Research Foundation, and the Mitsubishi Foundation to M.T., and by grants from the Uehara Memorial Foundation, the Sumitomo Foundation, and the Takeda Science Foundation to T.N. Author contributions: M.M., T.N., H.Mo., and M.T. conducted the experiments. M.M., T.N., H.Mi., and M.T. designed the experiments. M.M., T.N., and M.T. analyzed the data and wrote the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/26/eaay9778/DC1
REFERENCES AND NOTES
- 1.Sies H., Berndt C., Jones D. P., Oxidative stress. Annu. Rev. Biochem. 86, 715–748 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Ursini F., Maiorino M., Forman H. J., Redox homeostasis: The golden mean of healthy living. Redox Biol. 8, 205–215 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sies H., Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 11, 613–619 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisoschi A. M., Pop A., The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 97, 55–74 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C. J., Valko M., Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 38, 592–607 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S., Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 6, 183–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas D. C., The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol. Lett. 192, 88–96 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Brune B., Dehne N., Grossmann N., Jung M., Namgaladze D., Schmid T., von Knethen A., Weigert A., Redox control of inflammation in macrophages. Antioxid. Redox Signal. 19, 595–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyriakis J. M., Avruch J., Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 92, 689–737 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Takekawa M., Posas F., Saito H., A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 16, 4973–4982 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takekawa M., Saito H., A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Mita H., Tsutsui J., Takekawa M., Witten E. A., Saito H., Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 22, 4544–4555 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyake Z., Takekawa M., Ge Q., Saito H., Activation of MTK1/MEKK4 by GADD45 through induced N-C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol. Cell. Biol. 27, 2765–2776 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takekawa M., Kubota Y., Nakamura T., Ichikawa K., Regulation of stress-activated MAP kinase pathways during cell fate decisions. Nagoya J. Med. Sci. 73, 1–14 (2011). [PMC free article] [PubMed] [Google Scholar]
- 15.Chi H., Lu B., Takekawa M., Davis R. J., Flavell R. A., GADD45β/GADD45γ and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNγ production in T cells. EMBO J. 23, 1576–1586 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thalheimer F. B., Wingert S., De Giacomo P., Haetscher N., Rehage M., Brill B., Theis F. J., Hennighausen L., Schroeder T., Rieger M. A., Cytokine-regulated GADD45G induces differentiation and lineage selection in hematopoietic stem cells. Stem Cell Reports 3, 34–43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarkisian M. R., Siebzehnrubl D., Abnormal levels of Gadd45alpha in developing neocortex impair neurite outgrowth. PLOS ONE 7, e44207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warr N., Carre G.-A., Siggers P., Faleato J. V., Brixey R., Pope M., Bogani D., Childers M., Wells S., Scudamore C. L., Tedesco M., del Barco Barrantes I., Nebreda A. R., Trainor P. A., Greenfield A., Gadd45γ and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev. Cell 23, 1020–1031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bullard S. A., Seo S., Schilling B., Dyle M. C., Dierdorff J. M., Ebert S. M., De Lau A. D., Gibson B. W., Adams C. M., Gadd45a protein promotes skeletal muscle atrophy by forming a complex with the protein kinase MEKK4. J. Biol. Chem. 291, 17496–17509 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara A., Che Y.-S., Hanaoka R., Takeda H., Dawid I. B., Zebrafish GADD45β genes are involved in somite segmentation. Proc. Natl. Acad. Sci. U.S.A. 102, 361–366 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotamisligil G. S., Davis R. J., Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 8, a006072 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arimoto K., Fukuda H., Imajoh-Ohmi S., Saito H., Takekawa M., Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat. Cell Biol. 10, 1324–1332 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Chow J.-M., Shen S.-C., Wu C.-Y., Chen Y.-C., 12-o-Tetradecanoylphorbol 13-acetate prevents baicalein-induced apoptosis via activation of protein kinase C and JNKs in human leukemia cells. Apoptosis 11, 1999–2011 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Balsera M., Buchanan B. B., Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 140, 28–35 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Wynn R., Cocco M. J., Richards F. M., Mixed disulfide intermediates during the reduction of disulfides by Escherichia coli thioredoxin. Biochemistry 34, 11807–11813 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Morinaka A., Yamada M., Itofusa R., Funato Y., Yoshimura Y., Nakamura F., Yoshimura T., Kaibuchi K., Goshima Y., Hoshino M., Kamiguchi H., Miki H., Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci. Signal. 4, ra26 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Nordberg J., Zhong L., Holmgren A., Arnér E. S., Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. J. Biol. Chem. 273, 10835–10842 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H., Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17, 2596–2606 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura J.-J., Hübner A., Zhang C., Flavell R. A., Shokat K. M., Davis R. J., Chemical genetic analysis of the time course of signal transduction by JNK. Mol. Cell 21, 701–710 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Shen H.-M., Liu Z.-G., JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 40, 928–939 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Arthur J. S. C., Ley S. C., Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Rincón M., Davis R. J., Regulation of the immune response by stress-activated protein kinases. Immunol. Rev. 228, 212–224 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Chanput W., Mes J. J., Wichers H. J., THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 23, 37–45 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Lavieri R., Piccioli P., Carta S., Delfino L., Castellani P., Rubartelli A., TLR costimulation causes oxidative stress with unbalance of proinflammatory and anti-inflammatory cytokine production. J. Immunol. 192, 5373–5381 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Tobiume K., Saitoh M., Ichijo H., Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 191, 95–104 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M., Yamazaki S., Uematsu S., Sato S., Hemmi H., Hoshino K., Kaisho T., Kuwata H., Takeuchi O., Takeshige K., Saitoh T., Yamaoka S., Yamamoto N., Yamamoto S., Muta T., Takeda K., Akira S., Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IκBζ. Nature 430, 218–222 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Falvo J. V., Tsytsykova A. V., Goldfeld A. E., Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 11, 27–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saccani S., Pantano S., Natoli G., p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 3, 69–75 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Tanaka T., Narazaki M., Kishimoto T., IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbers C., Heink S., Korn T., Rose-John S., Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17, 395–412 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/26/eaay9778/DC1


